Nuts and Dried Fruits: An Update of Their Beneficial Effects on Type 2 Diabetes
Abstract
:1. Introduction
1.1. Nuts and Dried Fruits: The Concept
1.1.1. Nuts
1.1.2. Dried Fruits
1.2. Nutritional Composition of Nuts and Dried Fruits
1.3. Diet Quality in the Context of Nut and Dried Fruit Consumption
2. In Vivo and In Vitro Studies
2.1. Nuts
2.2. Dried Fruits
3. Epidemiological Studies on Nuts
4. Human Clinical Trials
4.1. Nuts
4.1.1. Acute Clinical Trials on Nuts
4.1.2. Chronic Clinical Trials on Nuts
4.2. Dried Fruits
4.2.1. Acute Clinical Trials on Dried Fruits
4.2.2. Chronic Clinical Trials on Dried Fruits
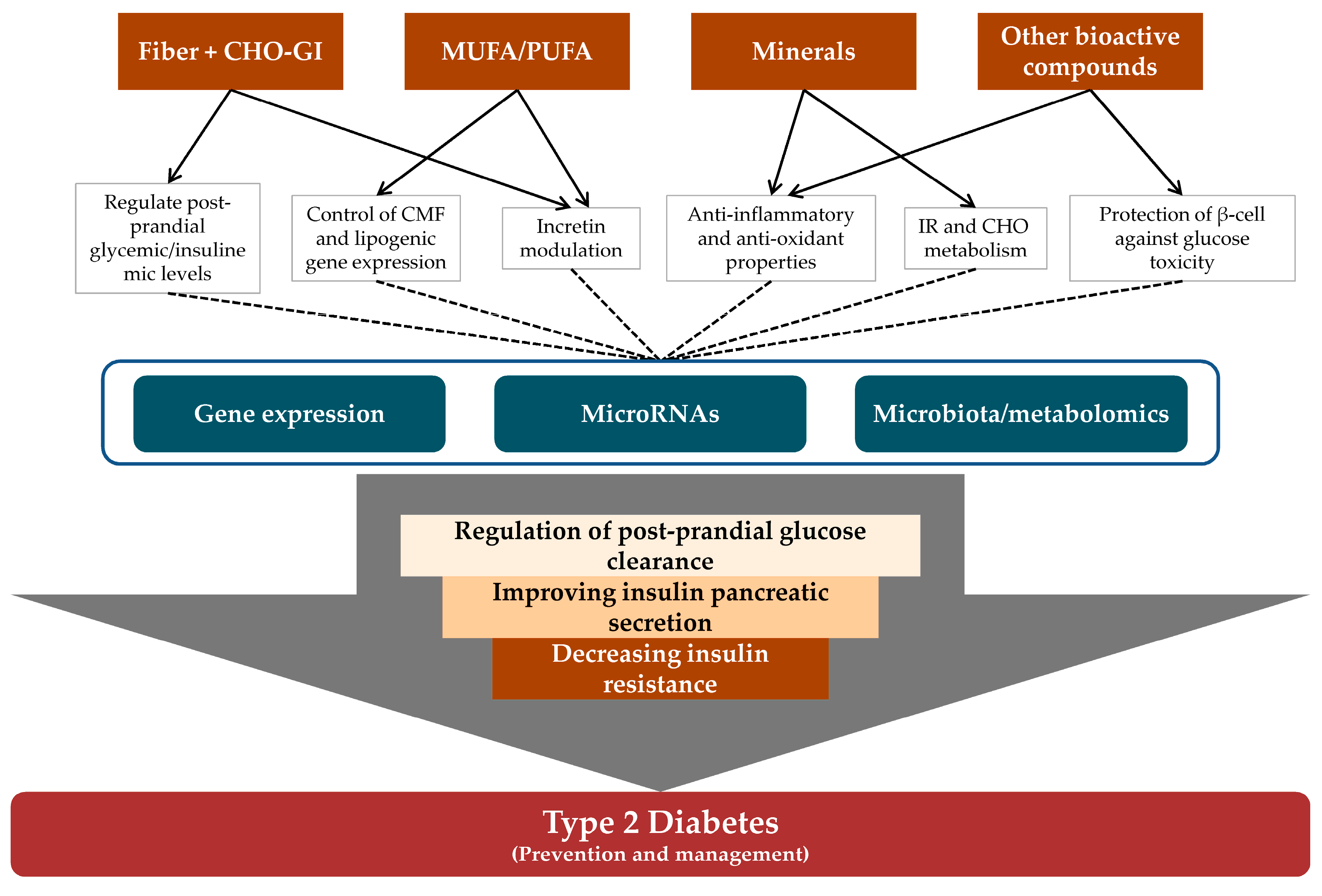
5. Potential Mechanisms Linking Nut and Dried Fruit Consumption to Glucose and Insulin Metabolism
5.1. Nut- and Dried Fruit-Related Nutrients and Their Role in Glucose and Insulin Metabolism
Fiber Content in Nuts and Dried Fruits
Carbohydrate Content—Glycaemic Index of Nuts and Dried Fruits
Fat Content in Nuts
Mineral Content in Nuts and Dried Fruits
Other Bioactive Compounds in Nuts and Dried Fruits
5.2. Cellular and Molecular Mechanisms Linking Nut and Dried Fruit Consumption and the Prevention and/or Management of T2D/IR
Gene Expression
MicroRNAs
Microbiota and Metabolomic Modulation
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPK | adenosine monophosphate-activated protein kinase |
| AT | aerobically trained |
| ATP | adenosine triphosphate |
| AOC | area over the curve |
| Apo | apolipoprotein |
| BMI | body mass index |
| BW | body weight |
| C | cholesterol |
| CHO | carbohydrate |
| CI | confidence interval |
| CMF | cellular membrane fluidity |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DF | dried fruit |
| EA | ellagic acid |
| EPIC | European Prospective Investigation into Cancer |
| FA | fatty acid |
| FBG | fasting blood glucose |
| FBP | fructose-1,6-bisphosphatase |
| G6Pase | glucose 6-phosphatase |
| GAE | gallic acid equivalents |
| GI | glycaemic index |
| GIP | gastric inhibitory polypeptide |
| GL | glycaemic load |
| GJ | grape juice |
| GLI | glibenclamide |
| GLP-1 | glucagon-like peptide-1 |
| GLUT | glucose transporter type |
| GPE | grape powder extract |
| HA | high almond |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein |
| HF | high fat |
| HFD | high-fat diet |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| HPFS | Health Professionals Follow-Up Study |
| IAUC | incremental area under the curve |
| ICAM-1 | intercellular adhesion molecule-1 |
| IGT | impaired glucose tolerance |
| IL-6 | interleukin-6 |
| IR | insulin resistance |
| LDL | low-density lipoprotein |
| LF | low-fat |
| LPS | lipopolysaccharide |
| MedDiet | Mediterranean diet |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| MetS | metabolic syndrome |
| MI | myocardial infarction |
| miRNA and miR | microRNA |
| M/F | male/female |
| MUFA | monounsaturated fatty acids |
| NA | not available |
| NAFLD | non-alcoholic fatty liver disease |
| NCEP | National Cholesterol Education Program |
| NHANES | National Health and Nutrition Examination Survey |
| NHS | Nurses’ Health Study |
| NLCS | Netherlands Cohort Study |
| NS | non-significant |
| Ob | obese |
| ORAC | oxygen radical absorbance capacity |
| Ow | overweight |
| PBMC | peripheral blood mononuclear cell |
| PF-4 | platelet factor-4 |
| PHS | Physicians' Health Study |
| PM | post-menopausal |
| PPAR | peroxisome proliferator-activated receptors |
| pre-D | pre-diabetes |
| PREDIMED | PREvención con DIeta MEDiterránea |
| PUFA | polyunsaturated fatty acids |
| PWE | polyphenol-rich walnut extract |
| Re | range |
| RETN | resistin |
| RCT | randomized clinical trial |
| RGR | relative glycaemic responses |
| RR | relative risk |
| RW | red wine |
| ROS | reactive oxygen species |
| S | sedentary |
| SBP | systolic blood pressure |
| SCCS | Southern Community Cohort Study |
| SCFA | short chain fatty acids |
| SFA | saturated fatty acids |
| SLC2A4 | solute carrier family 2 member 4 |
| SMHS | Shanghai Men's Health Study |
| STZ | streptozotocin |
| SWHS | Shanghai Women's Health Study |
| T2D | type 2 diabetes |
| TC | total cholesterol |
| TF | tissue factor |
| TG | triglycerides |
| TLGS | Tehran Lipid and Glucose Study |
| TNF-α | tumor necrosis factor-α |
| USDA | United States Department of Agriculture |
| VFM | visceral fat mass |
| WB | white bread |
| WC | waist circumference |
| WHtR | waist-to-height ratio |
References
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Ros, E. Nuts and CVD. Br. J. Nutr. 2015, 113 (Suppl.), S111–S120. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Salas-Huetos, A.; Salas-Salvadó, J. Mediterranean nuts: Origins, ancient medicinal benefits and symbolism. Public Health Nutr. 2011, 14, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Casas-Agustench, P.; Salas-Huetos, A. Cultural and historical aspects of Mediterranean nuts with emphasis on their attributed healthy and nutritional properties. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 1), S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F. The Mediterranean diet revisited: Evidence of its effectiveness grows. Curr. Opin. Cardiol. 2009, 24, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Shahidi, F. Composition, phytochemicals, and beneficial health effects of dried fruits: An overview. In Dried Fruits: Phytochemicals and Health Effects; Wiley-Blackwell: Oxford, UK, 2013; pp. 372–392. [Google Scholar]
- US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28 (revised), Version Current: May 2015. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 3 March 2017).
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [PubMed]
- Phillips, K.M.; Ruggio, D.M.; Ashraf-Khorassani, M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J. Agric. Food Chem. 2005, 53, 9436–9445. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Knekt, P.; Jarvinen, R.; Reunanen, A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Cadier, E.; Beulens, J.W.J.; van der A, D.L.; Spijkerman, A.M.W.; van der Schouw, Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hall, C.A. Composition and antioxidant activity of raisin extracts obtained from various solvents. Food Chem. 2008, 108, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Waters, A.R. Raisin consumption by humans: Effects on glycemia and insulinemia and cardiovascular risk factors. J. Food Sci. 2013, 78, A11–A17. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.E.; Keast, D.R.; Fulgoni, V.L.; Nicklas, T.A. Tree nut consumption improves nutrient intake and diet quality in US adults: An analysis of national health and nutrition examination survey (NHANES) 1999–2004. Asia Pac. J. Clin. Nutr. 2010, 19, 142–150. [Google Scholar] [PubMed]
- O’Neil, C.E.; Keast, D.R.; Nicklas, T.A.; Fulgoni, V.L. Out-of-hand nut consumption is associated with improved nutrient intake and health risk markers in US children and adults: National Health and Nutrition Examination Survey 1999–2004. Nutr. Res. 2012, 32, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Brown, R.; Gray, A.; Chisholm, A.; Delahunty, C. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J. Nutr. Metab. 2011, 2011, 357350. [Google Scholar] [CrossRef] [PubMed]
- McManus, K.; Antinoro, L.; Sacks, F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Keast, D.R.; O’Neil, C.E.; Jones, J.M. Dried fruit consumption is associated with improved diet quality and reduced obesity in US adults: National Health and Nutrition Examination Survey, 1999–2004. Nutr. Res. 2011, 31, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Wong, V.W.-S.; Chu, W.C.-W.; Wong, G.L.-H.; Li, L.S.; Leung, J.; Chim, A.M.-L.; Yeung, D.K.-W.; Sea, M.M.-M.; Woo, J.; et al. Diet-Quality Scores and Prevalence of Nonalcoholic Fatty Liver Disease: A Population Study Using Proton-Magnetic Resonance Spectroscopy. PLoS ONE 2015, 10, e0139310. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th Edition edDecember 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 10 March 2017).
- Bilbis, L.S.; Shehu, R.A.; Abubakar, M.G. Hypoglycemic and hypolipidemic effects of aqueous extract of Arachis hypogaea in normal and alloxan-induced diabetic rats. Phytomedicine 2002, 9, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ito, H.; Yoshida, T. Effect of the walnut polyphenol fraction on oxidative stress in type 2 diabetes mice. Biofactors 2004, 21, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Saravanan, R.; Pugalendi, K. V Effect of dietary substitution of groundnut oil on blood glucose, lipid profile, and redox status in streptozotocin-diabetic rats. Yale J. Biol. Med. 2006, 79, 9–17. [Google Scholar] [PubMed]
- Emekli-Alturfan, E.; Kasikci, E.; Yarat, A. Tissue factor activities of streptozotocin induced diabetic rat tissues and the effect of peanut consumption. Diabetes Metab. Res. Rev. 2007, 23, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Abdelmegeed, M.A.; Akbar, M.; Song, B.-J.J. Dietary walnut reduces hepatic triglyceride content in high-fat-fed mice via modulation of hepatic fatty acid metabolism and adipose tissue inflammation. J. Nutr. Biochem. 2016, 30, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Overman, A.; Bumrungpert, A.; Kennedy, A.; Martinez, K.; Chuang, C.-C.; West, T.; Dawson, B.; Jia, W.; McIntosh, M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int. J. Obes. (Lond.) 2010, 34, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Bumrungpert, A.; Kennedy, A.; Overman, A.; West, T.; Dawson, B.; McIntosh, M.K. Grape powder extract attenuates tumor necrosis factor α-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. Biochem. 2011, 22, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor—Mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadi, N.; Asadi-Shekaari, M.; Sheibani, V.; Jafari, M.; Shabani, M.; Asadi, A.R.; Tajadini, H.; Jarahi, M. Date fruit extract is a neuroprotective agent in diabetic peripheral neuropathy in streptozotocin-induced diabetic rats: A multimodal analysis. Oxid. Med. Cell. Longev. 2011, 2011, 976948. [Google Scholar] [CrossRef] [PubMed]
- Porto, L.C.S.; da Silva, J.; Ferraz, A.B.F.; Ethur, E.M.; Porto, C.D.L.; Marroni, N.P.; Picada, J.N. The Antidiabetic and Antihypercholesterolemic Effects of an Aqueous Extract from Pecan Shells in Wistar Rats. Plant Foods Hum. Nutr. 2015, 70, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Tédong, L.; Dzeufiet, P.D.D.; Dimo, T.; Asongalem, E.A.; Sokeng, S.N.; Flejou, J.-F.; Callard, P.; Kamtchouing, P. Acute and subchronic toxicity of Anacardium occidentale Linn (Anacardiaceae) leaves hexane extract in mice. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2006, 4, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Parkhideh, S.; Solhpour, A.; Madani, H.; Mahzouni, P.; Rahimi, P. Effect of ethanolic extract of Juglans regia L. on blood sugar in diabetes-induced rats. J. Med. Food 2008, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O. Laboratory evaluation of the hypoglycemic effect of Anacardium occidentale Linn (Anacardiaceae) stem-bark extracts in rats. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Egwim, E. Hypoglycemic potencies of crude ethanolic extracts of cashew roots and unripe pawpaw fruits in guinea pigs and rats. J. Herb. Pharmacother. 2005, 5, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Jelodar, G.; Mohsen, M.; Shahram, S. Effect of walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of alloxan induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2007, 4, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, E.K.; Gonzalez, A.; Garcia, C.; Tadros, J.H.; Chakraborty, G.; Toney, J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-alpha both in vitro and in vivo systems. Lipids Health Dis. 2009, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Adewale, O.F.; Isaac, O.; Tunmise, M.T.; Omoniyi, O. Palm oil and ground nut oil supplementation effects on blood glucose and antioxidant status in alloxan-induced diabetic rats. Pak. J. Pharm. Sci. 2016, 29, 83–87. [Google Scholar] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [PubMed]
- Chen, C.-Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2005, 135, 1366–1373. [Google Scholar] [PubMed]
- Wijeratne, S.S.K.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant polyphenols in almond and its coproducts. J. Agric. Food Chem. 2006, 54, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Tesoriere, L.; Butera, D.; Fazzari, M.; Monastero, M.; Allegra, M.; Livrea, M.A. Antioxidant activity of Sicilian pistachio (Pistacia vera L Var. Bronte) nut extract and its bioactive components. J. Agric. Food Chem. 2007, 55, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Tedong, L.; Madiraju, P.; Martineau, L.C.; Vallerand, D.; Arnason, J.T.; Desire, D.D.P.; Lavoie, L.; Kamtchouing, P.; Haddad, P.S. Hydro-ethanolic extract of cashew tree (Anacardium occidentale) nut and its principal compound, anacardic acid, stimulate glucose uptake in C2C12 muscle cells. Mol. Nutr. Food Res. 2010, 54, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, J.; Zareh, M.; Kamalinejad, M.; Pourahmad, J. Cytoprotective Effects of Hydrophilic and Lipophilic Extracts of Pistacia vera against Oxidative Versus Carbonyl Stress in Rat Hepatocytes. Iran. J. Pharm. Res. IJPR 2014, 13, 1263–1277. [Google Scholar] [PubMed]
- Aoun, M.; Michel, F.; Fouret, G.; Schlernitzauer, A.; Ollendorff, V.; Wrutniak-Cabello, C.; Cristol, J.-P.; Carbonneau, M.-A.; Coudray, C.; Feillet-Coudray, C. A grape polyphenol extract modulates muscle membrane fatty acid composition and lipid metabolism in high-fat--high-sucrose diet-fed rats. Br. J. Nutr. 2011, 106, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Weijers, R.N.M. Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr. Diabetes Rev. 2012, 8, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Soares de Moura, R.; da Costa, G.F.; Moreira, A.S.B.; Queiroz, E.F.; Moreira, D.D.C.; Garcia-Souza, E.P.; Resende, A.C.; Moura, A.S.; Teixeira, M.T. Vitis vinifera L. grape skin extract activates the insulin-signalling cascade and reduces hyperglycaemia in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2012, 64, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.H.; Giribabu, N.; Kassim, N.; Kumar, K.E.; Brahmayya, M.; Arya, A.; Salleh, N. Protective effect of aqueous seed extract of Vitis Vinifera against oxidative stress, inflammation and apoptosis in the pancreas of adult male rats with diabetes mellitus. Biomed. Pharmacother. 2016, 81, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, R.; Mann, T.R.; Spanevello, R.; Machado, M.M.; Zanini, D.; Pimentel, V.C.; Stefanello, N.; Martins, C.C.; Cardoso, A.M.; Bagatini, M.; et al. Moderate red wine and grape juice consumption modulates the hydrolysis of the adenine nucleotides and decreases platelet aggregation in streptozotocin-induced diabetic rats. Cell Biochem. Biophys. 2013, 65, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Salinas, R.; Decap, V.; Leguina, A.; Cáceres, P.; Perez, D.; Urquiaga, I.; Iturriaga, R.; Velarde, V. Antioxidant and anti hyperglycemic role of wine grape powder in rats fed with a high fructose diet. Biol. Res. 2015, 48, 53. [Google Scholar] [CrossRef] [PubMed]
- Overman, A.; Chuang, C.-C.; McIntosh, M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int. J. Obes. (Lond.) 2011, 35, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Steffen, L.M.; Ni, H.; Liu, K.; Jacobs, D.R. Dietary patterns and risk of incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008, 31, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.E.; Fulgoni, V.L.; Nicklas, T.A. Tree Nut consumption is associated with better adiposity measures and cardiovascular and metabolic syndrome health risk factors in U.S. Adults: NHANES 2005–2010. Nutr. J. 2015, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Ghorbani, Z.; Mirmiran, P.; Azizi, F. Nut consumption is associated with lower incidence of type 2 diabetes: The Tehran Lipid and Glucose Study. Diabetes Metab. 2017, 43, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Manson, J.E.; Stampfer, M.J.; Liu, S.; Willett, W.C.; Hu, F.B. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002, 288, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Gao, Y.-T.T.; Yang, G.; Li, H.-L.L.; Elasy, T.A.; Zheng, W.; Shu, X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008, 87, 162–167. [Google Scholar] [PubMed]
- Ibarrola-Jurado, N.; Bulló, M.; Guasch-Ferré, M.; Ros, E.; Martínez-González, M.A.; Corella, D.; Fiol, M.; Wärnberg, J.; Estruch, R.; Román, P.; et al. Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: The PREDIMED study. PLoS ONE 2013, 8, e57367. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Manson, J.E.; Willett, W.C.; Hu, F.B. Walnut consumption is associated with lower risk of type 2 diabetes in women. J. Nutr. 2013, 143, 512–518. [Google Scholar] [CrossRef] [PubMed]
- van den Brandt, P.A.; Schouten, L.J. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: A cohort study and meta-analysis. Int. J. Epidemiol. 2015, 44, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Boeing, H.; Drogan, D.; Schulze, M.B.; Feskens, E.J.; Amiano, P.; Barricarte, A.; Clavel-Chapelon, F.; de Lauzon-Guillain, B.; Fagherazzi, G.; et al. Consumption of fatty foods and incident type 2 diabetes in populations from eight European countries. Eur. J. Clin. Nutr. 2015, 69, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kochar, J.; Gaziano, J.M.; Djoussé, L. Nut consumption and risk of type II diabetes in the Physicians’ Health Study. Eur. J. Clin. Nutr. 2010, 64, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Pimpin, L.; Wu, J.H.Y.; Haskelberg, H.; Del Gobbo, L.; Mozaffarian, D. Is Butter Back? A Systematic Review and Meta-Analysis of Butter Consumption and Risk of Cardiovascular Disease, Diabetes, and Total Mortality. PLoS ONE 2016, 11, e0158118. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Josse, A.R.; Salvatore, S.; Brighenti, F.; Augustin, L.S.A.; Ellis, P.R.; Vidgen, E.; Rao, A.V. Almonds Decrease Postprandial Glycemia, Insulinemia, and Oxidative Damage in Healthy Individuals. J. Nutr. 2006, 136, 2987–2992. [Google Scholar] [PubMed]
- Josse, A.R.; Kendall, C.W.C.; Augustin, L.S.A.; Ellis, P.R.; Jenkins, D.J.A. Almonds and postprandial glycemia—A dose-response study. Metabolism. 2007, 56, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.M.; Considine, R.V.; Mattes, R.D.; Wong, J.; Jenkins, D.; Jiang, R.; Manson, J.; Stampfer, M.; Liu, S.; Willett, W.; et al. Acute and second-meal effects of almond form in impaired glucose tolerant adults: A randomized crossover trial. Nutr. Metab. (Lond.) 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Johnston, C.S. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A1c in individuals with well-controlled type 2 diabetes mellitus. Metabolism 2011, 60, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Crouch, M.A.; Slater, R.T. Almond “Appetizer” Effect on Glucose Tolerance Test (GTT) Results. J. Am. Board Fam. Med. 2016, 29, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; Josse, A.R.; Esfahani, A.; Jenkins, D.J.A. The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur. J. Clin. Nutr. 2011, 65, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; West, S.G.; Augustin, L.S.; Esfahani, A.; Vidgen, E.; Bashyam, B.; Sauder, K.A.; Campbell, J.; Chiavaroli, L.; Jenkins, A.L.; et al. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur. J. Clin. Nutr. 2014, 68, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.G.; Bordalo, L.A.; Rocha, A.L.C.; Freitas, D.M.O.; da Silva, M.V.L.; de Faria, V.C.; Martino, H.S.D.; Costa, N.M.B.; Alfenas, R.C. Ground roasted peanuts leads to a lower post-prandial glycemic response than raw peanuts. Nutr. Hosp. 2011, 26, 745–751. [Google Scholar] [PubMed]
- Moreira, A.P.B.; Teixeira, T.F.S.; Alves, R.D.M.; Peluzio, M.C.G.; Costa, N.M.B.; Bressan, J.; Mattes, R.; Alfenas, R.C.G. Effect of a high-fat meal containing conventional or high-oleic peanuts on post-prandial lipopolysaccharide concentrations in overweight/obese men. J. Hum. Nutr. Diet. 2016, 29, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; Esfahani, A.; Josse, A.R.; Augustin, L.S.A.; Vidgen, E.; Jenkins, D.J.A. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 1), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Most, M.M.; Lefevre, M.; Greenway, F.L.; Rood, J.C. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 1000–1006. [Google Scholar] [PubMed]
- Li, S.-C.; Liu, Y.-H.; Liu, J.-F.; Chang, W.-H.; Chen, C.-M.; Chen, C.-Y.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Damavandi, R.D.; Eghtesadi, S.; Shidfar, F.; Heydari, I.; Foroushani, A.R. Effects of hazelnuts consumption on fasting blood sugar and lipoproteins in patients with type 2 diabetes. J. Res. Med. Sci. 2013, 18, 314–321. [Google Scholar] [PubMed]
- Lasa, A.; Miranda, J.; Bulló, M.; Casas, R.; Salas-Salvadó, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutiérrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Parham, M.; Heidari, S.; Khorramirad, A.; Hozoori, M.; Hosseinzadeh, F.; Bakhtyari, L.; Vafaeimanesh, J. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: A randomized crossover trial. Rev. Diabet. Stud. 2014, 11, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M. Effect of Almond Supplementation on Glycemia and Cardiovascular Risk Factors in Asian Indians in North India with Type 2 Diabetes Mellitus: A 24–Week Study. Metab. Syndr. Relat. Disord. 2017, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bulló, M. Beneficial Effect of Pistachio Consumption on Glucose Metabolism, Insulin Resistance, Inflammation, and Related Metabolic Risk Markers: A Randomized Clinical Trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Josse, A.R.; Nguyen, T.H.; Faulkner, D.A.; Lapsley, K.G.; Singer, W. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjects: A randomized controlled crossover trial. Metabolism 2008, 57, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Flatt, S.W.; Natarajan, L.; Pakiz, B.; Quintana, E.L.; Heath, D.D.; Rana, B.K.; Rock, C.L. Effects of Diet Composition and Insulin Resistance Status on Plasma Lipid Levels in a Weight Loss Intervention in Women. J. Am. Heart Assoc. 2016, 5, e002771. [Google Scholar] [CrossRef] [PubMed]
- Claesson, A.-L.; Holm, G.; Ernersson, A.; Lindström, T.; Nystrom, F.H. Two weeks of overfeeding with candy, but not peanuts, increases insulin levels and body weight. Scand. J. Clin. Lab. Invest. 2009, 69, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.A.; Sabaté, J.M.; Iklé, D.N.; Cole, S.E.; Kandeel, F.R. Almonds vs. complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Gillen, L.J.; Patch, C.S.; Batterham, M.; Owen, A.; Baré, M.; Kennedy, M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004, 27, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Batterham, M.J.; Teuss, G.; Tan, S.-Y.; Dalton, S.; Quick, C.J.; Gillen, L.J.; Charlton, K.E. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur. J. Clin. Nutr. 2009, 63, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Njike, V.Y.; Millet, J.; Dutta, S.; Doughty, K.; Treu, J.A.; Katz, D.L. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care 2010, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Sweeney, L.L.; Liu, X.; Mantzoros, C.S. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity (Silver Spring) 2010, 18, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Liu, Y.; Lv, X.; Yang, W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr. J. 2012, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Davidhi, A.; Ma, Y.; Kavak, Y.; Bifulco, L.; Njike, V.Y. Effects of walnuts on endothelial function in overweight adults with visceral obesity: A randomized, controlled, crossover trial. J. Am. Coll. Nutr. 2012, 31, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Richmond, K.; Williams, S.; Mann, J.; Brown, R.; Chisholm, A. Markers of Cardiovascular Risk in Postmenopausal Women with Type 2 Diabetes Are Improved by the Daily Consumption of Almonds or Sunflower Kernels: A Feeding Study. ISRN Nutr. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Liu, Y.-H.; Chen, C.-M.; Chang, W.-H.; Chen, C.-Y.O. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: A randomized crossover controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Piotrowski, K.; Rau, T.; Waldmann, E.; Broedl, U.C.; Demmelmair, H.; Koletzko, B.; Stark, R.G.; Nagel, J.M.; Mantzoros, C.S.; et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: A randomized controlled cross-over clinical trial. Metabolism 2014, 63, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Oda, K.; Sabaté, J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr. J. 2014, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Sauder, K.A.; McCrea, C.E.; Ulbrecht, J.S.; Kris-Etherton, P.M.; West, S.G. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: A randomized trial. Metabolism 2015, 64, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Njike, V.Y.; Ayettey, R.; Petraro, P.; Treu, J.A.; Katz, D.L. Walnut ingestion in adults at risk for diabetes: Effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res. Care 2015, 3, e000115. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Celik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-week, randomized control trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Bleich, D.; Raghuwanshi, M.; Gould-Forgerite, S.; Gomes, J.; Monahan-Couch, L.; Oda, K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr. 2010, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Cabré-Vila, J.J.; Salas-Salvadó, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mejia, S.B.; Kendall, C.W.C.; Viguiliouk, E.; Augustin, L.S.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Maroleanu, A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of tree nuts on metabolic syndrome criteria: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2014, 4, e004660. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, O.; Winther, E.; Hermansen, K. Postprandial glucose and insulin responses to rolled oats ingested raw, cooked or as a mixture with raisins in normal subjects and type 2 diabetic patients. Diabet. Med. 1989, 6, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hertzler, S.R.; Byrne, H.K.; Mattern, C.O. Raisins are a low to moderate glycemic index food with a correspondingly low insulin index. Nutr. Res. 2008, 28, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Kanellos, P.T.; Kaliora, A.C.; Liaskos, C.; Tentolouris, N.K.; Perrea, D.; Karathanos, V.T. A study of glycemic response to Corinthian raisins in healthy subjects and in type 2 diabetes mellitus patients. Plant Foods Hum. Nutr. 2013, 68, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, A.; Lam, J.; Kendall, C.W.C. Acute effects of raisin consumption on glucose and insulin reponses in healthy individuals. J. Nutr. Sci. 2014, 3, e1. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Kanellos, P.T.; Gioxari, A.; Karathanos, V.T. Regulation of GIP and Ghrelin in Healthy Subjects Fed on Sun-Dried Raisins: A Pilot Study with a Crossover Trial Design. J. Med. Food 2017, 20, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Furchner-Evanson, A.; Petrisko, Y.; Howarth, L.; Nemoseck, T.; Kern, M. Type of snack influences satiety responses in adult women. Appetite 2010, 54, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, M.J.; Vaishnav, U.; Shrestha, S.; Torres-Gonzalez, M.; Wood, R.J.; Volek, J.S.; Fernandez, M.L. Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids Health Dis. 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.W.; Andreae, M.C.; Oliver Chen, C.-Y.; O’Keefe, S.F. Effect of raisin consumption on oxidative stress and inflammation in obesity. Diabetes Obes. Metab. 2008, 10, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Howarth, L.; Petrisko, Y.; Furchner-Evanson, A.; Nemoseck, T.; Kern, M. Snack Selection Influences Nutrient Intake, Triglycerides, and Bowel Habits of Adult Women: A Pilot Study. J. Am. Diet. Assoc. 2010, 110, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Kanellos, P.T.; Kaliora, A.C.; Tentolouris, N.K.; Argiana, V.; Perrea, D.; Kalogeropoulos, N.; Kountouri, A.M.; Karathanos, V.T. A pilot, randomized controlled trial to examine the health outcomes of raisin consumption in patients with diabetes. Nutrition 2014, 30, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.; Weiter, K.; Anderson, J. A randomized study of raisins versus alternative snacks on glycemic control and other cardiovascular risk factors in patients with type 2 diabetes mellitus. Phys. Sportsmed. 2015, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Weiter, K.M.; Christian, A.L.; Ritchey, M.B.; Bays, H.E. Raisins Compared with Other Snack Effects on Glycemia and Blood Pressure: A Randomized, Controlled Trial. Postgrad. Med. 2014, 126, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Dikeman, C.L.; Fahey, G.C. Viscosity as Related to Dietary Fiber: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Hopping, B.N.; Erber, E.; Grandinetti, A.; Verheus, M.; Kolonel, L.N.; Maskarinec, G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J. Nutr. 2010, 140, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C. The impact of nuts on diabetes and diabetes risk. Curr. Diab. Rep. 2005, 5, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Perez-Tilve, D. GLP-1 based therapeutics: Simultaneously combating T2DM and obesity. Front. Neurosci. 2015, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Bodnaruc, A.M.; Prud’homme, D.; Blanchet, R.; Giroux, I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr. Metab. (Lond.) 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, A.; Chiavaroli, L.; Srichaikul, K.; Augustin, L.S.A.; Sievenpiper, J.L.; Kendall, C.W.C.; Jenkins, D.J.A. The role of glycemic index and glycemic load in cardiovascular disease and its risk factors: A review of the recent literature. Curr. Atheroscler. Rep. 2014, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Storlien, L.H.; Baur, L.A.; Kriketos, A.D.; Pan, D.A.; Cooney, G.J.; Jenkins, A.B.; Calvert, G.D.; Campbell, L. V Dietary fats and insulin action. Diabetologia 1996, 39, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Haag, M.; Dippenaar, N.G. Dietary fats, fatty acids and insulin resistance: Short review of a multifaceted connection. Med. Sci. Monit. 2005, 11, RA359–RA367. [Google Scholar] [PubMed]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Segal-Isaacson, C.J.; Carello, E.; Wylie-Rosett, J. Dietary fats and diabetes mellitus: Is there a good fat? Curr. Diabetes Rep. 2001, 1, 161–169. [Google Scholar] [CrossRef]
- Rajaram, S.; Sabaté, J. Nuts, body weight and insulin resistance. Br. J. Nutr. 2006, 96 (Suppl. 2), S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L. Dietary interventions for metabolic syndrome: Role of modifying dietary fats. Curr. Diabetes Rep. 2009, 9, 43–50. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications - a review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.A.; Catania, A.S.; Ferreira, S.R. Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr. Rev. 2010, 68, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.P.; Sharma, R.; Bansal, D.D. Implications of magnesium deficiency in type 2 diabetes: A review. Biol. Trace Elem. Res. 2010, 134, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bulló, M.; Estruch, R.; Corella, D.; Martínez-González, M.A.; Ros, E.; Covas, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary magnesium intake is inversely associated with mortality in adults at high cardiovascular disease risk. J. Nutr. 2014, 144, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Current Understanding of Dietary Polyphenols and their Role in Health and Disease. Curr. Nutr. Food Sci. 2009, 5, 249–263. [Google Scholar] [CrossRef]
- Xiao, J.B.; Högger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B. Quercetin and Its Role in Chronic Diseases. In Advances in Experimental Medicine and Biology; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2016; Volume 929, pp. 377–387. [Google Scholar]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of Total Flavonoids, Myricetin, and Quercetin from Hovenia dulcis Thunb. As Inhibitors of α-Amylase and α-Glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms. Adv. Nutr. 2016, 7, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Nishii, S.; Zaima, N.; Moriyama, T.; Kawamura, Y. Ellagic acid improves hepatic steatosis and serum lipid composition through reduction of serum resistin levels and transcriptional activation of hepatic ppara in obese, diabetic KK-Ay mice. Biochem. Biophys. Res. Commun. 2013, 434, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Ellagic acid, an NF-κB inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem. Biol. Interact. 2014, 219, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Karachanak-Yankova, S.; Dimova, R.; Nikolova, D.; Nesheva, D.; Koprinarova, M.; Maslyankov, S.; Tafradjiska, R.; Gateva, P.; Velizarova, M.; Hammoudeh, Z.; et al. Epigenetic alterations in patients with type 2 diabetes mellitus. Balkan J. Med. Genet. 2015, 18, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Paluszczak, J.; Krajka-Kuźniak, V.; Baer-Dubowska, W. The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol. Lett. 2010, 192, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R. Mechanisms for the control of gene activity during development. Biol. Rev. Camb. Philos. Soc. 1990, 65, 431–471. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Claycombe, K.J.; Martinez, J.A.; Friso, S.; Schalinske, K.L. Nutritional Epigenomics: A Portal to Disease Prevention. Adv. Nutr. Int. Rev. J. 2013, 4, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Mukherjee, S.; Ray, D. Resveratrol and red wine, healthy heart and longevity. Heart Fail. Rev. 2010, 15, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamins, phytochemicals, diets, and their implementation in cancer chemoprevention. Crit. Rev. Food Sci. Nutr. 2004, 44, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Puchau, B.; Martínez, J.A. Fruit and vegetable consumption and proinflammatory gene expression from peripheral blood mononuclear cells in young adults: A translational study. Nutr. Metab. (Lond.) 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.C.; Chen, X.; Dreyfuss, G.; Eddy, S.R.; Griffiths-Jones, S.; Marshall, M.; et al. A uniform system for microRNA annotation. RNA 2003, 9, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Davis, C.D. The emerging role of microRNAs and nutrition in modulating health and disease. Annu. Rev. Nutr. 2014, 34, 305–336. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz Ros, J.A.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Cardona-Alvarado, M.I.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Sabater, M.; Fuentes-Batllevell, N.; Ramírez-Chávez, E.; Ricart, W.; Molina-Torres, J.; et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J. Nutr. Biochem. 2015, 26, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Giardina, S.; Salas-Salvadó, J.; Arcelin, P.; Bulló, M. Chronic pistachio intake modulates circulating microRNAs related to glucose metabolism and insulin resistance in prediabetic subjects. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.; Parkar, S.; Paturi, G.; Rosendale, D.; Blatchford, P. Modification of the Colonic Microbiota. Adv. Food Nutr. Res. 2013, 68, 205–217. [Google Scholar] [PubMed]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [PubMed]
- Nieman, D.C.; Scherr, J.; Luo, B.; Meaney, M.P.; Dréau, D.; Sha, W.; Dew, D.A.; Henson, D.A.; Pappan, K.L. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: A randomized, crossover trial. PLoS ONE 2014, 9, e113725. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Cañueto, D.; Giardina, S.; Salas-Salvadó, J.; Cañellas, N.; Correig, X.; Bulló, M. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in pre-diabetic subjects. J. Nutr. Biochem. 2017, 45, 48–53. [Google Scholar] [CrossRef] [PubMed]
| Nutrient | Almonds | Brazil Nuts b | Cashews | Hazelnuts | Macadamias | Peanuts | Pecans | Pine Nuts b | Pistachios | Walnuts c |
|---|---|---|---|---|---|---|---|---|---|---|
| Energy, Kcal | 579 | 659 | 553 | 628 | 718 | 567 | 691 | 673 | 560 | 654 |
| Water, g | 4.4 | 3.4 | 5.2 | 5.3 | 1.4 | 6.5 | 3.5 | 2.3 | 4.4 | 4.1 |
| Fat, g | 49.9 | 67.1 | 43.9 | 60.8 | 75.8 | 49.2 | 72.0 | 68.4 | 45.3 | 65.2 |
| SFA, g | 3.8 | 16.1 | 7.8 | 4.5 | 12.1 | 6.3 | 6.2 | 4.9 | 5.9 | 6.1 |
| MUFA, g | 31.6 | 23.9 | 23.8 | 45.7 | 58.9 | 24.4 | 40.8 | 18.8 | 23.3 | 9.0 |
| PUFA, g | 12.3 | 24.4 | 7.8 | 7.9 | 1.5 | 15.6 | 21.6 | 34.1 | 14.4 | 47.2 |
| Protein, g | 21.2 | 14.3 | 18.2 | 15.0 | 7.9 | 25.8 | 9.2 | 13.7 | 20.2 | 15.2 |
| CHO, g | 21.6 | 11.7 | 30.2 | 16.7 | 13.8 | 16.1 | 13.9 | 13.1 | 27.2 | 13.7 |
| Fiber, g | 12.5 | 7.5 | 3.3 | 9.7 | 8.6 | 8.5 | 9.6 | 3.7 | 10.6 | 6.7 |
| Ca, mg | 269 | 160 | 37 | 114 | 85 | 92 | 70 | 16 | 105 | 98 |
| Mg, mg | 270 | 376 | 292 | 163 | 130 | 168 | 121 | 251 | 121 | 158 |
| Na, mg | 1 | 3 | 12 | 0 | 5 | 18 | 0 | 2 | 1 | 2 |
| K, mg | 733 | 659 | 660 | 680 | 368 | 705 | 410 | 597 | 1025 | 441 |
| P, mg | 481 | 725 | 593 | 290 | 188 | 376 | 277 | 575 | 490 | 346 |
| Lutein-Zeaxanthin, µg | 1 | 0 | 22 | 92 | NA | 0 | 17 | 9 | 2903 | 9 |
| β-Carotene, µg | 1 | 0 | 0 | 11 | NA | 0 | 29 | 17 | 305 | 12 |
| α-Carotene, µg | 0 | 0 | 0 | 3 | NA | 0 | 0 | 0 | 10 | 0 |
| Phytosterols a, mg | 197 | 123.5 | 151 | 122 | 116 | NA | 158.8 | 236.1 | 214 | 110.2 |
| Total phenols, mg | 287 | 244 | 137 | 687 | 126 | 406 | 1284 | 32 | 867 | 1576 |
| Vitamin E (α-tocopherol), mg | 25.6 | 5.7 | 0.9 | 15.0 | 0.5 | 8.3 | 1.4 | 9.3 | 2.9 | 0.7 |
| Nutrient | Apples a | Apricots a | Currants (Zante) | Cranberries b | Dates c | Figs | Peaches a | Pears a | Plums/Prunes | Raisins d |
|---|---|---|---|---|---|---|---|---|---|---|
| Energy, Kcal | 243 | 241 | 283 | 308 | 282 | 249 | 239 | 262 | 240 | 299 |
| Water, g | 31.76 | 30.89 | 19.21 | 15.79 | 20.53 | 30.05 | 31.80 | 26.69 | 30.92 | 15.43 |
| Fat, g | 0.32 | 0.51 | 0.27 | 1.09 | 0.39 | 0.93 | 0.76 | 0.63 | 0.38 | 0.46 |
| CHO, g | 65.89 | 62.64 | 74.08 | 82.80 | 75.03 | 63.87 | 61.33 | 69.70 | 63.88 | 79.18 |
| Sugars, g | 57.19 | 53.44 | 67.28 | 72.56 | 63.35 | 47.92 | 41.74 | 62.20 | 38.13 | 59.19 |
| Fructose, g | NA | 12.47 | NA | 26.96 | 19.56 | 22.93 | 13.49 | NA | 12.45 | 29.68 |
| Protein, g | 0.93 | 3.39 | 4.08 | 0.17 | 2.45 | 3.30 | 3.61 | 1.87 | 2.18 | 3.07 |
| Fiber, g | 8.7 | 7.3 | 6.8 | 5.3 | 8.0 | 9.8 | 8.2 | 7.5 | 7.1 | 3.7 |
| Ca, mg | 14 | 55 | 86 | 9 | 39 | 162 | 28 | 34 | 43 | 50 |
| Fe, mg | 1.40 | 2.66 | 3.26 | 0.39 | 1.02 | 2.03 | 4.06 | 2.10 | 0.93 | 1.88 |
| Mg, mg | 16 | 32 | 41 | 4 | 43 | 68 | 42 | 33 | 41 | 32 |
| Na, mg | 87 | 10 | 8 | 5 | 2 | 10 | 7 | 6 | 2 | 11 |
| K, mg | 450 | 1162 | 892 | 49 | 656 | 680 | 996 | 533 | 732 | 749 |
| Cu, mg | 0.19 | 0.34 | 0.47 | 0.06 | 0.21 | 0.29 | 0.36 | 0.37 | 0.28 | 0.32 |
| β-carotene, µg | 0 | 2163 | 43 | 27 | 6 | 6 | 1074 | 2 | 394 | 0 |
| α-carotene, µg | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 57 | 0 |
| Lutein-Zeaxanthin, µg | 0 | 0 | 0 | 138 | 75 | 32 | 559 | 50 | 148 | 0 |
| Vitamin A, IU | 0 | 3604 | 73 | 46 | 10 | 10 | 2163 | 3 | 781 | 0 |
| Total phenols, mg GAE/100g e | 324 | 248 | NA | NA | 661 | 960 | 283 | 679 | 938 | 1065 |
| First Author (Year) [Reference] | Nut (Study Length) | Animal Model Used | Control | Intervention | Glucose and Insulin Metabolism Effects | Other Outcomes |
|---|---|---|---|---|---|---|
| Bilbis, L.S.; et al. (2002) [25] | Aqueous extract of peanut (21 days) | Alloxan-induced diabetic rats (n = 12) and non-diabetic rats (n = 12), divided into 3 equal groups | Non-diabetic with unrestricted standard diet and: (a) water ad libitum; (b) unrestricted access to drinking water and 2 mL of the extract 3 times/day; or (c) free access to the extract as the only drinking water. | Diabetic controls: treated as (a), (b) or (c) | The extract (alone or plus water) decreased FBG in both normal and alloxan-induced diabetic rats. | Significant decrease in serum TG, TC, HDL-C and LDL-C in both normal and alloxan-induced diabetic rats. |
| Fukuda, T.; et al. (2004) [26] | Polyphenol-rich walnut extract (PWE) (4 weeks) | db/db (n = 15) and C57BL/KsJ-db/db (n = 6) mice | Control db/db mice (n = 8) and C57BL/KsJ-db/+ m mice (n = 6, used for the blank group) were given water. | Experimental db/db mice (n = 7) received oral PWE (200 mg/kg BW) | Significant decrease in the level of urinary 8-hydroxy-2′-deoxyguanosin (in vivo marker of oxidative stress) in PWE-fed mice | Serum TG level was improved after PWE administration |
| Ramesh, B.; et al. (2006) [27] | Peanut oil (42 days) | Normal (n = 12) and STZ-diabetes induced (n = 18) Wistar rats | G1: Normal rats G3: Diabetic rats | G2: Normal rats + peanut oil diet (2%) G4: Diabetic rats + peanut oil diet (2%) G5: Diabetic rats + GLI (600 µg/kg BW) | Diabetic rats fed with peanut oil significantly reduce glucose, HbA1C, and G6Pase and FBP activities | Diabetic rats fed with peanut oil showed a small but significant reduction in TC, VLDL-C, LDL-C and TG and an increase in HDL-C. |
| Vassiliou, E.K.; et al. (2009) [41] | Peanut oil (21 days) | Male KKAy (n = 24) mice | KKA y mice fed with normal diet (11.4% fat) | Diabetic KKAy + HFD. Diabetic KKAy + HFD with peanut oil (0.70 mL/day). HFD is 58% fat. | Diabetic mice administered peanut oil had lower glucose levels than animals administered HFD alone. | |
| Choi, Y.; et al. (2016) [29] | Walnuts (20 weeks) | Male C57BL/6J mice (≥6 mice/group) | Regular rodent chow | HFD (45% energy-derived) with or without walnuts (21.5% energy-derived) | Glucose and insulin resistance tended to improve with walnut supplementation. | Walnut supplementation did not change the HFD-induced increase in BW or VFM. However, dietary walnuts significantly decreased the amounts of hepatic TG observed in HFD-fed mice. |
| Adewale, O.F.; et al. (2016) [42] | Peanut oil Palm oil (3 weeks) | Normal (n = 12) and alloxan-induced diabetic Wistar rats (n = 36) | Non-diabetic | Diabetic non-supplemented. Diabetic supplemented with PeO or PaO (200 mg/kg/day) | Significant reduction in blood glucose of supplemented groups (PeO + PaO) compared to the diabetic non-supplemented group. | Plasma Vitamins C and E and albumin levels were significantly increased in the supplemented groups versus the diabetic non-supplemented group. |
| First Author (Year) [Reference] | Study Name (Design) | Number of Subjects | Years of Follow-Up | Exposure | Findings |
|---|---|---|---|---|---|
| Jiang, R.; et al. (2002) [61] | NHS (Prospective) | 83,818 women | 16 | ≥5 times/week vs. never/almost never | Nut and peanut butter consumption was inversely associated with the risk of incident T2D. |
| Nettleton, J.A.; et al. (2008) [58] | MESA (Prospective) | 5011 men and women | 5 | Quintiles of low-risk food pattern | High intake of whole grains, fruit, nuts/seeds, and green leafy vegetables was inversely associated to the risk of incident T2D. |
| Villegas, R.; et al. (2008) [62] | SWHS (Prospective) | 64,227 women | 4.6 | Quintiles of peanut consumption | Consumption of peanuts was associated with a decreased risk of incident T2D. |
| Kochar, J.; et al. (2010) [67] | PHS I (Prospective) | 20,224 men | 19.2 | ≥7 servings of nuts/week vs. rarely or never consumers | No statistically significant association was found between nut consumption and T2D in either lean or overweight/obese subjects. |
| Ibarrola-Jurado, N.; et al. (2013) [63] | PREDIMED (Cross-sectional) | 7210 at high cardiovascular risk | Baseline | <1 serving/week, 1–3 servings/week and >3 servings/week | The upper category of nut consumption had a lower prevalence of T2D than the lowest category. |
| Pan, A.; et al. (2013) [64] | NHS, NHS II (Prospective) | 137,953 women | 10 | 1–3 servings/month, 1 serving/week, and ≥2 servings/week of walnuts vs. never/rarely | Higher walnut consumption is associated with a significantly lower risk of T2D incidence. |
| O’Neil, C.E.; et al. (2015) [59] | NHANES (Cross-sectional) | 14,386 men and women | 6 | Tree nut consumption compared with no consumption | Tree nut consumption was associated with lower HOMA-IR |
| Buijsse, B.; et al. (2015) [66] | EPIC-InterAct Study (Case-cohort) | 16,154 men and women | 12.3 Incident cases of T2D at 6.8 | Non-consumers vs. the middle tertile of consumption. | Consumption of nuts and seeds does not modify T2D risk under isocaloric conditions and independent from BMI. |
| Asghari, G.; et al. (2017) [60] | TLGS (prospective) | 1984 men and women | 6.2 ± 0.7 | ≥4 servings/week vs. 1 or <1 serving/week | Nut consumption was associated with a lower risk of T2D incidence. |
| First Author (Year) [Reference] | N° of Subjects (M/F) Type of Subject (Age in Years) | Type of Nut (Study Design) | Control Group | Intervention Group | Glucose and Insulin Metabolism Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Jenkins, D.J.; et al. (2006) [69] | 15 (7/8) Healthy subjects (26.3 ± 8.6) | Almonds (crossover) | 97 g of white bread |
| Almonds decrease postprandial glycaemia and insulinaemia. | Almonds are likely to decrease oxidative damage to serum proteins by decreasing glycaemic excursion and providing antioxidants. |
| Josse, A.R.; et al. (2007) [70] | 9 (7/2) Healthy subjects (27.8±6.9) | Almonds (crossover dose-response study) | White bread |
| The 90-g almond meal resulted in a significantly lower GI than the white bread control meal | |
| Mori, A.M.; et al. (2011) [71] | 14 (8/6) IGT (39.3 ± 10.9) | Almonds (crossover) | 75 g of available CHO (No almonds) | 75 g of available CHO from:
| Whole almonds significantly attenuated second-meal and daylong blood glucose IAUC. | GLP-1 concentrations did not significantly vary between treatments. |
| Kendall, C.W.; et al. (2011) [74] | 10 (3/7) Ow healthy subjects (48.3 ± 6.4) | Pistachios (crossover) | White bread | Study 1:
| Pistachios consumed alone had a minimal effect on postprandial glycaemia. Pistachios consumed with a carbohydrate meal attenuated the RGR. | |
| Cohen, A.E. and Johnston, C.S. (2011) [72] | 20 (6/14) Healthy subjects (n = 13) and T2D subjects (n = 7) (Healthy: 53.0 ± 3 and T2D: 66.0 ± 3.3) | Almonds (postprandial: crossover trial) | No almond meal | 28 g almonds enriched meal | The ingestion of almonds immediately before a starchy meal significantly reduced postprandial glycaemia by 30%. | |
| Kendall, C.W.; et al. (2011) [78] | 24 (11/13) Healthy (n = 14) and T2D subjects (n = 10) (Healthy: 36.0 ± 4 and T2D: 68.0 ± 2) | Mixed nuts (i.e., almonds, macadamias, walnuts, pistachios, hazelnuts and pecans) (crossover) | White bread | 3 doses of 30, 60 and 90 g of mixed nuts | Nuts improve short-term glycaemic control in patients with T2D. | |
| Reis, C.E.; et al. (2011) [76] | 13 (4/9) Healthy subjects (28.5 ± 10) | Peanuts (crossover) | Cheese sandwich | 63 g of:
| The ingestion of ground-roasted peanuts without skin for breakfast leads to a lower CHO intake and reduced postprandial glycaemic response. | |
| Moreira, A.P.; et al. (2014) [77] | 65 men Ow/Ob (Range: 18–50) | Conventional peanuts and high-oleic peanuts (parallel) | 56 g biscuit |
| Conventional peanut consumption was associated with decreased postprandial insulinaemia, which might be beneficial for saving β-cell function, independently of the influence on LPS concentrations. | |
| Kendall, C.W.; et al. (2014) [75] | 20 (8/12) Subjects with MetS (54.0 ± 8) | Pistachios (crossover) | Control 1: white bread Control 2: (white bread + butter + cheese) | Test meal 1: WB + 85 g of pistachios Test meal 2: 85 g of pistachios | Pistachio consumption reduced postprandial glycaemia compared with white bread. | Pistachio consumption increased GLP-1 levels compared with white bread. |
| Crouch, M.A. and Slater, R.T. (2016) [73] | 20 (13/7) Subjects with pre-diabetes * (Mean: 60.8) | Almonds (crossover) | No almonds | 12 units of dry-roasted almonds | A low-calorie almond preload “appetizer” decreased postprandial hyperglycemia. |
| First Author (Year) [Reference] | N° of Subjects (M/F) Type of Subjects (Age in Years) | Nut Study Design (Length of the Intervention) | Control Group | Intervention Group(s) | Glucose and Insulin Metabolism Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Lovejoy, J.C.; et al. (2002) [79] | 30 (13/17) T2D subjects (mean ± SEM: 53.8 ± 1.9) | Almonds Crossover (1 month per period) | HF-Control LF-Control | HF-HA LF-HA | No significant changes in glycaemia were observed. | Total cholesterol was lowest after the HF-HA diet. HDL-C was significantly decreased after the almond diet; however, no significant effect of fat source on LDL: HDL was reported. |
| Jenkins, D.J.A.; et al. (2008) [86] | 27 (15/12) Hyperlipidemic subjects (64 ± 9) | Almonds Crossover (1 month per period) | 147 ± 6 g/day of muffins | Almonds (73 ± 3 g/day) Half portion of almonds (37 ± 2 g/day) plus muffins (75 ± 3 g/day) Isoenergetic (mean, 423 Kcal/day) | No significant changes were observed in FBG, insulin, C-peptide, or HOMA-IR. The 24-h urinary C-peptide output, as a marker of 24-h insulin secretion, was significantly reduced by the half-and full-dose almonds in comparison to the control muffin diet after adjustment for urinary creatinine output. | There were no significant treatment differences in BW. |
| Claesson, A.L.; et al. 2009 [88] | 25 (11/14) Healthy subjects (range: 19–30) | Peanuts Parallel (2 weeks) | Addition of 20 kcal/kg-BW of candy to the regular caloric intake. | Addition of 20 kcal/kg-BW of roasted peanuts to the regular caloric intake. | Plasma-insulin and C-peptide increased in the candy group, but not in the peanut group. FBG was not modified. | Energy intake increased similarly in both groups. BW and WC increased significantly only in the candy group. At the end of the study LDL-C and ApoB/ApoA-1-ratio were higher in the candy group than in the peanut group. |
| Cohen, A.E.; et al. (2011) [72] | 13 (7/6) T2D subjects (66.0 ± 3.3) | Almonds Parallel (3 months) | Nut- free diet | Diet enriched with almonds (28 g, 5 times/week) | Significant reduction of HbA1c in the almond group compared to the nut-free diet group. | Chronic almond ingestion was associated with a reduction in BMI as compared with no change in the nut–free diet group. |
| Li, S.C.; et al. (2011) [80] | 20 (9/11) T2D subjects (Mean: 58) | Almonds Crossover (1 month per period) | NCEP step II diet (control diet); CHO (56 E%), protein (17 E%), and fat (27 E%). | Almonds were added to the control diet to replace 20% of total daily calorie intake. | Compared with subjects in the control diet, those in the almond diet reduced the levels of fasting insulin, FBG, and HOMA-IR. | Almond intake decreased TC, LDL-C, and LDL-C/HDL-C. The almond diet enhanced plasma α-tocopherol level compared with control diet. |
| Damavandi, R.D.; et al. (2013) [81] | 45 (15/33) Medicated T2D subjects (55.68 ± 7.74) | Hazelnuts Parallel (2 months) | Control diet | 10% of total daily calorie intake was replaced with hazelnuts | No significant differences in FBG between groups. | No changes in BMI were reported. Significant HDL-C reduction in control group was observed. Although the hazelnut group achieved a greater reduction in TG concentrations than the control group, these changes were non-significant. |
| Hernández-Alonso, P.; et al. (2014) [85] | 54 (29/25) Subjects with Pre-D Mean: 55 (range: 53.4–56.8) | Crossover (4 months per period) | Nut-free diet: the energy intake of other fatty foods, mostly olive oil, was adjusted to compensate for the energy from pistachios included in the PD. | Pistachio diet was supplemented with 2 ounces of pistachio (57 g/day) | FBG, insulin, and HOMA-IR decreased significantly after the chronic pistachio period compared with the nut-free period. | Fibrinogen, oxidized-LDL, and PF-4 significantly decreased under the pistachio period compared to the nut-free period, whereas GLP-1 increased. |
| Lasa, A.; et al. (2014) [82] | 191 (77/114) T2D subjects (Mean: 67) | Mixed nuts Parallel (1 year) | LFD | Mediterranean diets supplemented with either virgin olive oil or mixed nuts | Increased values of the adiponectin/leptin ratio and adiponectin/HOMA-IR ratio and decreased values of WC were observed in the three groups. | In both Mediterranean diet groups, but not in the LFD group, this was associated with a significant reduction in BW. |
| Parham, M.; et al. (2014) [83] | 44 (11/33) T2D subjects (Mean: 51) | Pistachios Crossover (3 months per period) | Previous diet without pistachios | Two snacks of 25 g pistachios/day | Marked decrease in HbA1c and FBG concentrations in the pistachio diet group compared with the control group. | There were no overall significant changes in BMI, blood pressure, HOMA-IR, or CRP concentrations. |
| Le, T.; et al. (2016) [87] | 213 women Ow/Ob subjects (Mean: 50) | Walnuts Parallel (1 year) | Control 1: a lower fat (20 E%), higher CHO (65 E%) diet. Control 2: lower CHO (45 E%), higher fat (35 E%) diet | Walnut-enriched diet: high fat (35 E%), lower CHO (45 E%) diet. | Insulin sensitivity and CRP levels improved after walnut-rich diet | TG decreased in all study arms at 6 months. The walnut-rich diet increased HDL-C more than either the lower fat or lower CHO diet. The walnut-rich diet also reduced LDL-C. |
| First Author (Year) [Reference] | N° of Subjects (M/F) Type of Subject (Age in Years) | Dried Fruit (Study Design) | Control Group | Intervention Group(s) | Glucose and Insulin Metabolism Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Rasmussen, O.; et al. (1989) [109] | 20 (9/11) Healthy (n = 11) and T2D subjects (n = 9) (Healthy: 30 ± 2; T2D subjects: 67 ± 2) | Raisins (crossover) | 75 g (healthy) or 50 g (T2D) of CHO | Raw rolled oats; oatmeal porridge or a mixture of raw rolled oats with raisins | Substitution of 25% of the starch meal with raisins (simple sugars) did not affect blood glucose or insulin responses | In normal and T2D subjects, the three meals produce similar glucose and insulin response curves. |
| Kim, Y.; et al. (2008) [110] | 10 S; 11 AT and 10 Pre-D (S (25.7 ± 1.3), AT (23.1 ± 1.0), Pre-D (50.0 ± 2.6)) | Raisins (crossover) | 50 g of available CHO from glucose | 50 g of available CHO from raisins | NS differences among groups. The GI of raisins seemed lower (≤55) in the S and P groups compared to moderate (GI, 56–69) in the A group. The insulinaemic index of raisins was not different among groups. | |
| Furchner-Evanson, A.; et al. (2010) [114] | 19 women ow subjects (39.2 ± 0.7) | Dried plums (crossover) | White bread (238 Kcal) | Dried plums (238 Kcal) Low-fat cookies (238 Kcal) | Dried plums elicited lower plasma glucose and insulin IAUC than low-fat cookies. | The satiety index IAUC was greater for the dried plums than low-fat cookies, and tended to promote a greater plasma ghrelin AOC |
| Kanellos, P.T.; et al. (2013) [111] | 30 (17/13) Healthy and T2D subjects (n = 15 each) (Healthy: 25.9 ± 0.8; T2D: 63.2 ± 1.7) | Corinthian raisins (crossover) | 50 g of glucose | 74 g of Corinthian raisins; 50 g of available CHO | Significantly different glucose peaks between raisins and glucose in healthy and in diabetic subjects. Glycaemic and insulinaemic responses were decreased after raisin consumption compared to glucose ingestion. | |
| Esfahani, A.; et al. (2014) [112] | 10 (4/6) Healthy subjects (39 ± 11) | Raisins (crossover) | 108 g of white bread; 50 g available CHO (consumed on two separate occasions) | R50: 69 g raisins; 50 g available CHO R20: 28 g raisins; 20 g available CHO | The raisin meals, R50 and R20, resulted in significantly reduced postprandial glucose and insulin responses compared with white bread | Raisins were determined to be low in GI, GL and insulinaemic index. |
| Kaliora, A.C.; et al. (2017) [113] | 10 Healthy normo-weight subjects (26.3 ± 0.8) | Raisins (crossover) | 50 g of glucose | 74 g of raisins; 50 g of available CHO | At 60 min, glucose and insulin levels were maximum in both groups. | GIP was lower after raising intake compared to glucose intake at 60 and 120 min postprandially. Ghrelin was lower after raisin compared to glucose intake at 120 and at 180 min post-ingestion. No differences were reported for GLP-1, apelin or obestatin in either trial. |
| First Author (Year) [Reference] | N° of Subjects (M/F) Type of Subject (Age in Years) | Study Design (Length of the Intervention) | Control Group | Intervention Group(s) | Glucose and Insulin Metabolism Outcomes | Other Outcomes |
|---|---|---|---|---|---|---|
| Puglisi, M.J.; et al. (2008) [115] | 34 (17/17 PM) Healthy (range: 50–70) | Raisin Parallel (6 weeks) | Walk (increase in the steps taken per day) | 150 g/day of raisins. Walk + 150 g/day of raisins | Changes in FBG and insulin values did not differ among intervention groups or from baseline. Plasma TNF-α decreased in the raisin group but no differences were reported between groups. | Plasma TC and LDL-C decreased in all the intervention groups. |
| Rankin, J.W.; et al. (2008) [116] | 17 (8/9) Ow (26.5 ± 7.6) | Raisin Crossover (2 weeks per period) | Jelly candy (264 Kcal/day) | 90 g/day raisins (264 Kcal/day) | NS changes in FBG or markers of inflammation or endothelial dysfunction after the raisin intervention. | Fasting protein-free ORAC was modestly higher after the raisin intervention than the jelly candy intervention. |
| Howarth, L.; et al. (2010) [117] | 26 women Ow/Ob (range: 25–54) | Dried plums Crossover (2 weeks per period) | Low-fat cookies (200 Kcal/day) | Dried plums (200 Kcal/day) | No changes were found in plasma glucose or insulin levels in any intervention. | Plasma TG concentration was unchanged by dried plum consumption and was higher after the consumption of low-fat cookies. Incorporation of dried plums or low-fat cookies into the diet did not alter energy intake or BW. |
| Anderson, J.W.; et al. (2014) [120] | 46 (21/25) Ow/Ob with Pre-D or at T2D risk. (snack (mean: 61.1), raisins (mean: 60.3)) | Raisins Parallel (12 weeks) | Snacks (300 Kcal/day) | 84 g/day of raisins (270 Kcal/day) | Fasting HbA1c levels were significantly reduced after raisin intake, whereas FBG and insulin levels were not significantly affected by the intake of raisins or snacks. Postprandial glucose levels were significantly reduced by raisin intake vs. snacks. | Raisin intake was associated with reductions in SBP and DBP. BW did not significantly change within or between groups. |
| Kanellos, P.T.; et al. (2014) [118] | 48 (25/23) T2D (raisins (63.7 ± 6.3), control (63 ± 8.5)) | Corinthian raisins Parallel (24 weeks) | Usual diet avoiding grapes and raisins | 36 g/day of Corinthian raisins | BW, glycaemic control, and lipid profile were not changed in either arm of the intervention. Patients in the CR arm reduced their DBP and increased their total antioxidant potential compared with baseline values and the control group. | No change in CRP was observed. A significant difference in plasma circulating p-hydroxybenzoic acid was observed between groups at the end of the trial. |
| Bays, H.; et al. (2015) [119] | 46 (19/27) T2D (mean: 58) | Dark raisins Parallel (12 weeks) | Snack group (300 Kcal/day) | 84 g/day of dark raisins group (270 Kcal/day) | Compared to the snack group, those who consumed raisins reduced their postprandial glucose levels, and an NS trend to a reduction in fasting glucose and HbA1c. NS changes in BW, fasting insulin, HOMA-IR or lipid profile between intervention groups. | Compared to alternative processed snacks, those who consumed raisins had a significant reduction in SBP but not a significant reduction in DBP. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Alonso, P.; Camacho-Barcia, L.; Bulló, M.; Salas-Salvadó, J. Nuts and Dried Fruits: An Update of Their Beneficial Effects on Type 2 Diabetes. Nutrients 2017, 9, 673. https://doi.org/10.3390/nu9070673
Hernández-Alonso P, Camacho-Barcia L, Bulló M, Salas-Salvadó J. Nuts and Dried Fruits: An Update of Their Beneficial Effects on Type 2 Diabetes. Nutrients. 2017; 9(7):673. https://doi.org/10.3390/nu9070673
Chicago/Turabian StyleHernández-Alonso, Pablo, Lucía Camacho-Barcia, Mònica Bulló, and Jordi Salas-Salvadó. 2017. "Nuts and Dried Fruits: An Update of Their Beneficial Effects on Type 2 Diabetes" Nutrients 9, no. 7: 673. https://doi.org/10.3390/nu9070673
APA StyleHernández-Alonso, P., Camacho-Barcia, L., Bulló, M., & Salas-Salvadó, J. (2017). Nuts and Dried Fruits: An Update of Their Beneficial Effects on Type 2 Diabetes. Nutrients, 9(7), 673. https://doi.org/10.3390/nu9070673







