Influence of Hot and Cold Environments on the Regulation of Energy Balance Following a Single Exercise Session: A Mini-Review
Abstract
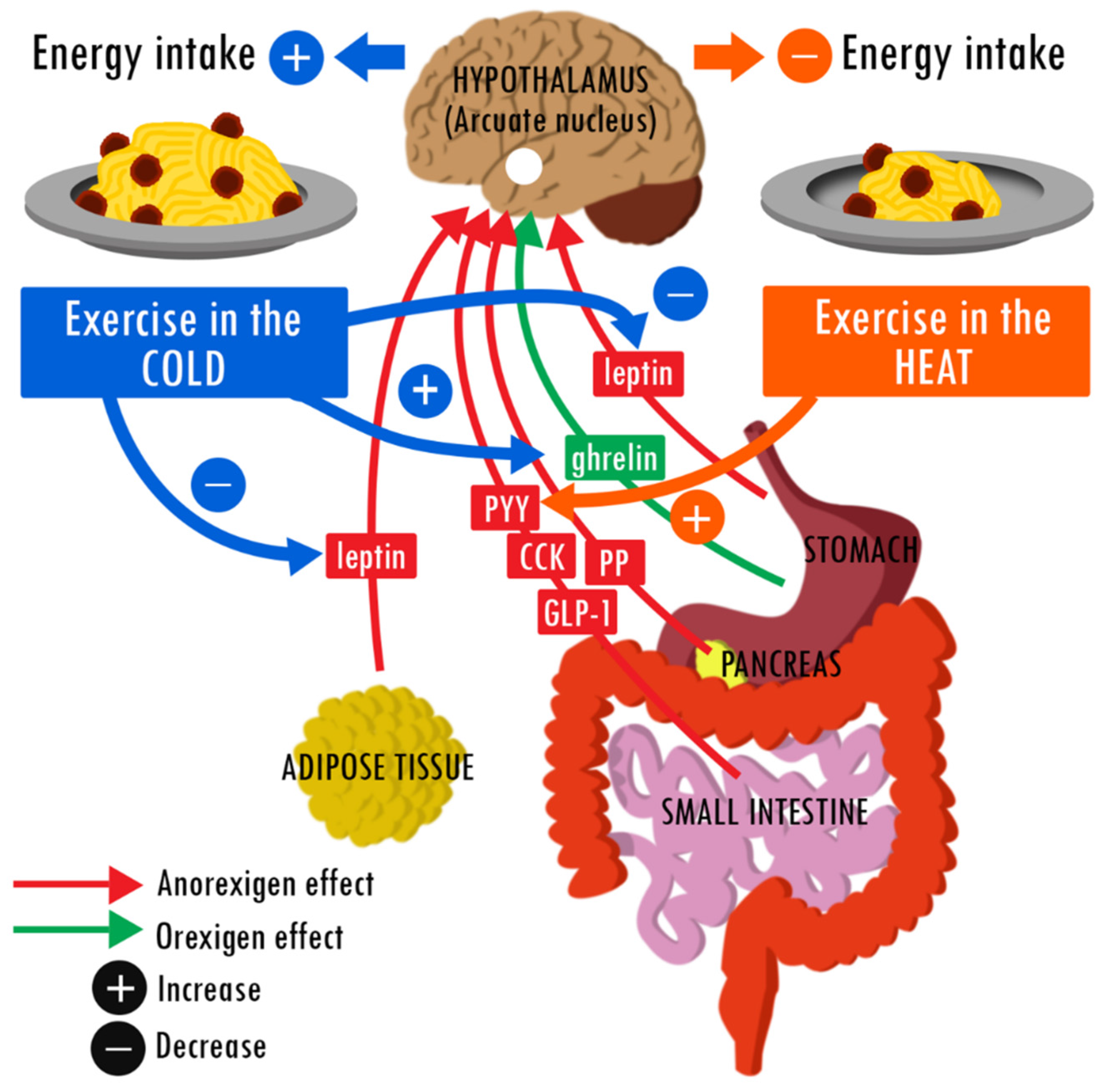
:1. Introduction
2. Effect of Exercise Sessions under Hot Conditions
2.1. Effect on Subjective Feeling of Appetite and Energy Intake
2.2. Effect on Appetite-Regulating Hormones
2.3. Specific Effect of Dehydration
3. Effect of Exercise Sessions under Cool Conditions
3.1. Effect on Subjective Feeling of Appetite and Energy Intake
3.2. Effect on Appetite-Regulating Hormones
4. Conclusions, Limitations, and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gurnani, M.; Birken, C.; Hamilton, J. Childhood Obesity: Causes, Consequences, and Management. Pediatr. Clin. N. Am. 2015, 62, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Yatsuya, H.; Li, Y.; Hilawe, E.H.; Ota, A.; Wang, C.; Chiang, C.; Zhang, Y.; Uemura, M.; Osako, A.; Ozaki, Y.; et al. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circ. J. 2014, 78, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Jebb, S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr. Rev. 2004, 62, S98–S104. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. (Lond.) 2015, 39, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16 (Suppl. 1), 67–76. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.L.; Delves, S.K.; Hill, N.E.; Cobley, R.; Brown, P.; Lanham-New, S.A.; Frost, G.; Brett, S.J.; Murphy, K.G.; Montain, S.J.; et al. Energy expenditure, nutritional status, body composition and physical fitness of Royal Marines during a 6-month operational deployment in Afghanistan. Br. J. Nutr. 2014, 112, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Holmes, M.E.; McAllister, M.J. Nutritional Considerations for Performance in Young Athletes. J. Sport. Med. 2015, 2015, 734649. [Google Scholar] [CrossRef] [PubMed]
- Saris, W.H.; van Erp-Baart, M.A.; Brouns, F.; Westerterp, K.R.; ten Hoor, F. Study on food intake and energy expenditure during extreme sustained exercise: The Tour de France. Int. J. Sports Med. 1989, 10 (Suppl. 1), S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 63, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.M.; Byrne, N.M.; King, N.A. Reproducibility of subjective appetite ratings and ad libitum test meal energy intake in overweight and obese males. Appetite 2014, 81, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, K.; Richard, D.; Tremblay, A. Reproducibility of energy and macronutrient intake and related substrate oxidation rates in a buffet-type meal. Br. J. Nutr. 2000, 83, 489–495. [Google Scholar] [PubMed]
- Thivel, D.; Genin, P.M.; Mathieu, M.E.; Pereira, B.; Metz, L. Reproducibility of an in-laboratory test meal to assess ad libitum energy intake in adolescents with obesity. Appetite 2016, 105, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Charlot, K.; Chapelot, D. Energy compensation after an aerobic exercise session in high-fat/low-fit and low-fat/high-fit young male subjects. Br. J. Nutr. 2013, 110, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Bryant, E.; Blundell, J.E.; King, N.A. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol. Behav. 2009, 97, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Blundell, J.E.; King, N.A. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br. J. Sports Med. 2014, 48, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Sepp, A.; Hughes, D.A.; Johnstone, A.M.; Horgan, G.W.; King, N.; Blundell, J. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. Eur. J. Clin. Nutr. 2002, 56, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, P.; Gibbons, C.; Hopkins, M.; King, N.; Finlayson, G.; Blundell, J. No sex difference in body fat in response to supervised and measured exercise. Med. Sci. Sports Exerc. 2013, 45, 351–358. [Google Scholar] [PubMed]
- King, N.A.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; Blundell, J.E. Individual variability following 12 weeks of supervised exercise: Identification and characterization of compensation for exercise-induced weight loss. Int. J. Obes. (Lond.) 2008, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.J.; Roberts, S.B. The effects of exercise on food intake and body fatness: A summary of published studies. Nutr. Rev. 2007, 65, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B. Energy balance and body composition in sports and exercise. J. Sports Sci. 2004, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J. Thermogenesis with relation to exercise and exercise-training. Acta Med. Scand. Suppl. 1986, 711, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Brobeck, J.R. Food intake as a mechanism of temperature regulation. Yale J. Biol. Med. 1948, 20, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Donhoffer, S.; Vonotzky, J. The effect of environmental temperature on food selection. Am. J. Physiol. 1947, 150, 329–333. [Google Scholar] [PubMed]
- Louis-Sylvestre, J. Adaptation of food ingestion to energy expenditure. Reprod. Nutr. Dev. 1987, 27, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, R.P.; Baumgard, L.H.; Suagee, J.K.; Sanders, S.R. Nutritional Interventions to Alleviate the Negative Consequences of Heat Stress. Adv. Nutr. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444. [Google Scholar] [CrossRef]
- An, J.J.; Liao, G.-Y.; Kinney, C.E.; Sahibzada, N.; Xu, B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 2015, 22, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Bi, S. Dorsomedial hypothalamic NPY modulation of adiposity and thermogenesis. Physiol. Behav. 2013, 121, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Gavini, C.K.; Jones, W.C., 2nd; Novak, C.M. Ventromedial hypothalamic melanocortin receptor activation: Regulation of activity energy expenditure and skeletal muscle thermogenesis. J. Physiol. 2016, 594, 5285–5301. [Google Scholar] [CrossRef] [PubMed]
- Shorten, A.L.; Wallman, K.E.; Guelfi, K.J. Acute effect of environmental temperature during exercise on subsequent energy intake in active men. Am. J. Clin. Nutr. 2009, 90, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Wasse, L.K.; King, J.A.; Stensel, D.J.; Sunderland, C. Effect of ambient temperature during acute aerobic exercise on short-term appetite, energy intake, and plasma acylated ghrelin in recreationally active males. Appl. Physiol. Nutr. Metab. 2013, 38, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Klausen, B.; Toubro, S.; Ranneries, C.; Rehfeld, J.F.; Holst, J.J.; Christensen, N.J.; Astrup, A. Increased intensity of a single exercise bout stimulates subsequent fat intake. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Thivel, D.; Isacco, L.; Montaurier, C.; Boirie, Y.; Duché, P.; Morio, B. The 24-h energy intake of obese adolescents is spontaneously reduced after intensive exercise: A randomized controlled trial in calorimetric chambers. PLoS ONE 2012, 7, e29840. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Charlot, K.; Henri, S.; Hardy-Dessources, M.-D.; Hue, O.; Antoine-Jonville, S. Effect of heat exposure and exercise on food intake regulation: A randomized crossover study in young healthy men. Metabolism 2016, 65, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Sasaki, H.; Tsuchiya, Y.; Goto, K. The influence of environmental temperature on appetite-related hormonal responses. J. Physiol. Anthropol. 2015, 34, 22. [Google Scholar] [CrossRef] [PubMed]
- Stensel, D. Exercise, appetite and appetite-regulating hormones: Implications for food intake and weight control. Ann. Nutr. Metab. 2010, 57 (Suppl. 2), 36–42. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, P.; Bendayan, M. A review on gastric leptin: The exocrine secretion of a gastric hormone. Anat. Cell Biol. 2012, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, P.J.; Sztefko, K.; Pizon, M. The effect of short-term cold and hot exposure on total plasma ghrelin concentrations in humans. Horm. Metab. Res. 2005, 37, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.L.; Zak, R.B.; Shute, R.J.; Heesch, M.W.S.; Dinan, N.E.; Bubak, M.P.; La Salle, D.B.; Slivka, D.R. Leptin, adiponectin, and ghrelin responses to endurance exercise in different ambient conditions. Temperature 2017, in press. [Google Scholar] [CrossRef]
- McCarthy, W.J.; Wang, M.C.; Roberts, C.K. Exercise and ambient temperature may influence energy balance via satiation processes. Am. J. Clin. Nutr. 2010, 91, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N. Physiological consequences of hypohydration: Exercise performance and thermoregulation. Med. Sci. Sports Exerc. 1992, 24, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Corney, R.A.; Horina, A.; Sunderland, C.; James, L.J. Effect of hydration status and fluid availability on ad-libitum energy intake of a semi-solid breakfast. Appetite 2015, 91, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Corney, R.A.; Sunderland, C.; James, L.J. The effect of hydration status on appetite and energy intake. J. Sports Sci. 2015, 33, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Guelfi, K.J.; Wallman, K.E.; Fairchild, T.J. Mild dehydration does not reduce postexercise appetite or energy intake. Med. Sci. Sports Exerc. 2012, 44, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Baillot, M.; Hue, O. Hydration and thermoregulation during a half-ironman performed in tropical climate. J. Sports Sci. Med. 2015, 14, 263–268. [Google Scholar] [PubMed]
- Goulet, E.D. Dehydration and endurance performance in competitive athletes. Nutr. Rev. 2012, 70 (Suppl. 2), S132–S136. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Bathalon, G.P.; Falco, C.M.; Kramer, F.M.; Morgan, C.A., 3rd; Niro, P. Severe decrements in cognition function and mood induced by sleep loss, heat, dehydration, and undernutrition during simulated combat. Biol. Psychiatry 2005, 57, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Bossingham, M.J.; Carnell, N.S.; Campbell, W.W. Water balance, hydration status, and fat-free mass hydration in younger and older adults. Am. J. Clin. Nutr. 2005, 81, 1342–1350. [Google Scholar] [PubMed]
- Rehrer, N.J.; Beckers, E.J.; Brouns, F.; ten Hoor, F.; Saris, W.H. Effects of dehydration on gastric emptying and gastrointestinal distress while running. Med. Sci. Sports Exerc. 1990, 22, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, M. Exercise in the cold. J. Sports Sci. 2004, 22, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.N.; Jay, O. Biophysical aspects of human thermoregulation during heat stress. Auton. Neurosci. 2016, 196, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; McDermott, B.P.; Lee, E.C.; Yeargin, S.W.; Armstrong, L.E.; Maresh, C.M. Cold water immersion: The gold standard for exertional heatstroke treatment. Exerc. Sport Sci. Rev. 2007, 35, 141–149. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Dressendorfer, R.H.; Holland, E.; McCoy, S.C.; Ferguson, M.A. Increased caloric intake soon after exercise in cold water. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Dressendorfer, R.H. Effect of internal body temperature on energy intake soon after aerobic exercise. Med. Sci. Sports Exerc. 1993, S42, 228. [Google Scholar] [CrossRef]
- Ebling, F.J.P. Hypothalamic control of seasonal changes in food intake and body weight. Front. Neuroendocrinol. 2015, 37, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Alonso-Calleja, C. Differences in reported winter and summer dietary intakes in young adults in Spain. Int. J. Food Sci. Nutr. 2005, 56, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.C.; Li, W.; Hafner, A.R.; Chiriboga, D.; Hebert, J.R.; Campbell, M.; Sarnie, M.; Ockene, I.S. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. Eur. J. Clin. Nutr. 2006, 60, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.R.; Sorensen, M.V.; Galloway, V.A.; Spencer, G.J.; Mosher, M.J.; Osipova, L.; Spitsyn, V.A. Climatic influences on basal metabolic rates among circumpolar populations. Am. J. Hum. Biol. 2002, 14, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, J.J.; Leonard, W.R.; Tarskaia, L.A.; Alekseev, V.P.; Krivoshapkin, V.G. Basal metabolic rate in the Yakut (Sakha) of Siberia. Am. J. Hum. Biol. 2005, 17, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Halse, R.E.; Wallman, K.E.; Guelfi, K.J. Postexercise water immersion increases short-term food intake in trained men. Med. Sci. Sports Exerc. 2011, 43, 632–638. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Wasse, L.K.; Stensel, D.J. The acute effects of swimming on appetite, food intake, and plasma acylated ghrelin. J. Obes. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, D.R.; Blannin, A.K. Effects of exercise in the cold on Ghrelin, PYY, and food intake in overweight adults. Med. Sci. Sports Exerc. 2015, 47, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zeyl, A.; Stocks, J.M.; Taylor, N.A.S.; Jenkins, A.B. Interactions between temperature and human leptin physiology in vivo and in vitro. Eur. J. Appl. Physiol. 2004, 92, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Pico, C.; Oliver, P.; Sanchez, J.; Palou, A. Gastric leptin: A putative role in the short-term regulation of food intake. Br. J. Nutr. 2003, 90, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Lees, S.J. Fundamental questions about genes, inactivity, and chronic diseases. Physiol. Genom. 2007, 28, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Kayser, B.; Saris, W.H.M.; Pichard, C. Effects of physical activity on food intake. Clin. Nutr. 2005, 24, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Ward, H.A.; Norat, T.; Luan, J.; May, A.M.; Weiderpass, E.; Sharp, S.J.; Overvad, K.; Ostergaard, J.N.; Tjonneland, A.; et al. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: The European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am. J. Clin. Nutr. 2015, 101, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Racinais, S.; Alonso, J.M.; Coutts, A.J.; Flouris, A.D.; Girard, O.; González-Alonso, J.; Hausswirth, C.; Jay, O.; Lee, J.K.; Mitchell, N.; et al. Consensus recommendations on training and competing in the heat. Sports Med. 2015, 45, 925–938. [Google Scholar] [CrossRef] [PubMed]
| Study | Participants | Temperature/Hygrometry | Exercise/Rest | Time between Exercise and Meals | Meals | Energy Intake (Absolute/Relative) | Subjective Feeling of Appetite | Hormones |
|---|---|---|---|---|---|---|---|---|
| Wasse et al. [34] | 11 lean M | 30 °C/50% | 60 min 65% VO2max | 120 and 330 min | Cold buffet | Yes/No | Yes | Acylated ghrelin |
| 20 °C/50% | 60 min 65% VO2max | |||||||
| Shorten et al. [33] | 11 lean M | 36 °C/30% | 40 min 70% VO2max | 45 min | Breakfast buffet | Yes/Yes | No | Acylated ghrelin, leptin, PP, PYY |
| 25 °C/30% | 40 min 70% VO2max | |||||||
| 25 °C/30% | Rest | |||||||
| Kojima et al. [38] | 11 lean M | 36 °C/40% | 30 min 65% VO2max | No meal | Yes | Total ghrelin, PYY | ||
| 24 °C/40% | 30 min 65% VO2max | |||||||
| Faure et al. [37] | 10 lean M | 31 °C/45% | 40 min 60% VO2max | 30 min | Sandwiches | Yes/Yes | Yes | Total ghrelin, PP, CCK |
| 31 °C/45% | Rest | |||||||
| 22 °C/55% | 40 min 60% VO2max | |||||||
| 22 °C/55% | Rest | |||||||
| Laursen et al. [42] | 11 M | 33 °C/60% | 60 min 60% Wmax | No meal | No | Adiponectin, total and acylated ghrelin, leptin | ||
| 20 °C/60% | 60 min 60% Wmax |
| Study | Participants | Temperature/Hygrometry | Exercise/Rest | Time between Exercise and Meals | Meals | Energy Intake (Absolute and Relative) | Subjective Feeling of Appetite | Hormones |
|---|---|---|---|---|---|---|---|---|
| Wasse et al. [34] | 10 lean M | 10 °C/50% | 60 min 65% VO2max | 120 and 330 min | Cold buffet | Yes/No | Yes | Acylated ghrelin |
| 20 °C/50% | 60 min 65% VO2max | |||||||
| White et al. [56] | 11 lean M | 33 °C (in water) | 45 min 60% VO2max | 45 min | Buffet | Yes/No | No | No |
| 20 °C (in water) | 45 min 60% VO2max | |||||||
| 25 °C/NC | Rest | |||||||
| Dressendorfer [57] | 6 lean M | 34 °C (in water) | 30 min 70% VO2max | NC | Sweet foods | Yes/No | No | No |
| 22 °C (in water) | 30 min 70% VO2max | |||||||
| 24 °C (on land)/NC | 30 min 70% VO2max | |||||||
| 24 °C/NC | Rest | |||||||
| Crabtree et al. [65] | 10 OW M & 6 OW F | 8 °C/40% | 45 min 55% VO2max | 45 min | Buffet | Yes/Yes | No | Total and acylated ghrelin, PYY |
| 20 °C/40% | 45 min 61% VO2max | |||||||
| Kojima et al. [38] | 11 M | 12 °C/40% | 30 min 65% VO2max | No meal | Yes | Total ghrelin, PYY | ||
| 12 °C/40% | 30 min 65% VO2max | |||||||
| Laursen et al. [42] | 11 M | 7 °C/60% | 60 min 60% Wmax | No meal | Adiponectin, total and acylated ghrelin, leptin | |||
| 20 °C/60% | 60 min 60% Wmax |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlot, K.; Faure, C.; Antoine-Jonville, S. Influence of Hot and Cold Environments on the Regulation of Energy Balance Following a Single Exercise Session: A Mini-Review. Nutrients 2017, 9, 592. https://doi.org/10.3390/nu9060592
Charlot K, Faure C, Antoine-Jonville S. Influence of Hot and Cold Environments on the Regulation of Energy Balance Following a Single Exercise Session: A Mini-Review. Nutrients. 2017; 9(6):592. https://doi.org/10.3390/nu9060592
Chicago/Turabian StyleCharlot, Keyne, Cécile Faure, and Sophie Antoine-Jonville. 2017. "Influence of Hot and Cold Environments on the Regulation of Energy Balance Following a Single Exercise Session: A Mini-Review" Nutrients 9, no. 6: 592. https://doi.org/10.3390/nu9060592
APA StyleCharlot, K., Faure, C., & Antoine-Jonville, S. (2017). Influence of Hot and Cold Environments on the Regulation of Energy Balance Following a Single Exercise Session: A Mini-Review. Nutrients, 9(6), 592. https://doi.org/10.3390/nu9060592





