Plantago asiatica L. Ameliorates Puromycin Aminonucleoside-Induced Nephrotic Syndrome by Suppressing Inflammation and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Seed of Plantago asiatica L.
2.2. Drugs and Chemicals
2.3. Animal Experiments and Treatment Protocol
2.4. Measurement of Urinary Protein Excretion
2.5. Measurement of Plasma Biochemical Parameters
2.6. Histopathological Staining of Renal Tissues
2.7. Immunohistochemical Staining of Renal Tissues
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of PAL on Proteinuria and Ascites
3.2. Effect of PAL on Plasma Parameters and Renal Function
3.3. Effect of PAL on Renal Morphology
3.4. Effect of PAL on Podocyte Injury
3.5. Effect of PAL on Inflammatory Markers in the Kidney
3.6. Effect of PAL on Apoptosis-Related Markers in Renal Tissues
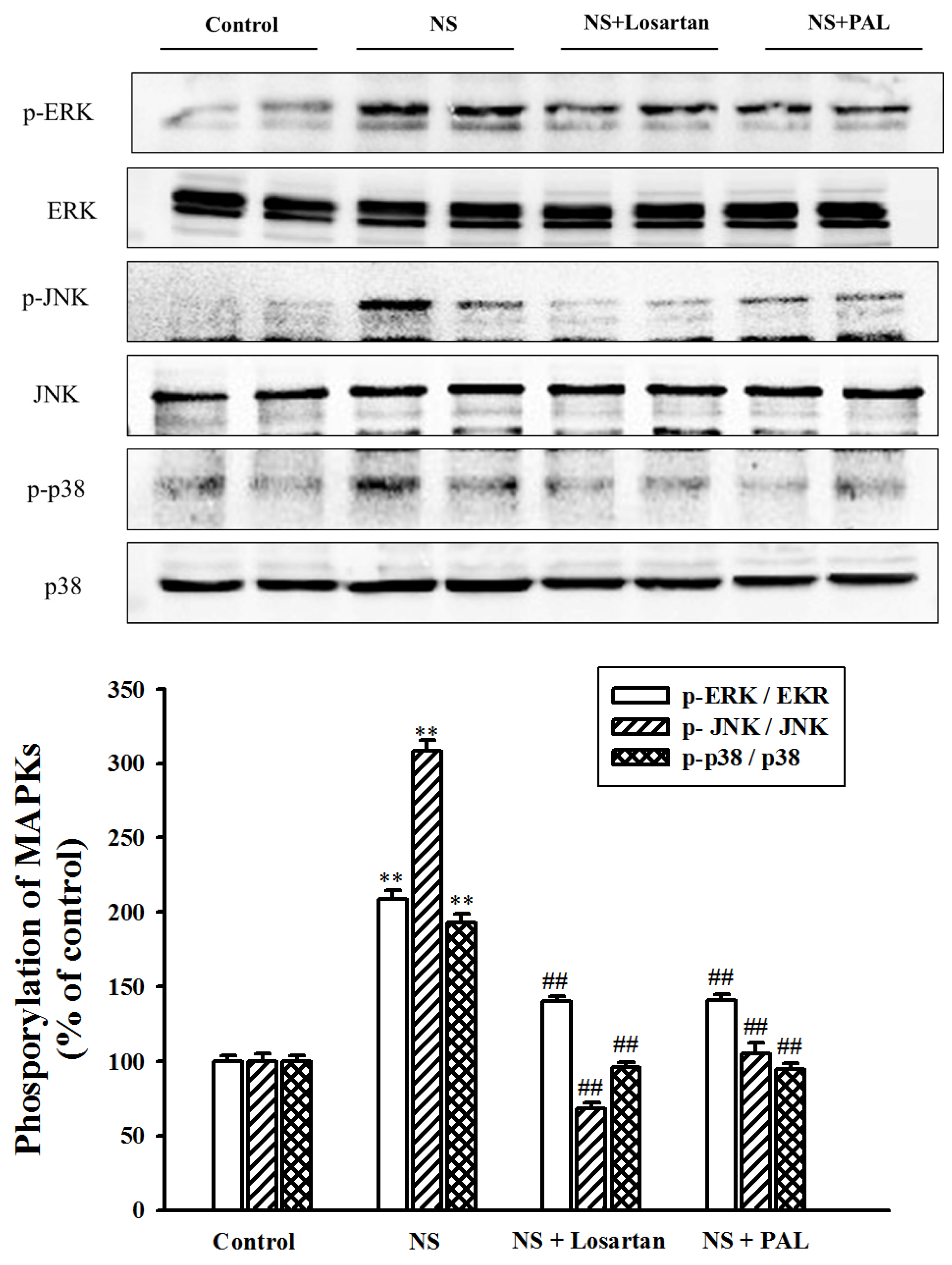
3.7. Effect of PAL on MAPK Signal Pathway in Renal Tissues
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orth, S.R.; Ritz, E. The nephrotic syndrome. N. Engl. J. Med. 1998, 338, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease from subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Bulucu, F.; Vural, A.; Aydin, A.; Sayal, A. Oxidative stress status in adults with nephrotic syndrome. Clin. Nephrol. 2000, 53, 169–173. [Google Scholar] [PubMed]
- Camici, M. The nephrotic syndrome is an immune inflammatory disorder. Med. Hypotheses 2007, 68, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Ryan, M.J. Immune and inflammatory role in renal disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [PubMed]
- Sakurai, H.; Hisada, Y.; Ueno, M.; Sugiura, M.; Kawashima, K.; Sugita, T. Activation of transcription factor NF-kappa B in experimental glomerulonephritis in rats. Biochim. Biophys. Acta 1996, 1316, 132–138. [Google Scholar] [CrossRef]
- Wang, Y.; Harris, D.C. Macrophages in renal disease. J. Am. Soc. Nephrol. 2011, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Santamaria, B.; Ruiz-Ortega, M.; Edido, J.; Ortiz, A. Mechanism of renal apoptosis in health and disease. J. Am. Soc. Nephrol. 2008, 19, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Abbate, M.; Remuzzi, G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol. Dial. Transplant. 2015, 30, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Hsiago, W.L.; Liu, L. The role of traditional Chinese herbal medicines in cancer therapy from TCM theory to mechanistic insights. Planta Med. 2010, 76, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Seo, N.; Yagi, H.; Ohtani, T.; Tokura, Y.; Takigawa, M.; Furukawa, F. Unique therapeutic effects of the Japanese-Chinese herbal medicine, Saireito, on Th1/Th2 cytokines balance of the autoimmunity of MRL/lpr mice. J. Dermatol. Sci. 2002, 28, 198–210. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S. Nephrotic syndrome and the TCM treatment. J. Tradit. Chin. Med. 2004, 24, 201–203. [Google Scholar] [PubMed]
- Wei, L.; Ye, R.; Luan, T.; Lu, R.; Chen, B. Long term effect of TCM decoctions in treatment of nephrotic syndrome. J. Tradit. Chin. Med. 2002, 22, 83–86. [Google Scholar] [PubMed]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Xu, C.; Luo, L.; Tan, R.X. Antidepressant effect of three traditional Chinese medicines in the learned helplessness model. J. Ethnopharmacol. 2004, 91, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.M.A.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-wahab, Y.H.A. Aqueous extracts of husks of Plantagoovata reduce hyperglycemia in type 1 and type 2 diabetes by inhibition of intestinal glucose absorption. Br. J. Nutr. 2006, 96, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Nie, S.P.; Zhou, C.; Wan, Y.; Xie, M.Y. Chemical characteristics and antioxidant activities of polysaccharide purified from the seeds of Plantago asiatica L. J. Sci. Food Agric. 2010, 90, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Patra, R.C.; Swarup, D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology 2005, 211, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, M.; Mukoyama, M.; Mori, K.; Suganami, T.; Sawai, K.; Yoshioka, T. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J. Am. Soc. Nephrol. 2005, 16, 2690–2701. [Google Scholar] [CrossRef] [PubMed]
- Gomez-chiari, M.; Ortiz, A.; Lerma, J.L.; Lopez-armada, M.J.; Mampaso, F.; Gonzalez, E. Involvement of tumor necrosis factor and platelet-activating factor in the pathogenesis of experimental nephrosis in rats. Lab. Investig. 1994, 70, 449–459. [Google Scholar]
- Ma, F.Y.; Liu, J.; Nikolic-paterson, D.J. The role of stress-activated protein kinase signaling in renal pathophysiology. Braz. J. Med. Biol. Res. 2009, 42, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Sever, J. Podocyte biology and pathogenesis of kidney disease. Annu. Rev. Med. 2013, 64, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Hidaka, T.; Takagi-akiba, M.; Trejo, J.A.O.; Sasaki, Y.; Wang, J.; Asanuma, K.; Tomino, Y. Podocin is translocated to cytoplasm in puromycinaminonucleoside nephrosis rats and in poor-prognosis patients with IgA nephropathy. Cell Tissue Res. 2015, 360, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Hiromura, K.; Tomioka, M.; Takahashi, S.; Maeshima, A.; Kaneko, Y.; Kuroiwa, T.; Nojiam, Y. The immunosuppressive drug mizoribine directly prevents podocyte injury in puromycinaminonucleoside nephrosis. Nephron Exp. Nephrol. 2010, 116, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bohr, D.C.; Koch, M.; Kritzenberger, M.; Fuchshofer, R.; Tamm, E.R. Increase expression of olfactomedin-1 and myocilin in podocytes during puromycinaminonucleoside nephrosis. Nephrol. Dial. Transplant. 2011, 26, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Coward, R.J.; Foster, R.R.; Patton, D.; Ni, L.; Lennon, R.; Bates, D.O.; Harper, S.J.; Mathieson, P.W.; Saleem, M.A. Nephrotic plasma alters slit diaphragm dependent signaling and translocatesnephrin, podocin and CD2 associated protein in cultured human podocytes. J. Am. Soc. Nephrol. 2015, 16, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Romero, M.; Soto, H.; Mosquera, J. Increase apoptosis in acute puromycinaminonucleoside nephrosis. Exp. Nephrol. 2001, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Wang, S.; Liu, W.; Haig, A.; Zhang, Z.X.; Jevnikar, A.M. Glycyrrhizic acid ameliorates HMGB1-mediated cell death and inflammation after renal ischemia reperfusion injury. Am. J. Nephrol. 2014, 40, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Ohse, T.; Inagi, R.; Tanaka, T.; Ota, T.; Miyata, T.; Kojima, I. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 2006, 70, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Mechanisms and consequences of hypertriglyceridemia and cellular lipid accumulation in chronic kidney disease and metabolic syndrome. Histol. Histopathol. 2011, 26, 1599–1610. [Google Scholar] [PubMed]
- Soriano, M.E.; Scorrano, L. The interplay between BCL-2family proteins and mitochondrial morphology in the regulation of apoptosis. Adv. Exp. Med. Biol. 2010, 687, 97–114. [Google Scholar] [PubMed]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulating during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.; Karandikar, M.; Berman, K.; Cobb, M. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [PubMed]
- Singh, K.; Ray, R.; Sharma, A.; Gupta, R.; Bagga, A.; Dinda, A.K. Peritubular capillaries and renal function in pediatric idiopathic nephrotic syndrome. Saudi J. Kidney Dis. Transplant. 2013, 24, 942–949. [Google Scholar]
- Chang, L.; Karin, M. Mammalian MAP kinase signaling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.; Chun, K.; Cha, H.; Han, S.; Keum, Y.; Park, K.; Lee, S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Jiang, K.; Chen, X.; Zhu, Z.; Qiu, C.; Li, C.; Deng, G. Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Int. Immunopharmacol. 2016, 351, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Morooka, H.; Bonventre, J.V.; Pombo, M.C.; Kyriakis, J.M.; Force, T. Ischemia and reperfusion enhance ATF-2 and c-Jun binding to cAMP response elements and to an AP-1 binding site from the c-Jun promoter. J. Biol. Chem. 1995, 270, 30084–30092. [Google Scholar] [PubMed]
- Park, S.J.; Jeong, K.S. Cell-type-specific activation of mitogen-activated protein kinasesin PAN-induced progressive renal disease in rats. Biochem. Biophys. Res. Commun. 2004, 323, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.; Wei, C.; Fernandez, I.; Li, J.; Tardi, N.J.; Tracy, M.; Wadhwani, S.; Cao, Y.; Peev, V.; Zloza, A.; et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat. Med. 2017, 23, 100–106. [Google Scholar] [CrossRef] [PubMed]
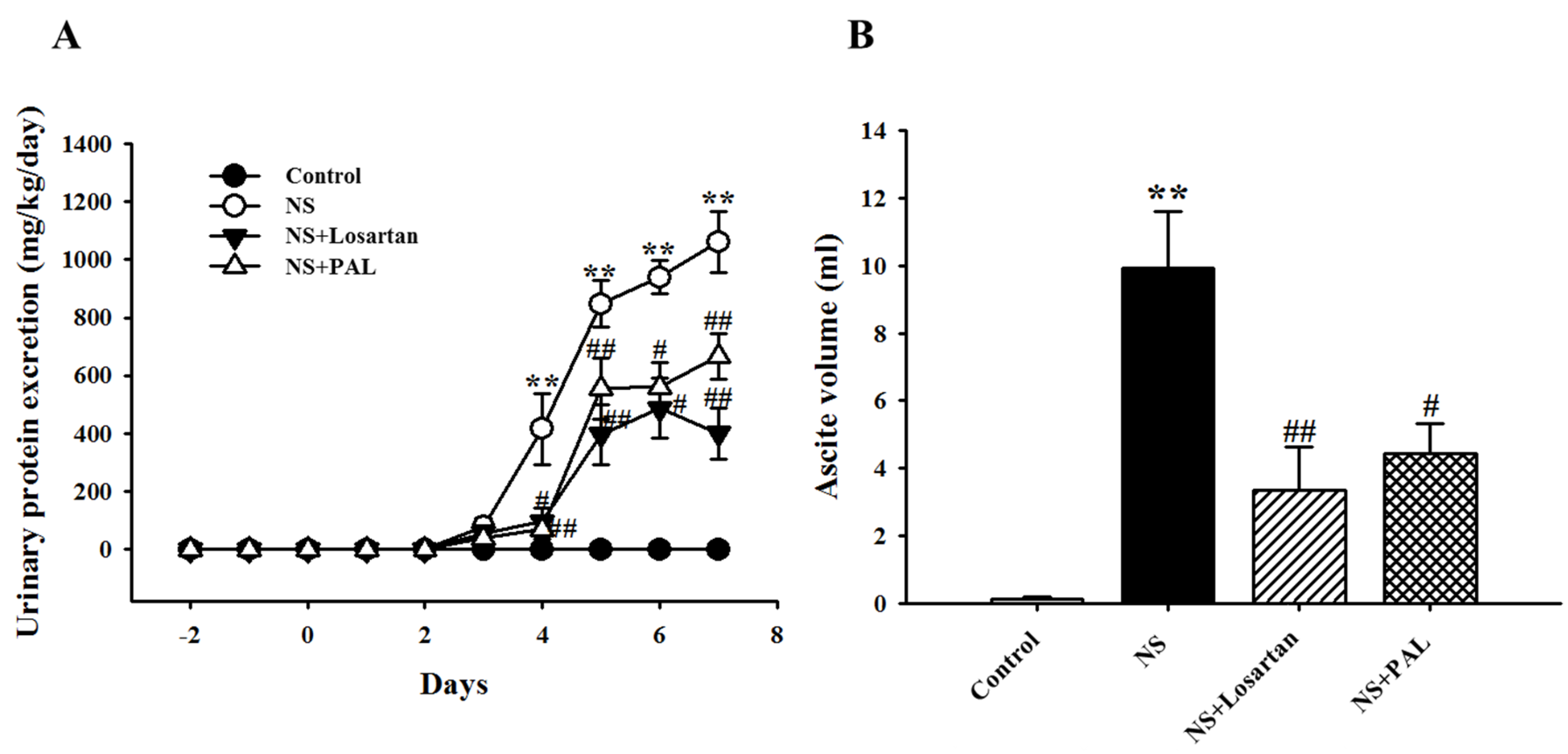
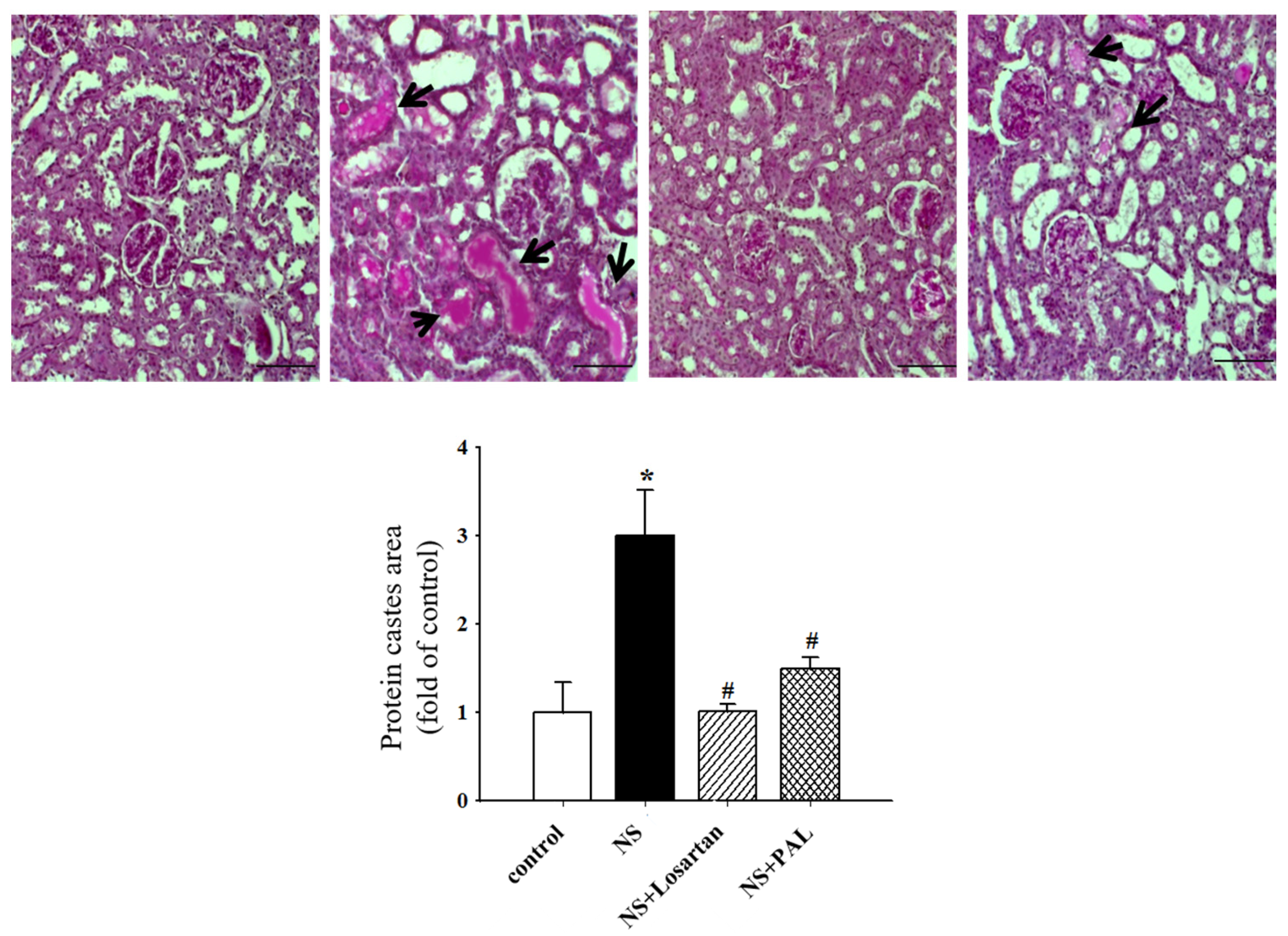
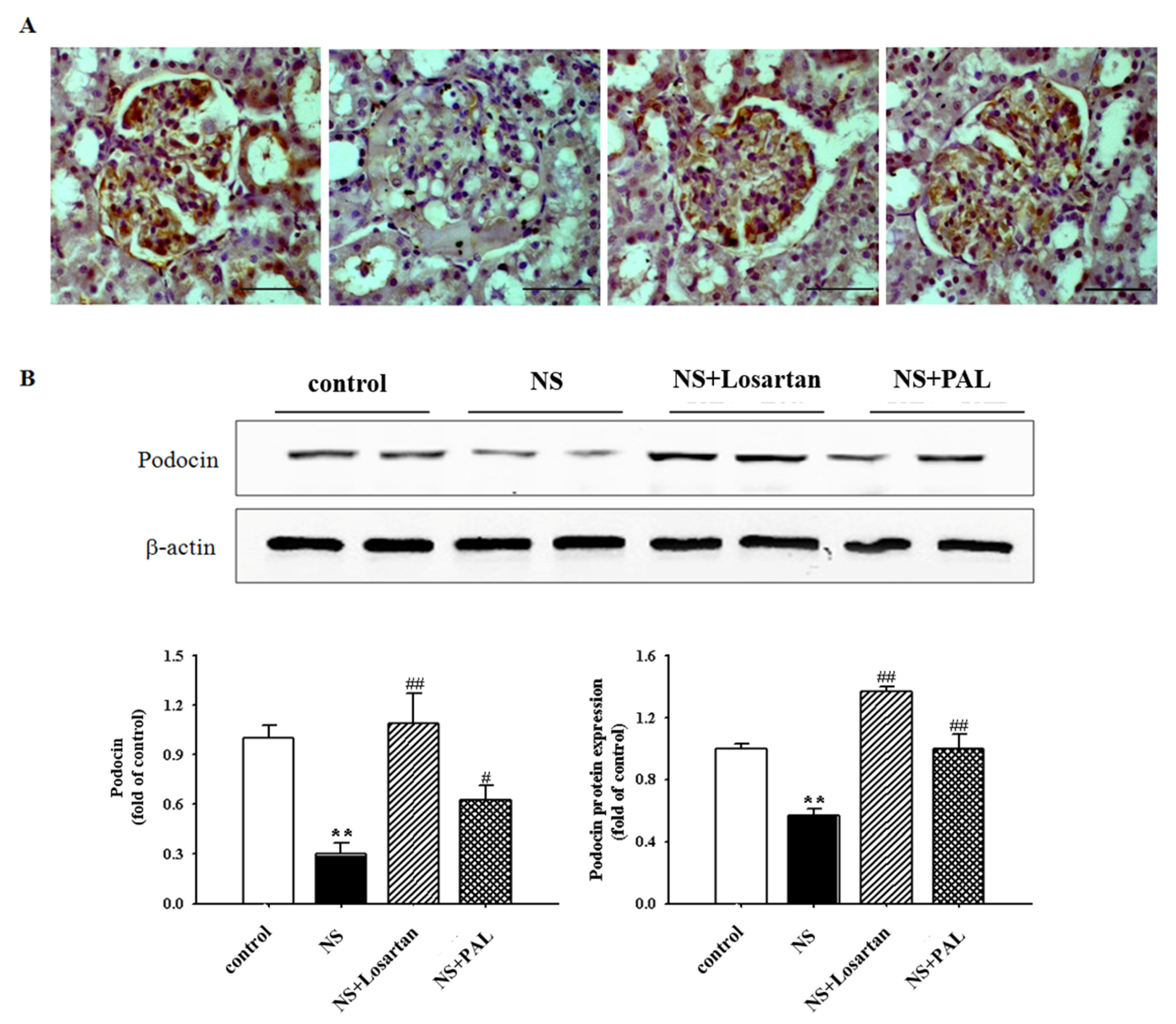

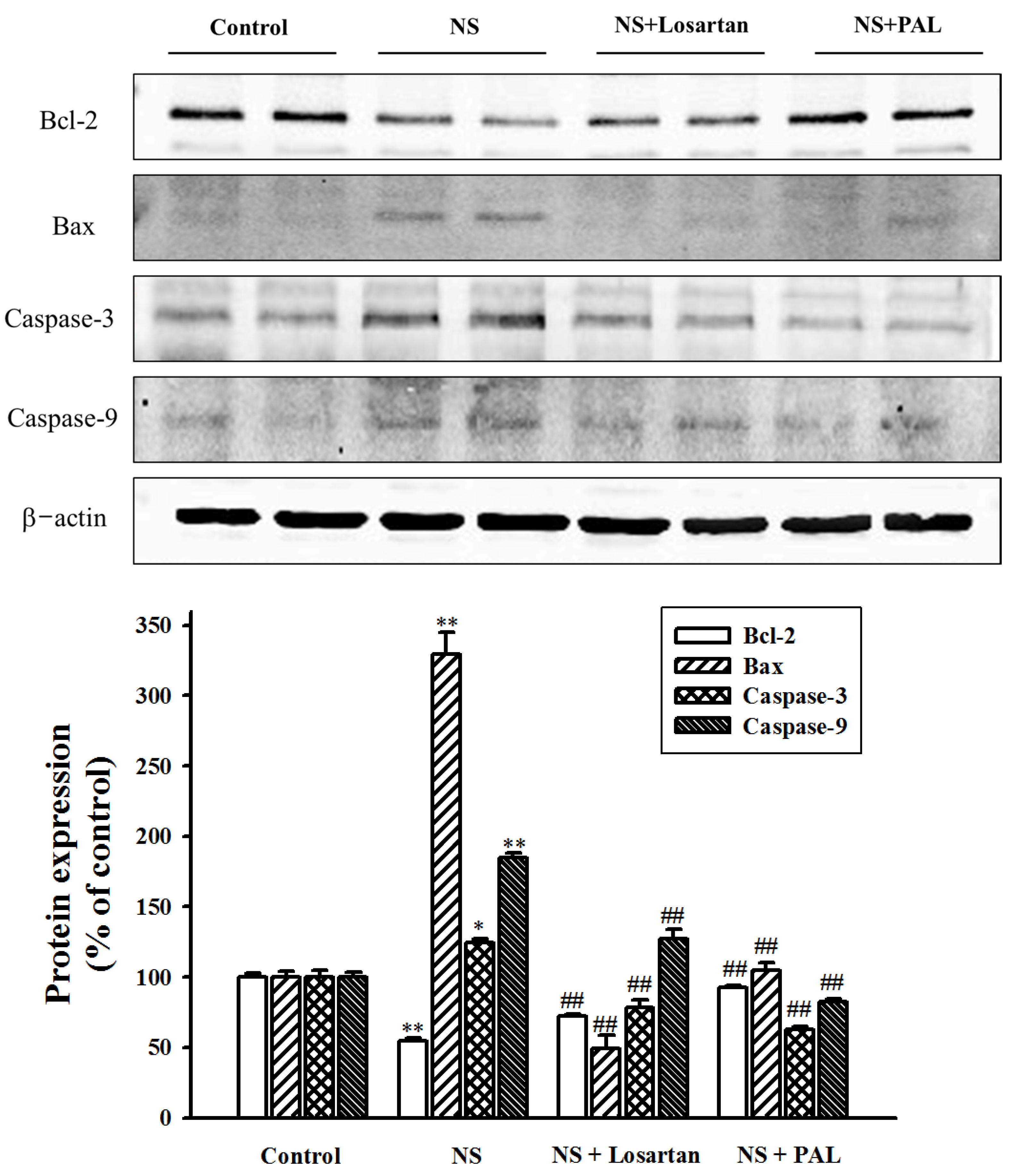
| Groups | Control | NS | NS | |
|---|---|---|---|---|
| Losartan | PAL | |||
| T-Cho (mg/dL) | 63.4 ± 3.2 | 397.8 ± 2.3 ** | 258.2 ± 38.4 # | 301.8 ± 34.0 # |
| TG (mg/dL) | 97.6 ± 16.1 | 196.6 ± 3.4 ** | 352.3 ± 65.3 # | 355.2 ± 41.1 ## |
| LDL-c (mg/dL) | 20.8 ± 3.6 | 171.2 ± 9.0 ** | 90.6 ± 20.9 ## | 110.6 ± 22.0 # |
| Groups | Control | NS | NS | |
|---|---|---|---|---|
| Losartan | PAL | |||
| BUN (mg/dL) | 13.7 ± 0.8 | 56.4 ± 5.5 ** | 33.9 ± 6.3 # | 30.8 ± 3.6 ## |
| T-pro (mg/dL) | 5.6 ± 0.1 | 3.6 ± 0.1 ** | 4.8 ± 0.3 ## | 4.2 ± 0.2 # |
| Alb (mg/dL) | 4.1 ± 0.1 | 1.1 ± 0.2 ** | 2.9 ± 0.4 ## | 2.2 ± 0.3 ## |
| Plasma creatinine (mg/dL) | 0.11 ± 0.01 | 0.25 ± 0.03 ** | 0.13 ± 0.02 ## | 0.15 ± 0.01 ## |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kho, M.C.; Park, J.H.; Han, B.H.; Tan, R.; Yoon, J.J.; Kim, H.Y.; Ahn, Y.M.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Plantago asiatica L. Ameliorates Puromycin Aminonucleoside-Induced Nephrotic Syndrome by Suppressing Inflammation and Apoptosis. Nutrients 2017, 9, 386. https://doi.org/10.3390/nu9040386
Kho MC, Park JH, Han BH, Tan R, Yoon JJ, Kim HY, Ahn YM, Lee YJ, Kang DG, Lee HS. Plantago asiatica L. Ameliorates Puromycin Aminonucleoside-Induced Nephrotic Syndrome by Suppressing Inflammation and Apoptosis. Nutrients. 2017; 9(4):386. https://doi.org/10.3390/nu9040386
Chicago/Turabian StyleKho, Min Chul, Ji Hun Park, Byung Hyuk Han, Rui Tan, Jung Joo Yoon, Hye Yoom Kim, You Mee Ahn, Yun Jung Lee, Dae Gill Kang, and Ho Sub Lee. 2017. "Plantago asiatica L. Ameliorates Puromycin Aminonucleoside-Induced Nephrotic Syndrome by Suppressing Inflammation and Apoptosis" Nutrients 9, no. 4: 386. https://doi.org/10.3390/nu9040386
APA StyleKho, M. C., Park, J. H., Han, B. H., Tan, R., Yoon, J. J., Kim, H. Y., Ahn, Y. M., Lee, Y. J., Kang, D. G., & Lee, H. S. (2017). Plantago asiatica L. Ameliorates Puromycin Aminonucleoside-Induced Nephrotic Syndrome by Suppressing Inflammation and Apoptosis. Nutrients, 9(4), 386. https://doi.org/10.3390/nu9040386




