Inhibition of VEGF-Induced VEGFR-2 Activation and HUVEC Migration by Melatonin and Other Bioactive Indolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Study Compounds
2.3. Treatments of HUVECs
2.4. Phosphorylated VEGFR-2 ELISA
2.5. Western Blot Analysis for VEGR-2
2.6. Migration Wound-Healing Assay
2.7. Statistical Analysis
3. Results
3.1. Inhibition of VEGF-Induced VEGFR-2 Activation by Melatonin
3.2. Inhibitory Effect of Other Indolic Related Compounds on VEGF-Induced VEGR-2 Activation
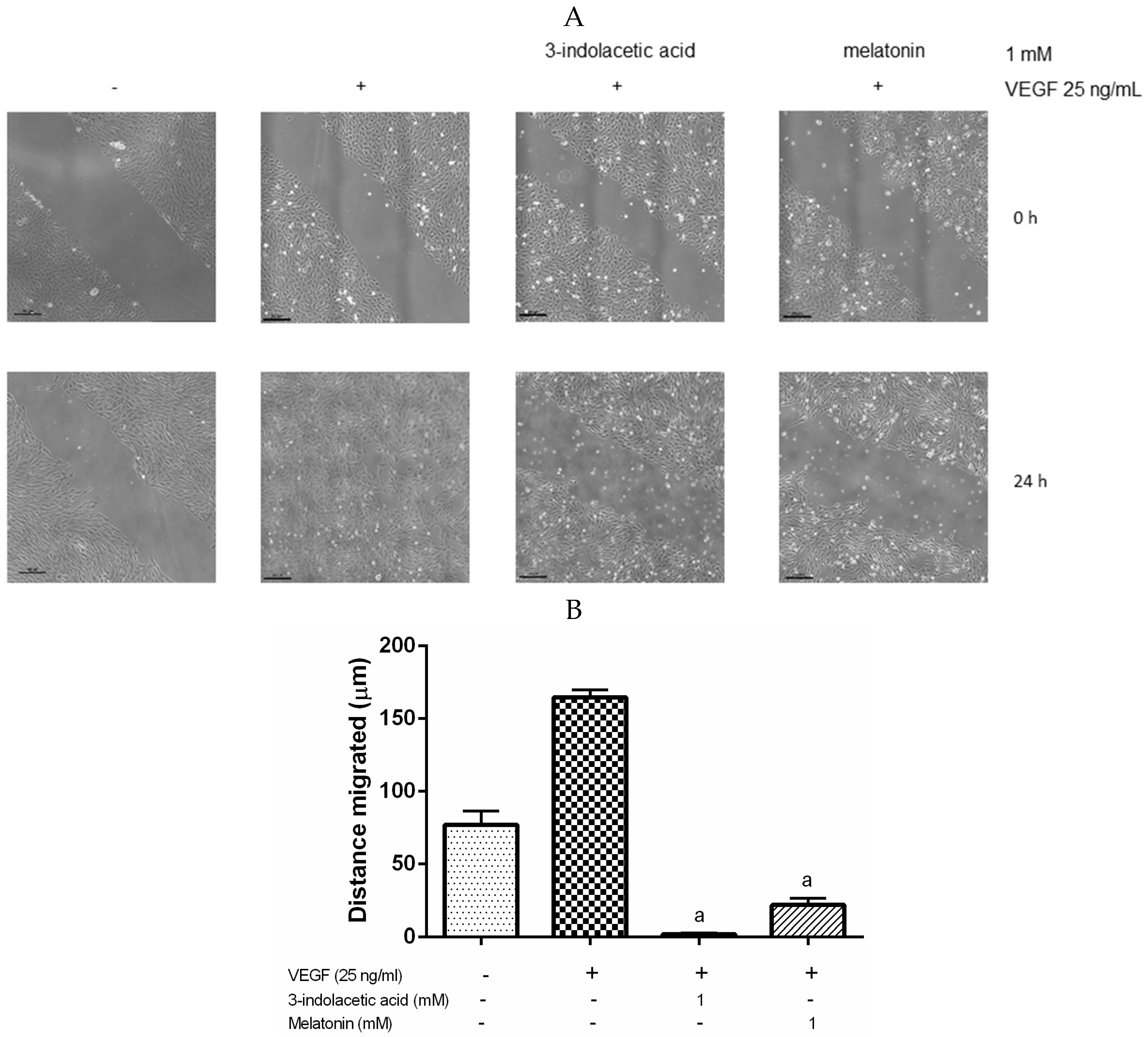
3.3. Effects of Melatonin and 3-Indolacetic Acid on HUVECs Migration
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Celletti, F.L.; Waugh, J.M.; Amabile, P.G.; Brendolan, A.; Hilfiker, P.R.; Dake, M.D. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat. Med. 2001, 7, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J. The vascular endothelial growth factor (VEGF) signaling pathway: A therapeutic target in patients with hematologic malignancies. Oncologist 2001, 6, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J. Nutraceuticals as anti-angiogenic agents: Hopes and reality. J. Physiol. Pharmacol. 2005, 1, 51–67. [Google Scholar]
- Cebe-Suarez, S.; Zehnder-Fjallman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol. Life Sci. 2006, 63, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.M.; Figg, W.D. Angiogenesis inhibitors: Current strategies and future prospects. CA-Cancer J. Clin. 2010, 60, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 2007, 19, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, L.; Kreuger, J.; Holmborn, K.; Lundin, L.; Eriksson, I.; Kjellén, L.; Claesson-Welsh, L. Heparan Sulfate in Trans Potentiates VEGFR-Mediated Angiogenesis. Dev. Cell 2006, 10, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, G.B.; Limberg, B.J.; Rosenbaum, J.S. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF165 and VEGF121. J. Biol. Chem. 2001, 276, 25520–25531. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Simons, M.; Martin, J.F.; Zachary, I.C. Role of angiogenesis in cardiovascular disease—A critical appraisal. Circulation 2005, 112, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; van de Water, L.; Brown, L.F.; Nagy, J.A.; Yeo, K.-T.; Yeo, T.-K.; Berse, B.; Jackman, R.W.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metast. Rev. 1993, 12, 303–324. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, L.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kancho, R.; Hara, M.; Sazuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Murch, S.J.; Simmons, C.B.; Saxena, P.K. Melatonin in feverfew and other medicinal plants. Lancet 1997, 350, 1598–1599. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Chen, G.; Huo, Y.; Tan, D.X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Zhou, Z.; Coelho Cruz, M.H.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.T.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wang, L.; Tan, D.X.; Zhao, Y.; Zheng, X.D.; Chen, H.; Li, Q.T.; Zuo, B.X.; Kong, J. Identification of genes for melatonin synthetic enzymes in “Red Fuji” apple (Malus domesticus Borkh. cv. Red) and their expression and melatonin production during fruit development. J. Pineal Res. 2013, 55, 443–451. [Google Scholar] [PubMed]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid-liquid extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Mas, A. Melatonin and other tryptophan metabolites produced by yeasts: Implications in cardiovascular and neurodegenerative diseases. Front. Microbiol. 2016, 6, 1565. [Google Scholar] [CrossRef] [PubMed]
- De la Puerta, C.; Carrascosa-Salmoral, M.P.; García-Luna, P.P.; Lardone, P.J.; Herrera, J.L.; Fernández-Montesinos, R.; Guerrero, J.M.; Pozo, D. Melatoninis a phytochemical in olive oil. Food Chem. 2007, 104, 609–612. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; García-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Stürtz, M.; Cerezo, A.B.; Cantos, E.; García-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersiconesculentum) and strawberries (Fragariaananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Varoni, E.M. The good health of Bacchus: Melatonin in grapes, the unveiled myth. LWT-Food Sci. Technol. 2016, 65, 758–761. [Google Scholar] [CrossRef]
- Vigentini, I.; Gardana, C.; Francassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast contribution to melatonin, melatonin isomers and tryptophan ethyl ester during alcoholic fermentation of grape musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef] [PubMed]
- The EFSA Comprehensive European Food Consumption Database. Available online: https://www.efsa.europa.eu/en/food-consumption/comprehensive-database (accessed on 25 November 2016).
- Di, W.-L.; Kadva, A.; Johnston, A.; Silman, R. Variable Bioavailability of Oral Melatonin. N. Engl. J. Med. 1997, 336, 1028–1029. [Google Scholar] [CrossRef] [PubMed]
- Fourtillan, J.B.; Brisson, A.M.; Gobin, P.; Ingrand, I.; Decourt, J.; Girault, J. Bioavailability of melatonin in humans after daytime administration of D7 melatonin. Biopharm. Drug Dispos. 2000, 21, 15–22. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar]
- Andersen, L.P.H.; Werner, M.D.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol. Toxicol. 2016, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Leal, A.; Alvarez-Fernandez, M.A.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcıa-Parrilla, M.C. Quality control and determination of melatonin in food supplements. J. Food Compost. Anal. 2016, 45, 80–86. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EC) 178/2002 of the European Parliament and of the Council laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Commun. 2002, L31, 1–24. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Union 2002, L183, 51–57. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EU) No. 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2002, L136, 1–40. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 5, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Rovelli, F.; Malugani, F.; Bucovec, R.; Conti, A.; Maestroni, G.J.M. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol. Lett. 2001, 22, 45–47. [Google Scholar] [PubMed]
- Girotti, L.; Lago, M.; Ianovsky, O.; Elizari, M.V.; Dini, A.; PerezLloret, S.; Albornoz, L.E.; Cardinali, D.P. Low urinary 6-sulfatoxymelatonin levels in patients with severe congestive heart failure. Endocrine 2003, 22, 245–248. [Google Scholar] [CrossRef]
- Jonas, M.; Garfinkel, D.; Zisapel, N.; Laudon, M.; Grossman, E. Impaired nocturnal melatonin secretion in non-dipper hypertensive patients. Blood Press. 2003, 12, 19–24. [Google Scholar] [PubMed]
- Simko, F.; Paulis, L. Melatonin as a potential antihypertensive treatment. J. Pineal Res. 2007, 42, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A. The circadian melatonin rhythm and its modulation: Possible impact on hypertension. Hypertension 2009, 27, S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hidalgo, M.; Guerrero, J.M.; Villegas, J.M.; Packham, G.; de la Lastra, C.A. Melatonin, a natural programmed cell death inducer in cancer. Curr. Med. Chem. 2012, 19, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, A.; Abreu-Gonzñalez, P.; Sanchez-Sanchez, J.J.; Kaski, J.C.; Reiter, R.J. Melatonin and circadian biology in human cardiovascular disease. J. Pineal Res. 2010, 49, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-García, V.; González, A.; Alonso-González, C.; Martínez-Campa, C.; Cos, S. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc. Res. 2013, 87, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Yu, M.; Peng, X.; Dong, L.; Yang, Z. Melatonin prevents human pancreatic carcinoma cell PANC-1-induced human umbilical vein endothelial cell proliferation and migration by inhibiting vascular endothelial growth factor expression. J. Pineal Res. 2012, 52, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-García, V.; González, A.; Alonso-González, C.; Martínez-Campa, C.; Cos, S. Regulation of vascular endothelial growth factor by melatonin in human breast cancer cells. J. Pineal Res. 2013, 54, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin and the von Hippel–Lindau/HIF-1 oxygen sensing mechanism: A review. Biochim. Biophys. Acta 2016, 1865, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Wolf Maciel, J.M.; Carvalho Ferreira, L.; Ferreira Da Silva, R.; Pires De Campos Zuccari, D.A. Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncol. Lett. 2016, 12, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Cui, P.; Yu, M.; Han, J.; Li, H.; Xiu, R. Melatonin modulates the expression of VEGF and HIF-1α induced by CoCl2 in cultured cancer cells. J. Pineal Res. 2008, 44, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, M.S.; Suh, S.I.; Baek, W.K. Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J. Pineal Res. 2009, 46, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.N.; Rodrigues, R.V.; Jardim-Perassi, B.V.; Moschetta, M.G.; Lopes, J.R.; Colombo, J.; de Campos Zuccari, D.A.P. Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin. Anti-Cancer Agents Med. Chem. 2014, 14, 1302–1311. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, H.J.; Jeong, S.J.; Lee, H.J.; Kim, H.S.; Chen, C.Y.; Lee, E.O.; Kim, S.H. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1alpha inactivation in hypoxic PC-3 prostate cancer cells. J. Pineal Res. 2011, 51, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Yu, M.; Lou, Z.; Dai, M.; Han, J.; Xiu, R.; Yang, Z. Intracellular signaling pathways involved in cell growth inhibition of human umbilical vein endothelial cells by melatonin. J. Pineal Res. 2008, 44, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Luo, Z.; Zhang, H.; Su, Y.; Li, A.; Li, H.; Zhang, J.; Yang, Z.; Xiu, R. Effect and mechanism of melatonin’s action on the proliferation of human umbilical vein endothelial cells. J. Pineal Res. 2006, 41, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.F.; Ng, T.B.; Liu, W.K. Antiproliferative effect of pineal indoles on cultured tumor cell lines. J. Pineal Res. 1993, 14, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.Y.; Jagota, A. Melatonin has differential effects on age-induced stoichiometric changes in daily chronomics of serotonin metabolism in SCN of male Wistar rats. Biogerontology 2015, 16, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Stevens, A.P.; Dettmer, K.; Gottfried, E.; Hoves, S.; Kreutz, M.; Holler, E.; Canelas, A.B.; Kema, I.; Oefner, P.J. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Dalmazzo, L.F.F.; Santana-Lemos, B.A.; Jácomo, R.H.; Garcia, A.B.; Rego, E.M.; da Fonseca, L.M.; Falcão, R.P. Antibody-targeted horseradish peroxidase associated with indole-3-acetic acid induces apoptosis in vitro in hematological malignancies. Leuk. Res. 2011, 35, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Pakala, R.; Willerson, J.T.; Benedict, C.R. Mitogenic effect of serotonin on vascular endothelial cells. Circulation 1994, 90, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; Garcia-Parrilla, M.C. Validation of an analytical method to determine melatonin and compounds related to L-tryptophan metabolism using UHPLC/HRMS. Food Anal. Methods 2016, 9, 3327–3336. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; Garcia-Parrilla, M.C. Melatonin and derived L-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Moyle, C.W.A.; Cerezo, A.B.; Winterbone, M.S.; Hollands, W.J.; Aleexev, Y.; Needs, P.W.; Kroon, P.A. Potent inhibition of VEGFR-2 activation by tight binding of green tea epigallocatechin gallate and apple procyanidins to VEGF: Relevance to angiogenesis. Mol. Nutr. Food Res. 2015, 59, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Winterbone, M.S.; Moyle, C.W.A.; Needs, P.W.; Kroon, P.A. Molecular structure-function relationship of dietary polyphenols for inhibiting VEGF-induced VEGFR-2 activity. Mol. Nutr. Food Res. 2015, 59, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; van Kuppevelt, T.H.; Belting, M. ScFv Anti-Heparan Sulfate Antibodies Unexpectedly Activate Endothelial and Cancer Cells through p38 MAPK: Implications for Antibody-Based Targeting of Heparan Sulfate Proteoglycans in Cancer. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cernaro, V.; Medici, M.A.; Leonello, G.; Buemi, A.; Kohnke, F.H.; Villari, A.; Santoro, D.; Buemi, M. Auxin induces cell proliferation in an experimental model of mammalian renal tubular epithelial cells. Ren. Fail. 2015, 37, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, V.C.; Shivanna, S.; Martorell, J.; Balcells, M.; Bosch, I.; Kolandaivelu, K.; Edelman, E.R. Uremic Serum and Solutes Increase Post–Vascular Interventional Thrombotic Risk Through Altered Stability of Smooth Muscle Cell Tissue Factor. Circulation 2013, 127, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Jourde-Chiche, N.; Dou, L.; Sabatier, F.; Calaf, R.; Cerini, C.; Robert, S.; Camoin-Jau, L.; Charpiot, P.; Argiles, A.; Dignat-George, F.; Brunet, P. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J. Thromb. Haemost. 2009, 7, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, H.A.M.; van den Heuvel, L.P.; Ringens, L.H.J.; Dankers, A.C.A.; Russel, F.G.M.; Wetzels, J.F.M.; Hoenderop, J.G.; Masereeuw, R. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS ONE 2011, 6, e18438. [Google Scholar] [CrossRef] [PubMed]
- Greco, O.; Dachs, G.U.; Tozer, G.M.; Kanthou, C. Mechanisms of cytotoxicity induced by horseradish peroxidase/indole-3-acetic acid gene therapy. J. Cell. Biochem. 2002, 87, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Ryu, J.S.; Li, H.; Park, W.-J.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Sohn, U.D.; Kim, D.-S. UVB-activated indole-3-acetic acid induces apoptosis of PC-3 prostate cancer cells. Anticancer Res. 2010, 30, 4607–4612. [Google Scholar] [PubMed]
- Kim, D.-S.; Jeon, S.-E.; Park, K.-C. Oxidation of indole-3-acetic acid by horseradish peroxidase induces apoptosis in G361 human melanoma cells. Cell Signal. 2004, 16, 81–88. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, P.; Guo, G.; Wang, L. Combination of cytokinin and auxin induces apoptosis, cell cycle progression arrest and blockage of the Akt pathway in HeLa cells. Mol. Med. Rep. 2015, 12, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, D.; Shepro, D.; Peterson, S.; Hechtman, H.B. Serotonin, histamine, and norepinephrine mediation of endothelial and vascular smooth muscle cell movement. Am. J. Physiol. 1985, 248, C252–C257. [Google Scholar] [PubMed]
- Matsusaka, S.; Wakabayashi, I. 5-Hydroxytryptamine as a potent migration enhancer of human aortic endothelial cells. FEBS Lett. 2005, 579, 6721–6725. [Google Scholar] [CrossRef] [PubMed]
- Pakala, R. Serotonin and thromboxane A2 stimulate platelet-derived microparticle-induced smooth muscle cell proliferation. Cardiovasc. Radiat. Med. 2004, 5, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pakala, R.; Benedict, C.R. Effect of serotonin and thromboxane A2 on endothelial cell proliferation: Effect of specific receptor antagonists. J. Lab. Clin. Med. 1998, 131, 527–537. [Google Scholar] [CrossRef]
- Zamani, A.; Qua, Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett. 2012, 586, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Dizeyi, N.; Bjartell, A.; Nilsson, E.; Hansson, J.; Gadaleanu, V.; Cross, N.; Abrahamsson, P.-A. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate 2004, 59, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, E.J.; Shabbir, M.; Mikhailidis, D.P.; Thompson, C.S.; Mumtaz, F.H. The role of serotonin (5-hydroxytryptamine 1A and 1B) receptors in prostate cancer cell proliferation. J. Urol. 2006, 176, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.G.; Fesce, R.; Vlcentini, L.M. Mitogenic effect of serotonin in human small cell lung carcinoma cells via both 5-HT1A and 5-HT1D receptors. Eur. J. Pharmacol. 1995, 291, 209–211. [Google Scholar] [CrossRef]
- Oufkir, T.; Arseneault, M.; Sanderson, J.T.; Vaillancourt, C. The 5-HT2A serotonin receptor enhances cell viability, affects cell cycle progression and activates MEK-ERK1/2 and JAK2-STAT3 signalling pathways in human choriocarcinoma cell lines. Placenta 2010, 31, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Sonier, B.; Arseneault, M.; Lavigne, C.; Ouellette, R.; Vaillancourt, C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem. Biophys. Res. Commun. 2006, 343, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Soll, C.; Jang, J.H.; Riener, M.O.; Moritz, W.; Wild, P.J.; Graf, R.; Clavien, P.A. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology 2010, 51, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, I.; Kidd, M.; Gustafsson, B.I.; Svejda, B.; Joseph, R.; Pfragner, R.; Modlin, I.M. Auto-regulatory effects of serotonin on proliferation and signalling pathways in lung and small intestine neuroendocrine tumor cell lines. Cancer 2009, 115, 4934–4945. [Google Scholar] [CrossRef] [PubMed]
- Lübbe, A.S.; Huhnt, W. Microvessel diameters of human colon adenocarcinoma during acute treatment with serotonin. Int. J. Microcirc. 1994, 14, 218–225. [Google Scholar] [CrossRef]
- Vicaut, E.; Laemmel, E.; Stiicker, O. Impact of serotonin on tumour growth. Ann. Med. 2000, 32, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and Cancer: What Is the Link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Ebihara, S.; Yamanda, S.; Niu, K.; Okazaki, T.; Sora, I.; Arai, H. Depletion of serotonin and selective inhibition of 2β receptor suppressed tumor angiogenesis by inhibiting endothelial nitric oxide synthase and extracellular signal-regulated kinase ½ phosphorylation. Neoplasia 2009, 11, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Tsuno, N.H.; Shuno, Y.; Sasaki, K.; Hongo, K.; Okaji, Y.; Sunami, E.; Kitayama, J.; Takahashi, K.; Nagawa, H. Antiangiogenic effect of a selective 5-HT4 receptor agonist. J. Surg. Res. 2010, 159, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Basilicata, M.F.; de Simone, M.; Del Giudice, C.; Anastasio, A.; Sorriento, D.; Saviano, M.; Del Gatto, A.; Trimarco, B.; Pedone, C.; et al. Evaluation of the anti-angiogenic properties of the new selective aVb3 integrin antagonist RGDechiHCit. J. Transl. Med. 2011, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Wronska, A.; Uryu, K.; Diacovo, T.G.; Gao, M.; Marx, S.O.; Kitajewski, J.; Chilton, J.M.; Akat, K.M.; Tuschl, T.; et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J. Clin. Investig. 2014, 124, 4102–4114. [Google Scholar] [CrossRef] [PubMed]
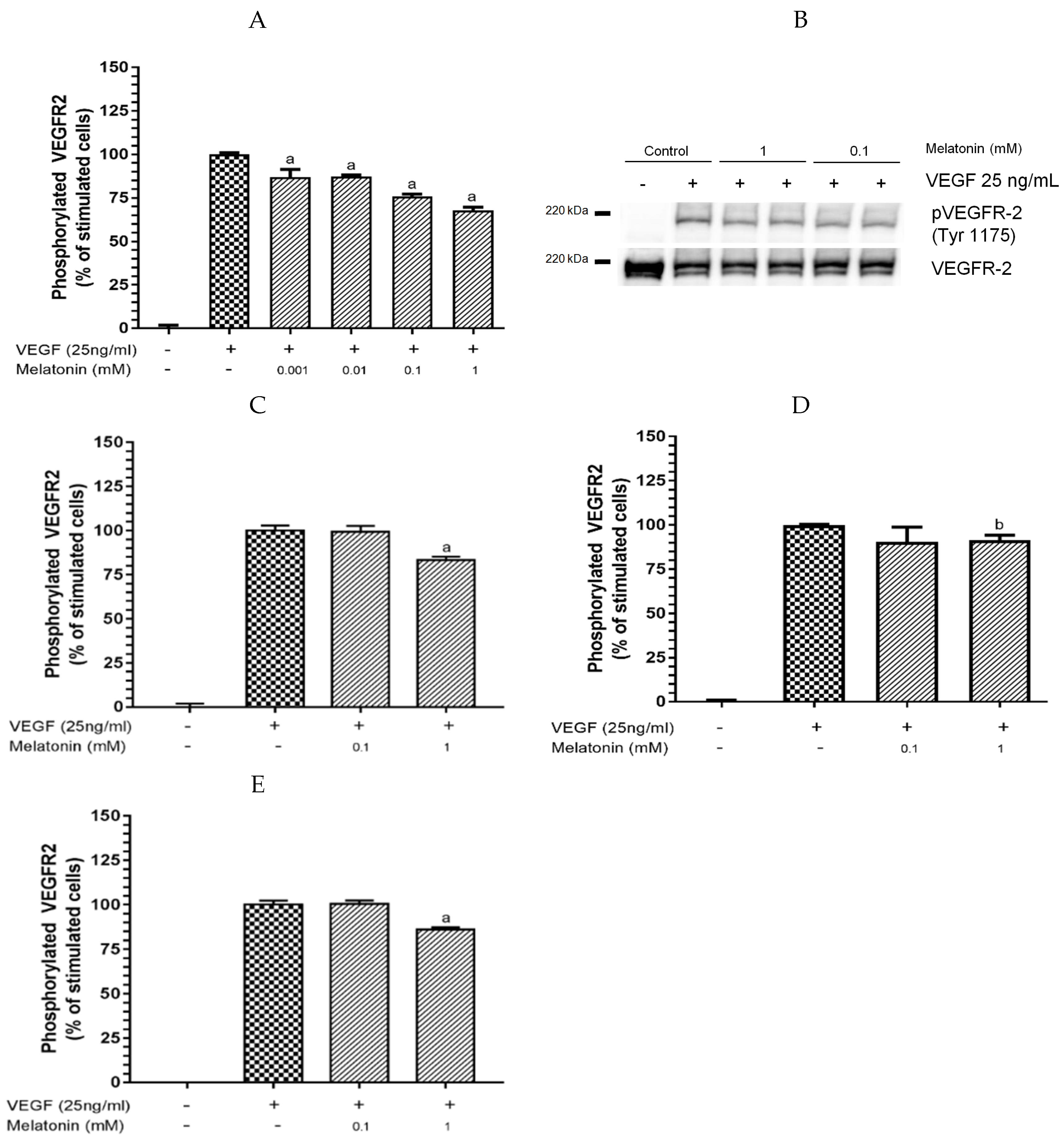
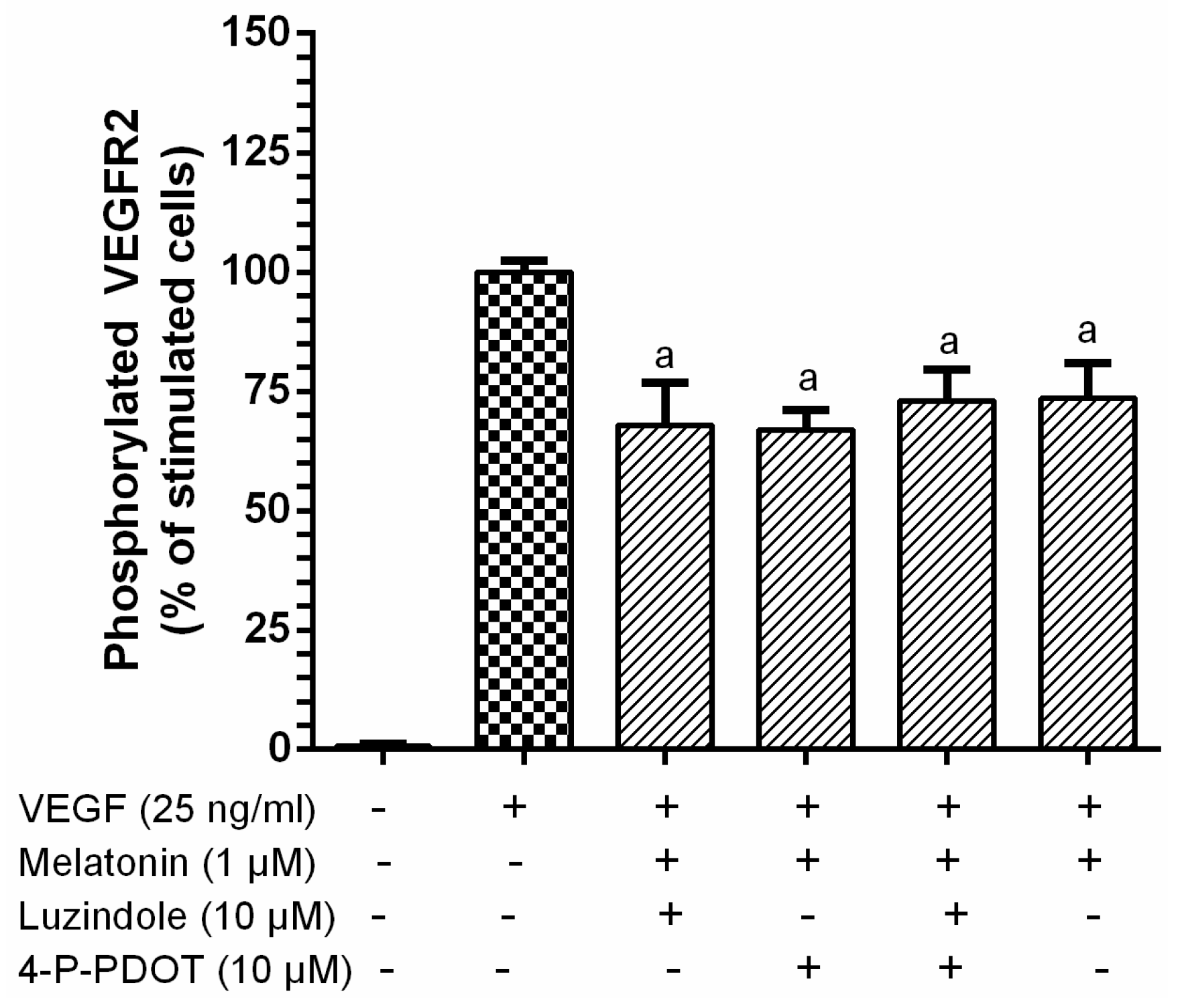
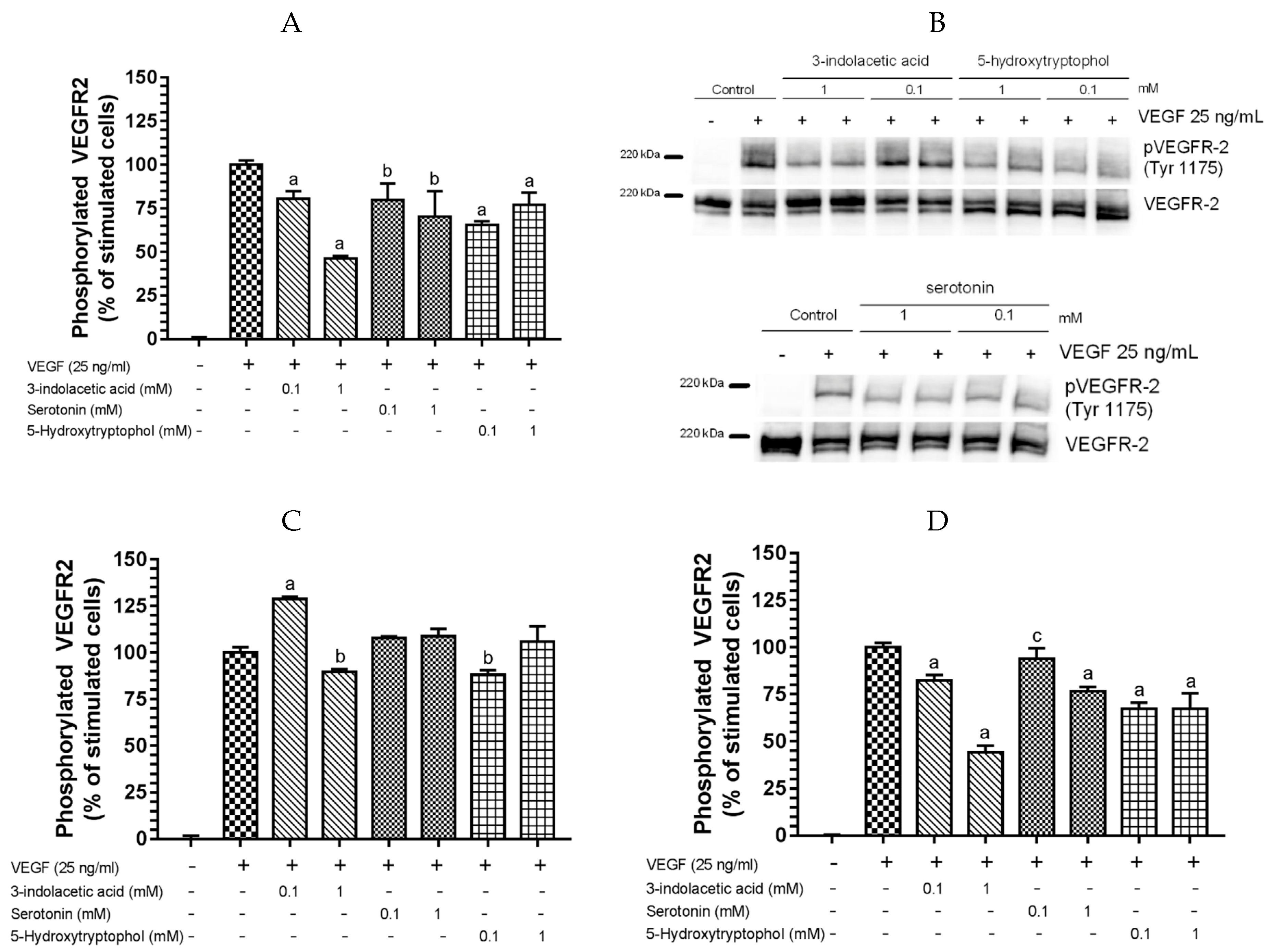
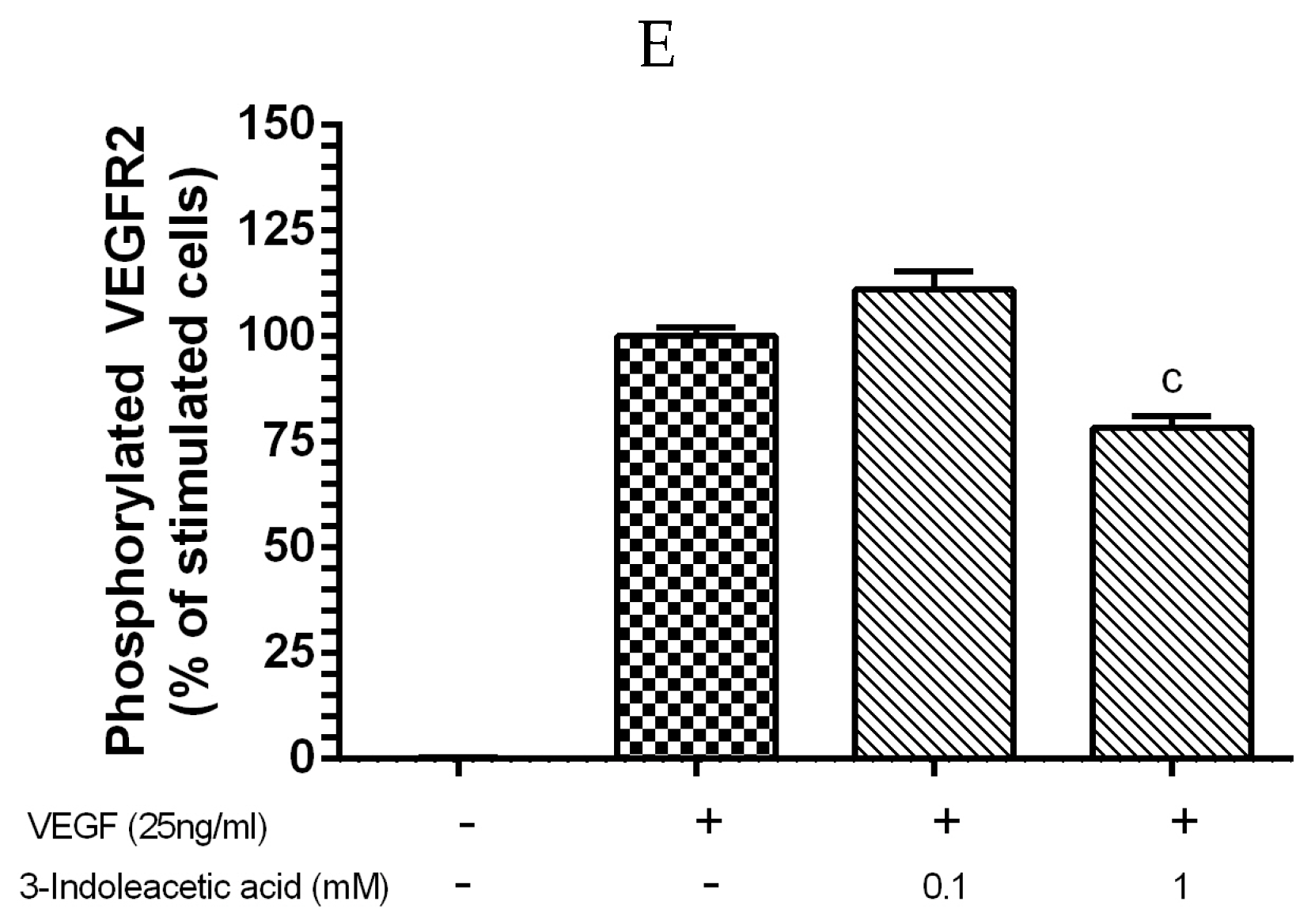
| Compounds | Concentrations (mM) | % Inhibition | IC50 (mM) | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Melatonin | 0.001 | 13.12 ± 4.47 | ND | ND | ND |
| 0.01 | 12.85 ± 0.95 | ND | ND | ||
| 0.1 | 24.36 ± 1.57 | 0.68 ± 3.35 | NI | ||
| 1 | 32.15 ± 1.87 | 16.66 ± 1.9 | 13.86 ± 1.12 | ||
| 3-Indolacetic acid | 0.1 | 19.41 ± 4.19 | NI | 17.68 ± 2.99 | 0.9704 (0.7174–1.313) |
| 1 | 53.56 ± 1.39 | 10.34 ± 1.55 | 55.94 ± 3.64 | ||
| Serotonin | 0.1 | 20.21 ± 9.47 | NI | 6.16 ± 5.66 | ND |
| 1 | 29.56 ± 14.36 | NI | 23.33 ± 2.29 | ||
| 5-hydroxytryptophol | 0.1 | 34.37 ± 2.11 | 11.95 ± 2.50 | 32.51 ± 3.08 | ND |
| 1 | 22.99 ± 7.02 | NI | 32.58 ± 8.09 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerezo, A.B.; Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Troncoso, A.M.; García-Parrilla, M.C. Inhibition of VEGF-Induced VEGFR-2 Activation and HUVEC Migration by Melatonin and Other Bioactive Indolic Compounds. Nutrients 2017, 9, 249. https://doi.org/10.3390/nu9030249
Cerezo AB, Hornedo-Ortega R, Álvarez-Fernández MA, Troncoso AM, García-Parrilla MC. Inhibition of VEGF-Induced VEGFR-2 Activation and HUVEC Migration by Melatonin and Other Bioactive Indolic Compounds. Nutrients. 2017; 9(3):249. https://doi.org/10.3390/nu9030249
Chicago/Turabian StyleCerezo, Ana B., Ruth Hornedo-Ortega, M. Antonia Álvarez-Fernández, Ana M. Troncoso, and M. Carmen García-Parrilla. 2017. "Inhibition of VEGF-Induced VEGFR-2 Activation and HUVEC Migration by Melatonin and Other Bioactive Indolic Compounds" Nutrients 9, no. 3: 249. https://doi.org/10.3390/nu9030249
APA StyleCerezo, A. B., Hornedo-Ortega, R., Álvarez-Fernández, M. A., Troncoso, A. M., & García-Parrilla, M. C. (2017). Inhibition of VEGF-Induced VEGFR-2 Activation and HUVEC Migration by Melatonin and Other Bioactive Indolic Compounds. Nutrients, 9(3), 249. https://doi.org/10.3390/nu9030249






