Effect of Monthly, High-Dose, Long-Term Vitamin D on Lung Function: A Randomized Controlled Trial
Abstract
1. Introduction
2. Material and Methods
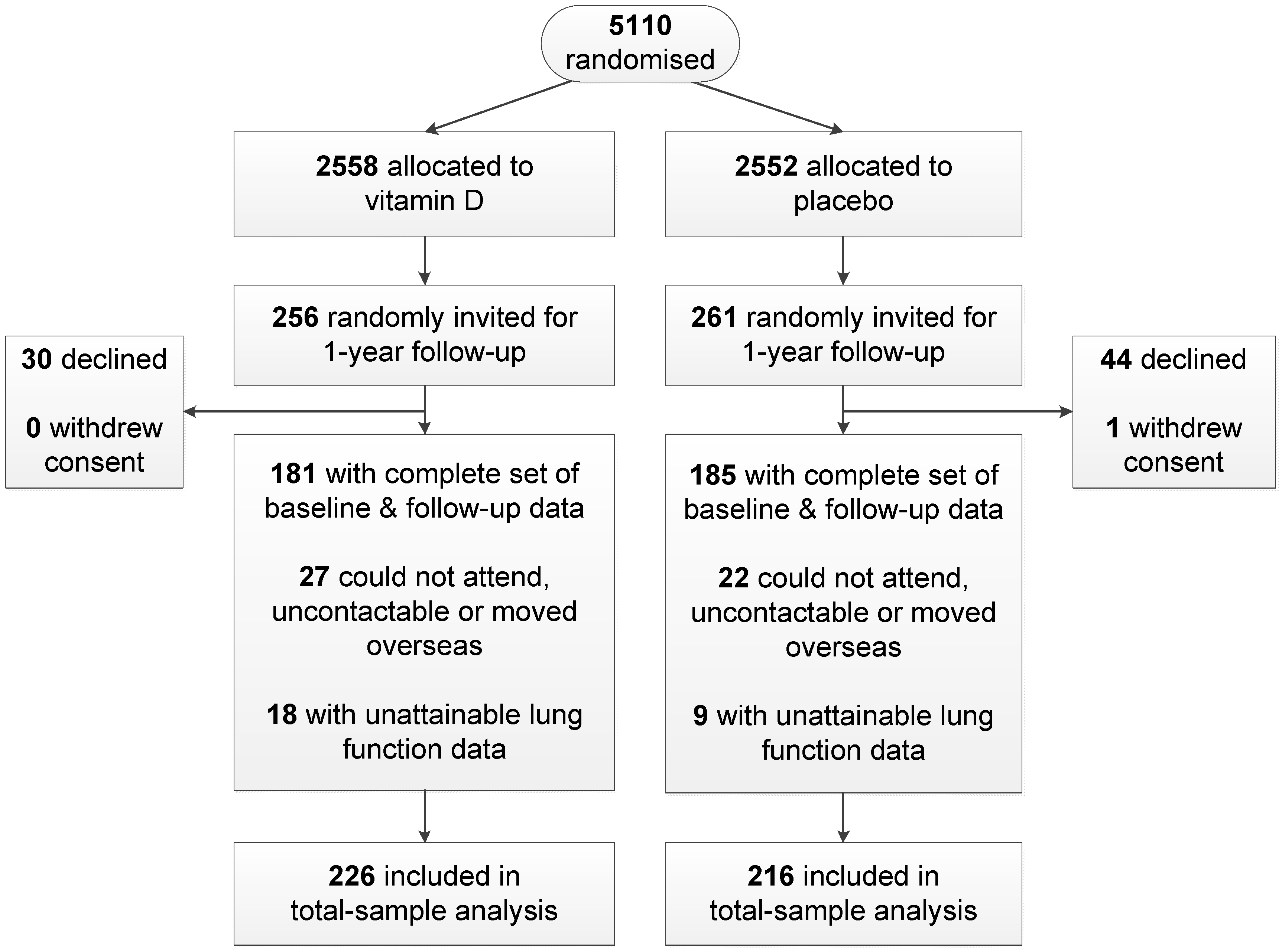
2.1. Participants
2.2. Vitamin D Intervention
2.3. Non-Lung Function Measures
2.4. Lung Function Measures
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Black, P.N.; Scragg, R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.E.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Vitamin D deficiency, smoking, and lung function in the normative aging study. Am. J. Respir. Crit. Care Med. 2012, 186, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Price, O.J.; Hull, J.H.; Howatson, G.; Robson-Ansley, P.; Ansley, L. Vitamin D and omega-3 polyunsaturated fatty acid supplementation in athletes with exercise-induced bronchoconstriction: A pilot study. Expert Rev. Respir. Med. 2015, 9, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.A.J.; Shoushtari, M.H. The effects of vitamin D supplementation on pulmonary function of chronic obstructive pulmonary disease patients, before and after clinical trial. Diseases 2015, 3, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2012, 156, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A.; et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDA randomized clinical trial. JAMA 2014, 311, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Gholami, M.; Anbari, K.; Ghanadi, K.; Bachari, E.C.; Azargon, A. Effects of vitamin D intake on FEV1 and COPD exacerbation: A randomized clinical trial study. Glob. J. Health Sci. 2015, 7, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Arshi, S.; Fallahpour, M.; Nabavi, M.; Bemanian, M.H.; Javad-Mousavi, S.A.; Nojomi, M.; Esmaeilzadeh, H.; Molatefi, R.; Rekabi, M.; Jalali, F.; et al. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthma. Ann. Allergy Asthma Immunol. 2014, 113, 404–409. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.C.; van Roon, E.N.H.; Storm, H.; Veeger, N.J.G.M.; Zwinderman, A.H.; Hiemstra, P.S.; Bel, E.H.D.; Ten Brinke, A. Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J. Allergy Clin. Immunol. 2015, 135, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; MacLaughlin, B.D.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Kilpin, K.; McLaughlin, D.; Fletcher, G.; Mein, C.A.; et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015, 70, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Selim, S.; Abbassi, M.M.; Sabry, N.A. Effect of alfacalcidol on the pulmonary function of adult asthmatic patients: A randomized trial. Ann. Allergy Asthma Immunol. 2017, 118, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Nageswari, A.D.; Rajanandh, M.G.; Priyanka, R.K.; Rajasekhar, P. Effect of vitamin D3 on mild to moderate persistent asthmatic patients: A randomized controlled pilot study. Perspect. Clin. Res. 2014, 5, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A., Jr.; Budinger, G.R.S.; Escobar, G.J.; Hansel, N.N.; Hanson, C.K.; Huffnagle, G.B.; Buist, A.S. Promotion of lung health: NHLBI workshop on the primary prevention of chronic lung diseases. Ann. Am. Thorac. Soc. 2014, 11, S125–S138. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Iodice, S.; Pilz, S.; Grant, W.B.; Bagnardi, V.; Gandini, S. Vitamin D deficiency and mortality risk in the general population: A meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2012, 95, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Manson, J.E.; Pilz, S.; März, W.; Michaëlsson, K.; Lundqvist, A.; Jassal, S.K.; Barrett-Connor, E.; Zhang, C.; et al. Circulating 25-Hydroxy-Vitamin D and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Larose, T.L.; Brumpton, B.M.; Langhammer, A.; Camargo, C.A., Jr.; Chen, Y.; Romundstad, P.; Mai, X.M. Serum 25-hydroxyvitamin D level, smoking and lung function in adults: The HUNT Study. Eur. Respir. J. 2015, 46, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Lange, P.; Bojesen, S.E.; Freiberg, J.J.; Nordestgaard, B.G. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax 2014, 69, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bartz, T.M.; Chittoor, G.; Eiriksdottir, G.; Manichaikul, A.W.; Sun, F.; Terzikhan, N.; Zhou, X.; Booth, S.L.; Brusselle, G.G.; et al. Large meta-analysis provides evidence for an association of serum vitamin D with pulmonary function. bioRxiv 2017. [Google Scholar] [CrossRef]
- Scragg, R.; Waayer, D.; Stewart, A.W.; Lawes, C.M.M.; Toop, L.; Murphy, J.; Khaw, K.T.; Camargo, C.A., Jr. The Vitamin D Assessment (ViDA) Study: Design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J. Steroid Biochem. Mol. Biol. 2016, 164, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Sorkness, C.A.; Li, J.T.; Marcus, P.; Murray, J.J.; Nathan, R.A.; Kosinski, M.; Pendergraft, T.B.; Jhingran, P. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J. Allergy Clin. Immunol. 2006, 117, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.; Sheahan, D.; Mackey, B.; Jacobsen, C. Use of Asthma Control Test (ACT) affects New Zealand primary care doctors’ perception of asthma control. N. Z. Med. J. 2011, 124, 99–101. [Google Scholar] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive (2017 Report). Available online: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ (accessed on 2 March 2017).
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.C.; Shoben, A.; Levin, G.P.; Robinson-Cohen, C.; Hoofnagle, A.N.; Swords-Jenny, N.; Ix, J.H.; Budoff, M.; Lutsey, P.L.; Siscovick, D.S.; et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2013, 97, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.S.; Hunninghake, G.W. Smoking disrupts vitamin D metabolism in the lungs. Am. J. Respir. Crit. Care Med. 2010, 181. [Google Scholar] [CrossRef]
- Haley, K.J.; Manoli, S.E.; Tantisira, K.G.; Litonjua, A.A.; Nguyen, P.; Kobzik, L.; Weiss, S.T. Maternal smoking causes abnormal expression of the vitamin D receptor. Am. J. Respir. Crit. Care Med. 2009, 179. [Google Scholar] [CrossRef]
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic triad in COPD: Oxidative stress, protease-antiprotease imbalance, and inflammation. Int. J. Chronic Obstruct. Pulm. Dis. 2011, 6, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D. Proteolysis in the lung. Eur. Respir. J. 2003, 22, 30s–32s. [Google Scholar] [CrossRef]
- Mortaz, E.; Masjedi, M.R.; Rahman, I. Outcome of smoking cessation on airway remodeling and pulmonary inflammation in COPD patients. Tanaffos 2011, 10, 7–11. [Google Scholar] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Baek, M.S.; Yoon, D.S.; Park, J.S.; Yoon, B.W.; Oh, B.S.; Park, J.; Kim, H.J. Vitamin D inhibits expression and activity of matrix metalloproteinase in human lung fibroblasts (HFL-1) cells. Tuberc. Respir. Dis. 2014, 77, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.Y.; Yang, F.L.; Wu, W.T.; Chung, C.H.; Lee, R.P.; Yang, W.T.; Subeq, Y.M.; Liao, K.W. Vitamin D3 reduces tissue damage and oxidative stress caused by exhaustive exercise. Int. J. Med. Sci. 2016, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.C.W.; Ho, S.P.; Ho, A.S.S.; Law, B.K.W.; Cheung, A.H.K.; Ho, J.C.M.; Ip, M.S.M.; Chan-Yeung, M.M.W. Sustained elevation of systemic oxidative stress and inflammation in exacerbation and remission of asthma. ISRN Allergy 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.F. Minimal clinically important differences in COPD lung function. COPD 2005, 2, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Beeh, K.M.; Chapman, K.R.; Decramer, M.; Mahler, D.A.; Wedzicha, J.A. Minimal clinically important differences in pharmacological trials. Am. J. Respir. Crit. Care Med. 2014, 189, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Vaz Fragoso, C.A.; Concato, J.; McAvay, G.; Klar Yaggi, H.; van Ness, P.H.; Gill, T.M. Staging the severity of chronic obstructive pulmonary disease in older persons based on spirometric Z-scores. J. Am. Geriatr. Soc. 2011, 59, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, H.K.; Baek, S.; Jung, J.-Y.; Kim, Y.S. Development of a spirometry T-score in the general population. Int. J. Chron. Obstruct. Pulm. Dis. 2016, 11, 369–379. [Google Scholar]
- Ministry of Health New Zealand. Enrollment in a Primary Health Organisation. Available online: http://www.health.govt.nz/our-work/primary-health-care/about-primary-health-organisations/enrolment-primary-health-organisation (accessed on 17 November 2017).
- Dijkman, B.; Kooistra, B.; Bhandari, M.; Archibald, S.; Baillie, F.; Cadeddu, M.; Cinà, C.; Farrokhyar, F.; Goldsmith, C.H.; Haines, T.; et al. How to work with a subgroup analysis. Can. J. Surg. 2009, 52, 515–522. [Google Scholar] [PubMed]
- Nessvi, S.; Johansson, L.; Jopson, J.; Stewart, A.; Reeder, A.; McKenzie, R.; Scragg, R.K. Association of 25-hydroxyvitamin D3 levels in adult New Zealanders with ethnicity, skin color and self-reported skin sensitivity to sun exposure. Photochem. Photobiol. 2011, 87, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.J.; Beasley, R. Hospital admissions for chronic obstructive pulmonary disease in New Zealand. N. Z. Med. J. 2015, 128, 23–35. [Google Scholar] [PubMed]
- Kaiser, L.D. Stratification of randomization is not required for a pre-specified subgroup analysis. Pharm. Stat. 2013, 12, 43–47. [Google Scholar] [CrossRef] [PubMed]
| Variable | Vitamin D | Placebo |
|---|---|---|
| n | 226 | 216 |
| Days from randomization to follow-up 1 | 401 ± 29 | 402 ± 30 |
| Age (years) 1 | 64.6 ± 8.4 | 65.4 ± 9.0 |
| Male sex (n (%)) | 140 (62) | 117 (54) |
| Ethnicity | ||
| European/Other (n (%)) | 172 (76) | 169 (78) |
| Maori (n (%)) | 15 (7) | 13 (6) |
| Pacific (n (%)) | 22 (10) | 16 (7) |
| South Asian (n (%)) | 17 (8) | 18 (8) |
| Asthma (n (%)) | 28 (12) | 36 (17) |
| ACT score, median ± IQR | 23.5 ± 4.5 | 21.5 ± 5.5 |
| COPD (n (%)) | 40 (18) | 37 (17) |
| GOLD stage 1 (n (%)) | 15 (7) | 14 (6) |
| GOLD stage 2 (n (%)) | 19 (8) | 18 (8) |
| GOLD stage 3 (n (%)) | 4 (2) | 2 (1) |
| GOLD stage 4 (n (%)) | 2 (1) | 3 (1) |
| Body mass index (kg/m2) 1 | 28.6 ± 5.2 | 28.5 ± 4.9 |
| 25-hydroxyvitamin D | ||
| Observed 1 | 61.5 ± 24.4 | 61.4 ± 23.7 |
| Deseasonalised 1 | 66.0 ± 23.7 | 65.5 ± 23.3 |
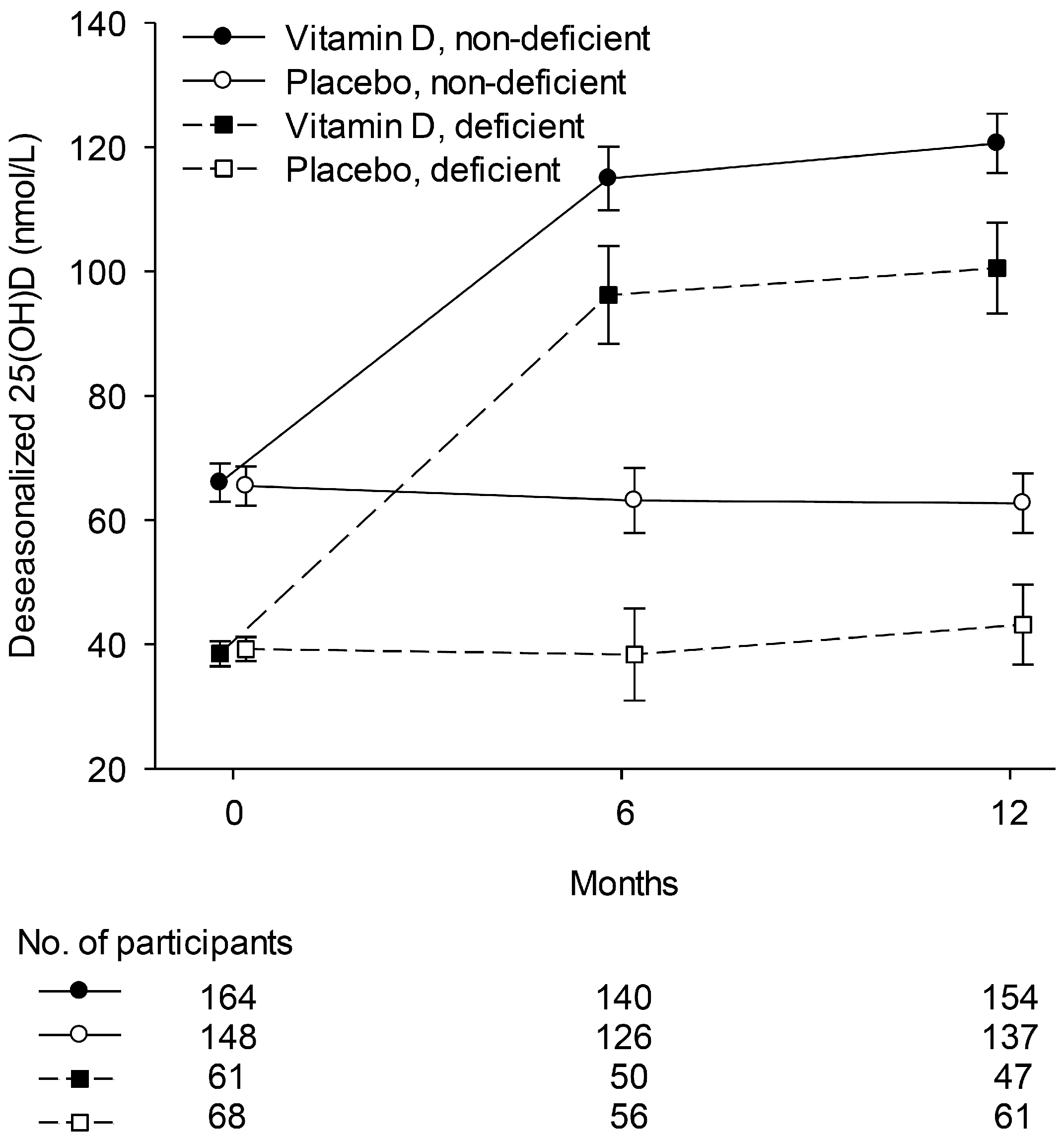
| Deseasonalised < 50 nmol/L (n (%)) | 61 (27) | 68 (31) |
| Lung function medication (n (%)) | 21 (9) | 27 (13) |
| On vitamin D supplements at baseline (n (%)) | 27 (12) | 22 (10) |
| Smoking | ||
| Never-smoker (n (%)) | 122 (54) | 103 (48) |
| Ex-smoker (n (%)) | 83 (37) | 95 (44) |
| Current smoker (n (%)) | 21 (9) | 18 (8) |
| Sun exposure (hours/day) | ||
| <1 (n (%)) | 34 (15) | 29 (13) |
| 1–2 (n (%)) | 113 (50) | 118 (55) |
| >2 (n (%)) | 79 (35) | 69 (32) |
| Sample | n | Mean (Standard Deviation) | Change from Baseline, Vitamin D Minus Placebo | |||||
|---|---|---|---|---|---|---|---|---|
| Vitamin D Group | Placebo Group | Vitamin D Group | Placebo Group | |||||
| Baseline | Follow-Up | Baseline | Follow-Up | Mean (95% CI) | p-Value | |||
| Total | 226 | 216 | 2242 (684) | 2313 (687) | 2370 (754) | 2325 (737) | 16 (−19, 51) | 0.38 |
| Vitamin D-deficient 1 | 61 | 68 | 2363 (597) | 2363 (620) | 2201 (777) | 2162 (774) | 39 (−28, 107) | 0.25 |
| Asthma/COPD | 54 | 59 | 1869 (600) | 1861 (623) | 1951 (735) | 1903 (691) | 40 (−33, 112) | 0.28 |
| Vitamin D-deficient 1 + asthma/COPD | 16 | 27 | 2023 (563) | 2079 (574) | 1914 (896) | 1861 (844) | 109 (−15, 233) | 0.08 |
| Ever-smoker | 104 | 113 | 2241 (725) | 2232 (750) | 2262 (733) | 2197 (693) | 57 (4, 109) | 0.03 |
| Ever-smoker + vitamin D-deficient 1 | 26 | 28 | 2348 (641) | 2378 (781) | 1912 (616) | 1821 (514) | 122 (8, 236) | 0.04 |
| Ever-smoker + asthma/COPD | 25 | 35 | 1538 (532) | 1632 (565) | 1775 (657) | 1709 (582) | 160 (53, 268) | 0.004 |
| Sample | n | Mean (Standard Deviation) | Change from Baseline, Vitamin D Minus Placebo | |||||
|---|---|---|---|---|---|---|---|---|
| Vitamin D Group | Placebo Group | Vitamin D Group | Placebo Group | |||||
| Baseline | Follow-UP | Baseline | Follow-Up | Mean (95% CI) | p-Value | |||
| Total | 226 | 216 | 3078 (858) | 3060 (861) | 3107 (945) | 3093 (910) | −5 (−49, 39) | 0.83 |
| Vitamin D-deficient 1 | 61 | 68 | 3098 (774) | 3085 (783) | 2961 (992) | 2941 (949) | 7 (−82, 95) | 0.88 |
| Asthma/COPD | 54 | 59 | 2885 (833) | 2863 (891) | 2889 (976) | 2868 (918) | 0 (−96, 96) | 0.99 |
| Vitamin D-deficient 1 + asthma/COPD | 16 | 27 | 2917 (757) | 2967 (770) | 2836 (1172) | 2817 (1110) | 69 (−109, 246) | 0.44 |
| Ever-smoker | 104 | 113 | 2777 (846) | 2979 (882) | 3015 (901) | 2976 (840) | 42 (−19, 102) | 0.17 |
| Ever-smoker + vitamin D-deficient 1 | 26 | 28 | 3062 (805) | 3091 (942) | 2659 (780) | 2573 (617) | 115 (−14, 243) | 0.08 |
| Ever-smoker + asthma/COPD | 25 | 35 | 2423 (718) | 2486 (639) | 2621 (840) | 2598 (770) | 86 (−52, 225) | 0.22 |
| Sample | n | Mean (Standard Deviation) | Change from Baseline, Vitamin D Minus Placebo | |||||
|---|---|---|---|---|---|---|---|---|
| Vitamin D Group | Placebo Group | Vitamin D Group | Placebo Group | |||||
| Baseline | Follow-Up | Baseline | Follow-Up | Mean (95% CI) | p-Value | |||
| Total | 226 | 216 | 76.4 (7.7) | 76.0 (7.6) | 76.6 (7.4) | 75.4 (7.0) | 0.7 (−0.1, 1.5) | 0.07 |
| Vitamin D-deficient 1 | 61 | 68 | 76.6 (6.5) | 76.8 (7.0) | 74.3 (8.6) | 73.4 (8.0) | 1.2 (−0.5, 2.9) | 0.16 |
| Asthma/COPD | 54 | 59 | 65.9 (8.6) | 66.1 (9.4) | 68.0 (8.6) | 66.8 (8.2) | 1.4 (−0.2, 3.0) | 0.08 |
| Vitamin D-deficient 1 + asthma/COPD | 16 | 27 | 69.7 (9.4) | 70.2 (9.8) | 67.3 (9.9) | 66.0 (8.0) | 1.9 (−0.8, 4.6) | 0.16 |
| Ever-smoker | 104 | 113 | 75.2 (7.8) | 75.0 (7.7) | 75.3 (8.1) | 74.0 (7.7) | 1.1 (0.0, 2.5) | 0.05 |
| Ever-smoker + vitamin D-deficient 1 | 26 | 28 | 77.6 (6.0) | 77.4 (6.6) | 72.6 (11.0) | 71.5 (8.8) | 0.9 (−1.4, 3.2) | 0.42 |
| Ever-smoker + asthma/COPD | 25 | 35 | 63.2 (8.2) | 64.5 (10.5) | 66.5 (9.6) | 64.8 (8.8) | 3.0 (0.7, 5.4) | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sluyter, J.D.; Camargo, C.A., Jr.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.-T.; Scragg, R. Effect of Monthly, High-Dose, Long-Term Vitamin D on Lung Function: A Randomized Controlled Trial. Nutrients 2017, 9, 1353. https://doi.org/10.3390/nu9121353
Sluyter JD, Camargo CA Jr., Waayer D, Lawes CMM, Toop L, Khaw K-T, Scragg R. Effect of Monthly, High-Dose, Long-Term Vitamin D on Lung Function: A Randomized Controlled Trial. Nutrients. 2017; 9(12):1353. https://doi.org/10.3390/nu9121353
Chicago/Turabian StyleSluyter, John D., Carlos A. Camargo, Jr., Debbie Waayer, Carlene M. M. Lawes, Les Toop, Kay-Tee Khaw, and Robert Scragg. 2017. "Effect of Monthly, High-Dose, Long-Term Vitamin D on Lung Function: A Randomized Controlled Trial" Nutrients 9, no. 12: 1353. https://doi.org/10.3390/nu9121353
APA StyleSluyter, J. D., Camargo, C. A., Jr., Waayer, D., Lawes, C. M. M., Toop, L., Khaw, K.-T., & Scragg, R. (2017). Effect of Monthly, High-Dose, Long-Term Vitamin D on Lung Function: A Randomized Controlled Trial. Nutrients, 9(12), 1353. https://doi.org/10.3390/nu9121353





