Effects of Melatonin on Glucose Homeostasis, Antioxidant Ability, and Adipokine Secretion in ICR Mice with NA/STZ-Induced Hyperglycemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Induction of Hyperglycemia
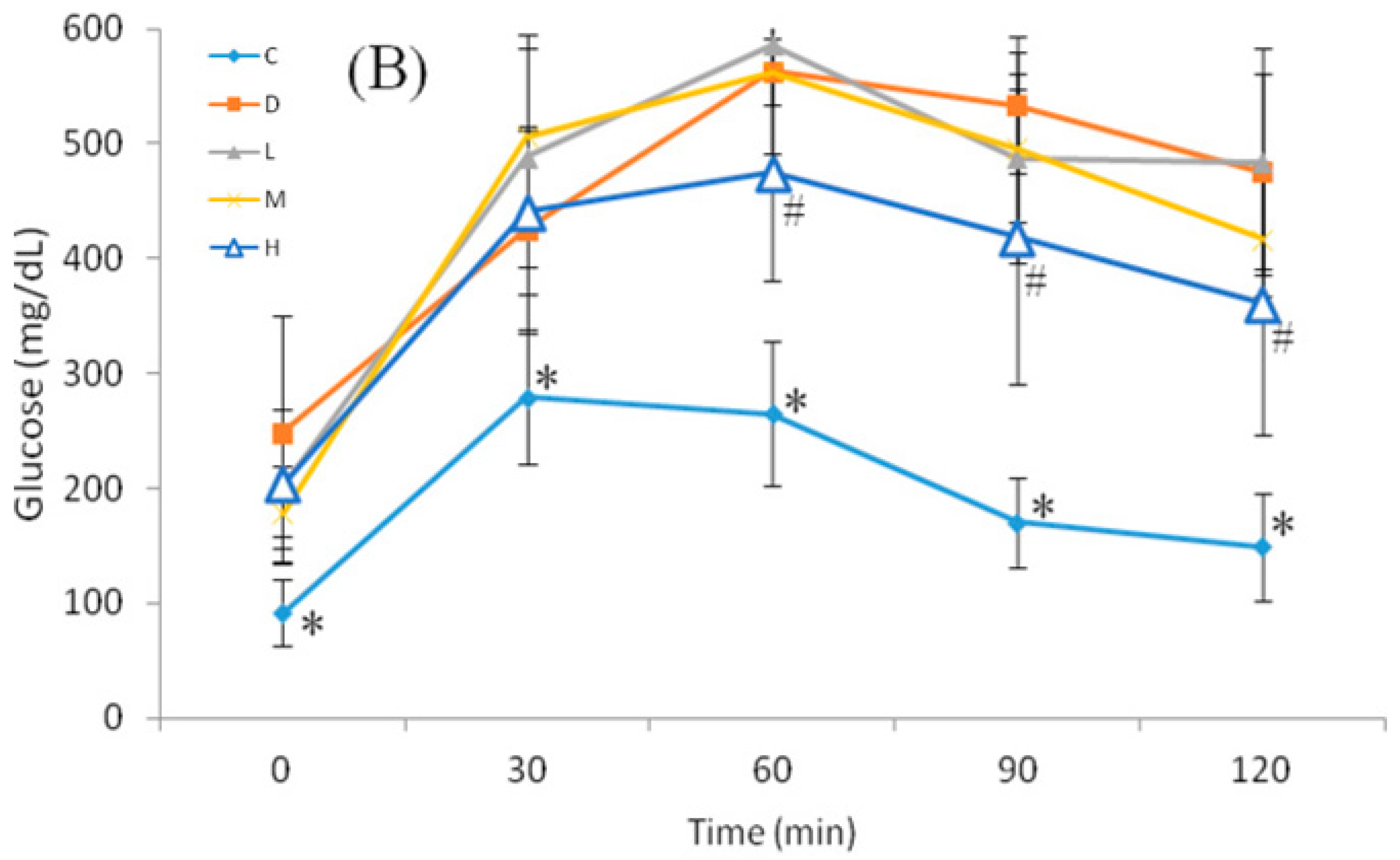
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Pharmacokinetics of Melatonin
2.6. Biochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Body, Fat Mass, and Organ Weights
3.2. Water Intake
3.3. Fasting Glucose, Plasma Insulin, and HOMA-IR
3.4. Glucose Homeostasis
3.5. Liver and Plasma Lipid Profiles
3.6. Oxidative Stress Biomarkers
3.7. Plasma Adipokines and Melatonin
3.8. Pharmacokinetics of Melatonin and Plasma Melatonin
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | an analysis of variance |
| BW | body weight |
| DM | diabetes mellitus |
| ELISA | enzyme-linked immunosorbent assay |
| FFA | fatty acid |
| FBG | fasting blood glucose |
| GPx | glutathione peroxidase |
| HOMA-IR | homeostasis model assessment for insulin resistance |
| IACUC | nstitutional Animal Care and Use Committee |
| MDA | Malondialdehyde |
| NA | nicotinamide |
| OGTT | Oral glucose tolerance test |
| ROS | reactive oxygen species |
| STZ | streptozotocin |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid-reactive substance |
| ZDF | Zucker diabetic fatty |
References
- Chan, J.C.; Malik, V.; Jia, W.; Kadowaki, T.; Yajnik, C.S.; Yoon, K.-H.; Hu, F.B. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-H.; Lee, J.-H.; Kim, J.-W.; Cho, J.H.; Choi, Y.-H.; Ko, S.-H.; Zimmet, P.; Son, H.-Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006, 368, 1681–1688. [Google Scholar] [CrossRef]
- Yeh, C.-J.; Chang, H.-Y.; Pan, W.-H. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: From NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac. J. Clin. Nutr. 2011, 20, 292–300. [Google Scholar] [PubMed]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 1993, 977–986. [Google Scholar]
- Wei, M.; Gaskill, S.P.; Haffner, S.M.; Stern, M.P. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality: The San Antonio heart study. Diabetes Care 1998, 21, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Garrel, C.; Fowler, P.A.; Al-Gubory, K.H. Developmental changes in antioxidant enzymatic defences against oxidative stress in sheep placentomes. J. Endocrinol. 2010, 205, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.; Fuentes-Broto, L.; Paredes, S.; Reiter, R. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Benloucif, S.; Burgess, H.J.; Klerman, E.B.; Lewy, A.J.; Middleton, B.; Murphy, P.J.; Parry, B.L.; Revell, V.L. Measuring melatonin in humans. J. Clin. Sleep Med. 2008, 4, 66–69. [Google Scholar] [PubMed]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 2009, 13, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Rosado, I.; Ruiz, R.; Figueroa, A.; Zen, N.; Fernández-Vázquez, G. Melatonin improves glucose homeostasis in young zucker diabetic fatty rats. J. Pineal Res. 2012, 52, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Paulis, L.; Simko, F. Blood pressure modulation and cardiovascular protection by melatonin: Potential mechanisms behind. Physiol. Res. 2007, 56, 671. [Google Scholar] [PubMed]
- Singh, M.; Jadhav, H.R. Melatonin: Functions and ligands. Drug Discov. Today 2014, 19, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, T.C.; Lellis-Santos, C.; Jesus, D.S.; Taneda, M.; Rodrigues, S.C.; Amaral, F.G.; Lopes, A.M.S.; Cipolla-Neto, J.; Bordin, S.; Anhe, G.F. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology 2011, 152, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.R. Effect of melatonin on cholesterol absorption in rats. J. Pineal Res. 2007, 42, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Esrefoglu, M.; Vardi, N.; Taslidere, E.; Ozerol, E.; Tanbek, K. Melatonin, quercetin and resveratrol attenuates oxidative hepatocellular injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2015, 34, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Schein, P.S.; McMenamin, M.G.; Cooney, D.A. Streptozotocin diabetes. Correlation with extent of depression of pancreatic islet nicotinamide adenine dinucleotide. J. Clin. Investig. 1974, 54, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, K.-D.; Fehsel, K.; Sommer, A.; Rodriguez, M.-L.; Kolb-Bachofen, V. Nitric oxide generation during cellular metabolization of the diabetogenic n-methyl-n-nitroso-urea streptozotozin contributes to islet cell DNA damage. Biol. Chem. Hoppe Seyler 1995, 376, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Takahashi, A.; Saito, I.; Kawanishi, S. Site-specific DNA methylation and apoptosis: Induction by diabetogenic streptozotocin. Biochem. Pharmacol. 1999, 57, 881–887. [Google Scholar] [CrossRef]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Adipocyte dysfunction in rats with streptozotocin–nicotinamide-induced diabetes. Int. J. Exp. Pathol. 2014, 95, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Park, J.-E.; Shin, E.J.; Shon, Y.H. Modulation of glucose metabolism by balanced deep-sea water ameliorates hyperglycemia and pancreatic function in streptozotocin-induced diabetic mice. PLoS ONE 2014, 9, e102095. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.-I.; Seo, K.-I.; Cho, H.W.; Kim, M.-J.; Park, E.-M.; Lee, M.-K. Effects of ursolic acid on glucose metabolism, the polyol pathway and dyslipidemia in non-obese type 2 diabetic mice. Indian J. Exp. Biol. 2014, 52, 683–691. [Google Scholar] [PubMed]
- She, M.; Hou, H.; Wang, Z.; Zhang, C.; Laudon, M.; Yin, W. Melatonin rescues 3T3-L1 adipocytes from ffa-induced insulin resistance by inhibiting phosphorylation of IRS-1 on ser307. Biochimie 2014, 103, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.G.; Turati, A.O.; Barone, M.; Scialfa, J.H.; Carmo Buonfiglio, D.; Peres, R.; Peliciari-Garcia, R.A.; Afeche, S.C.; Lima, L.; Scavone, C. Melatonin synthesis impairment as a new deleterious outcome of diabetes-derived hyperglycemia. J. Pineal Res. 2014, 57, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Huisamen, B.; Strijdom, H.; Blackhurst, D.; Lochner, A. Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J. Pineal Res. 2014, 57, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Prunet-Marcassus, B.; Desbazeille, M.; Bros, A.; Louche, K.; Delagrange, P.; Renard, P.; Casteilla, L.; Penicaud, L. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 2003, 144, 5347–5352. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Du Toit, E.F.; Blackhurst, D.; Marais, D.; Lochner, A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J. Pineal Res. 2011, 50, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, A.; Ohta, Y.; Ohashi, K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J. Pineal Res. 2012, 52, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Sartori, C.; Dessen, P.; Mathieu, C.; Monney, A.; Bloch, J.; Nicod, P.; Scherrer, U.; Duplain, H. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 2009, 150, 5311–5317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Meng, H.Z.; Yang, R.F.; Yang, M.W.; Sun, G.H.; Liu, J.H.; Shi, P.X.; Liu, F.; Yang, B. Melatonin suppresses autophagy in type 2 diabetic osteoporosis. Oncotarget 2016, 7, 52179–52194. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Kumar, A.; Kaundal, R.K.; Gulati, A.; Sharma, S.S. Functional and biochemical evidence indicating beneficial effect of melatonin and nicotinamide alone and in combination in experimental diabetic neuropathy. Neuropharmacology 2010, 58, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Klepac, N.; Rudes, Z.; Klepac, R. Effects of melatonin on plasma oxidative stress in rats with streptozotocin induced diabetes. Biomed. Pharmacother. 2006, 60, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Amniattalab, A.; Malekinejad, H.; Rezabakhsh, A.; Rokhsartalab-Azar, S.; Alizade-Fanalou, S. Silymarin: A novel natural agent to restore defective pancreatic beta cells in streptozotocin (stz)-induced diabetic rats. Iran. J. Pharm. Res. 2016, 15, 493–500. [Google Scholar] [PubMed]
- Elbe, H.; Vardi, N.; Esrefoglu, M.; Ates, B.; Yologlu, S.; Taskapan, C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum. Exp. Toxicol. 2015, 34, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Bibak, B.; Khalili, M.; Rajaei, Z.; Soukhtanloo, M.; Hadjzadeh, M.A.; Hayatdavoudi, P. Effects of melatonin on biochemical factors and food and water consumption in diabetic rats. Adv. Biomed. Res. 2014, 3, 173. [Google Scholar] [PubMed]
- Allagui, M.S.; Feriani, A.; Bouoni, Z.; Alimi, H.; Murat, J.C.; El Feki, A. Protective effects of vitamins (c and e) and melatonin co-administration on hematological and hepatic functions and oxidative stress in alloxan-induced diabetic rats. J. Physiol. Biochem. 2014, 70, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Al-Shali, K.Z.; Hegele, R.A. Hypertriglyceridemia: Its etiology, effects and treatment. CMAJ 2007, 176, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Navarro-Alarcon, M.; Ruiz, R.; Abuhamadah, S.; El-Mir, M.Y.; Vazquez, G.F. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J. Pineal Res. 2011, 50, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Stampfer, M.J.; Rifai, N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein (a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001, 285, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Salmanoglu, D.S.; Gurpinar, T.; Vural, K.; Ekerbicer, N.; Dariverenli, E.; Var, A. Melatonin and l-carnitin improves endothelial disfunction and oxidative stress in type 2 diabetic rats. Redox Biol. 2016, 8, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Vural, H.; Sabuncu, T.; Arslan, S.O.; Aksoy, N. Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J. Pineal Res. 2001, 31, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, N.; Vural, H.; Sabuncu, T.; Aksoy, S. Effects of melatonin on oxidative-antioxidative status of tissues in streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2003, 21, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Sudnikovich, E.J.; Maksimchik, Y.Z.; Zabrodskaya, S.V.; Kubyshin, V.L.; Lapshina, E.A.; Bryszewska, M.; Reiter, R.J.; Zavodnik, I.B. Melatonin attenuates metabolic disorders due to streptozotocin-induced diabetes in rats. Eur. J. Pharmacol. 2007, 569, 180–187. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.; Andreotti, S.; Farias Tda, S.; Torres-Leal, F.L.; de Proenca, A.R.; Campana, A.B.; de Souza, A.H.; Sertie, R.A.; Carpinelli, A.R.; Cipolla-Neto, J.; et al. Metabolic disorders and adipose tissue insulin responsiveness in neonatally STZ-induced diabetic rats are improved by long-term melatonin treatment. Endocrinology 2012, 153, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Raselli, S.; Grigore, L.; Garlaschelli, K.; Dozio, E.; Magni, P.; Catapano, A.L. Leptin: Adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke 2007, 38, 2844–2846. [Google Scholar] [CrossRef] [PubMed]

| Group | C | D | L | M | H |
|---|---|---|---|---|---|
| Fasting body weight (g) | 36.47 ± 1.65 a | 36.18 ± 1.01 a,b | 34.47 ± 1.79 b,c | 35.35 ± 1.44 b,c | 33.80 ± 2.24 c |
| Initial body weight (g) | 37.28 ± 2.09 | 39.12 ± 1.91 | 37.41 ± 2.08 | 39.27 ± 1.19 | 37.28 ± 2.43 |
| Final body weight (g) | 38.83 ± 1.55 | 40.71 ± 1.38 | 38.28 ± 1.66 | 39.80 ± 2.06 | 38.70 ± 2.66 |
| Total weight gain (g) | 1.15 ± 2.07 | 1.65 ± 1.70 | 1.06 ± 0.36 | 0.61 ± 0.91 | 1.31 ± 0.18 |
| Liver weight (g) | 1.75 ± 0.18 a | 2.06 ± 0.24 b | 1.90 ± 0.13 a,b | 1.84 ± 0.10 a | 1.92 ± 0.18 a,b |
| Kidney weight (g) | 0.66 ± 0.08 | 0.65 ± 0.09 | 0.63 ± 0.08 | 0.62 ± 0.05 | 0.62 ± 0.07 |
| Epididymal fat mass weight (g) | 0.52 ± 0.13 a | 0.32 ± 0.21 b | 0.34 ± 0.11 b | 0.32 ± 0.07 b | 0.24 ± 0.08 b |
| Relative liver weight (g/100 g BW) | 4.79 ± 0.34 a | 5.70 ± 0.56 c | 5.54 ± 0.47 b,c | 5.22 ± 0.25 a,b | 5.69 ± 0.34 c |
| Relative kidney weight (g/100 g BW) | 1.81 ± 0.18 | 1.79 ± 0.20 | 1.83 ± 0.19 | 1.78 ± 0.12 | 1.84 ± 0.15 |
| Relative epididymal fat mass weight (g/100 g BW) | 1.42 ± 0.37 a | 0.91 ± 0.58 b | 1.00 ± 0.32 b | 0.92 ± 0.19 b | 0.71 ± 0.25 b |
| Group | C | D | L | M | H |
|---|---|---|---|---|---|
| Water intake (mL/day) | |||||
| Week 2 | 7.70 ± 0.89 a | 18.89 ± 0.52 b | 20.95 ± 1.86 b | 18.86 ± 1.41 b | 23.97 ± 3.82 c |
| Week 4 | 6.95 ± 0.49 a | 22.04 ± 1.50 *,b | 23.33 ± 1.25 *,b,c | 21.88 ± 0.79 *,b | 24.41 ± 1.21 c |
| Week 6 | 6.38 ± 0.37 *,a | 26.06 ± 4.19 *, b | 24.30 ± 3.02 *,b | 22.38 ± 2.32 *,b | 25.01 ± 3.69 b |
| Total water gain (mL/day) | −1.04 ± 0.04 a | 7.18 ± 14.10 c | 3.34 ± 2.95 b | 3.52 ± 1.55 b | 1.03 ± 7.4 a,b |
| Group | C | D | L | M | H |
|---|---|---|---|---|---|
| Fasting glucose (mg/dL) | |||||
| Week 0 | 80.6 ± 27.6 | 124.6 ± 22.5 | 115.7 ± 37.2 | 109.6 ± 15.9 | 115.5 ± 67.3 |
| Week 6 | 91.5 ± 29.2 a | 249.1 ± 101.1 b | 214.8 ± 80.8 b | 178.1 ± 41.5 b | 203.5 ± 45.2 b |
| Change in fasting glucose (mg/dL) | 9.0 ± 25.2 a | 124.5 ± 42.2 b | 107.8 ± 68.8 b,c | 80.2 ± 50.8 c | 74.3 ± 32.1 c |
| Plasma insulin (µU/mL ) | 9.21 ± 0.71 a | 8.45 ± 0.56 b | 8.23 ± 0.58 b | 8.48 ± 0.53 a,b | 9.43 ± 0.94 a |
| HOMA-IR | 2.04 ± 0.67 a | 5.33 ± 2.32 b | 4.40 ± 1.65 b | 3.7 ± 0.75 b | 4.60 ± 0.80 b |
| Group | C | D | L | M | H |
|---|---|---|---|---|---|
| AUC | |||||
| Week 0 | 21,474 ± 6855 a | 44,128 ± 5314 b | 45,042 ± 5681 b | 43,616 ± 6426 b | 42,042 ± 5558 b |
| Week 6 | 25,040 ± 5530 a | 56,508 ± 6158 b | 57,173 ± 4865 b | 55,865 ± 5394 b | 48,500 ± 9433 c |
| Increase of AUC values | 3566 ± 4484 a | 13,036 ± 2892 b | 12,130 ± 2358 b | 12,249 ± 3540 b | 6457 ± 4309 a |
| Group | C | D | L | M | H |
|---|---|---|---|---|---|
| Liver | |||||
| Triglyceride (mg/g tissue) | 18.5 ± 4.7 a | 16.4 ± 3.0 a | 10.4 ± 1.5 b | 10.0 ± 2.2 b | 9.3 ± 2.2 b |
| Cholesterol (mg/g tissue ) | 3.24 ± 0.96 a | 5.69 ± 0.98 b | 3.22 ± 0.75 a | 3.16 ± 0.51 a | 3.14 ± 0.70 a |
| Plasma | |||||
| Triglyceride (mg/dL) | 78.0 ± 15.4 a | 114.2 ± 15.0 b | 103.1 ± 19.7 b | 95.8 ± 14.6 a,b | 81.5 ± 18.8 a |
| Cholesterol (mg/dL) | 74.4 ± 12.5 a | 116.4 ± 12.5 c | 94.5 ± 17.8 b | 91.3 ± 12.3 b | 90.8 ± 10.7 b |
| HDL-C (mg/dL) | 38.0 ± 11.2 a | 39.2 ± 9.1 a | 39.0 ± 6.1 a | 45.8 ± 13.0 a | 59.6 ± 7.6 b |
| LDL-C (mg/dL) | 22.0 ± 9.9 a,b | 54.4 ± 10.7 c | 35.0± 17.5 b | 28.9 ± 9.6 b | 14.9 ± 6.2 a |
| Total Cholesterol/HDL-C ratio | 2.12 ± 0.55 a | 3.53 ± 0.67 b | 2.48 ± 0.65 a | 2.08 ± 0.29 a | 1.53 ± 0.18 c |
| LDL-C/HDL-C ratio | 0.67 ± 0.43 a,c | 1.83 ± 0.56 b | 0.93 ± 0.54 a | 0.64 ± 0.25 a,c | 0.25 ± 0.12 c |
| Group | C | D | L | M | H |
|---|---|---|---|---|---|
| Liver | |||||
| MDA (nmol/g tissue) | 91.2 ± 16.1 a | 127.2 ± 10.7 b | 93.1 ± 18.5 a | 95.4 ± 14.7 a | 84.4 ± 22.3 a |
| SOD (U/mg protein) | 2.18 ± 0.96 a | 1.12 ± 0.11 b | 1.43 ± 0.30 b,c | 1.52 ± 0.47 b,c | 1.75 ± 0.26 a,c |
| GPx (nmol/min/mg protein) | 5.60 ± 1.00 a | 3.79 ± 0.43 b | 4.04 ± 0.63 b | 4.16 ± 0.76 b | 4.03 ± 0.42 b |
| Plasma | |||||
| MDA (µM) | 31.1 ± 7.6 a | 66.7 ± 16.6 b | 58.3 ± 13.3 b,c | 45.3 ± 14.5 c | 47.3 ± 4.97 c |
| Group | C | D | L | M | H |
|---|---|---|---|---|---|
| Adiponectin (µg/mL) | 3.98 ± 0.60 a | 2.14 ± 0.21 b | 2.82 ± 0.75 c | 2.79 ± 0.44 c | 2.90 ± 0.45 c |
| Leptin (ng/mL) | 5.98 ± 2.61 a | 4.94 ± 1.69 a | 3.96 ± 1.40 a,b | 2.08 ± 1.41 b | 2.41 ± 1.91 b |
| leptin/adiponectin ratio | 1.72 ± 0.92 a,b | 2.01 ± 0.65 a | 1.27 ± 0.24 b | 0.53 ± 0.32 c | 0.61 ± 0.68 c |
| Melatonin (pg/mL) | 60.4 ± 7.9 a | 50.3 ± 5.8 b | 120.5 ± 11.7 c | 148.6 ± 17.4 d | 134.8 ± 12.2 d |
| Group | 10 mg/kg of BW | 50 mg/kg of BW | p value | |
|---|---|---|---|---|
| Melatonin level after oral administration of melatonin (µg/mL) | ||||
| 10 min | Melatoninconcentration (μg/mL) | 0.82 ± 0.10 | 3.62 ± 0.36 | <0.01 |
| 30 min | 1.82 ± 0.12 | 8.04 ± 0.59 | <0.01 | |
| 60 min | 0.86 ± 0.02 | 3.84 ± 0.22 | <0.01 | |
| 120 min | 0.17 ± 0.03 | 0.87 ± 0.09 | <0.01 | |
| AUC (µg * min/mL) | 102.0 ± 4.8 | 455.1 ± 25.2 | <0.01 | |
| Ke (1/h) | 1.62 ± 0.13 | 1.48 ± 0.15 | 0.126 | |
| Half-life (h) | 0.43 ± 0.04 | 0.47 ± 0.05 | 0.122 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, C.-C.; Lin, S.-H.; Chang, J.-S.; Chien, Y.-W. Effects of Melatonin on Glucose Homeostasis, Antioxidant Ability, and Adipokine Secretion in ICR Mice with NA/STZ-Induced Hyperglycemia. Nutrients 2017, 9, 1187. https://doi.org/10.3390/nu9111187
Lo C-C, Lin S-H, Chang J-S, Chien Y-W. Effects of Melatonin on Glucose Homeostasis, Antioxidant Ability, and Adipokine Secretion in ICR Mice with NA/STZ-Induced Hyperglycemia. Nutrients. 2017; 9(11):1187. https://doi.org/10.3390/nu9111187
Chicago/Turabian StyleLo, Chung-Cheng, Shyh-Hsiang Lin, Jung-Su Chang, and Yi-Wen Chien. 2017. "Effects of Melatonin on Glucose Homeostasis, Antioxidant Ability, and Adipokine Secretion in ICR Mice with NA/STZ-Induced Hyperglycemia" Nutrients 9, no. 11: 1187. https://doi.org/10.3390/nu9111187
APA StyleLo, C.-C., Lin, S.-H., Chang, J.-S., & Chien, Y.-W. (2017). Effects of Melatonin on Glucose Homeostasis, Antioxidant Ability, and Adipokine Secretion in ICR Mice with NA/STZ-Induced Hyperglycemia. Nutrients, 9(11), 1187. https://doi.org/10.3390/nu9111187






