Coptidis Rhizoma Prevents Heat Stress-Induced Brain Damage and Cognitive Impairment in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
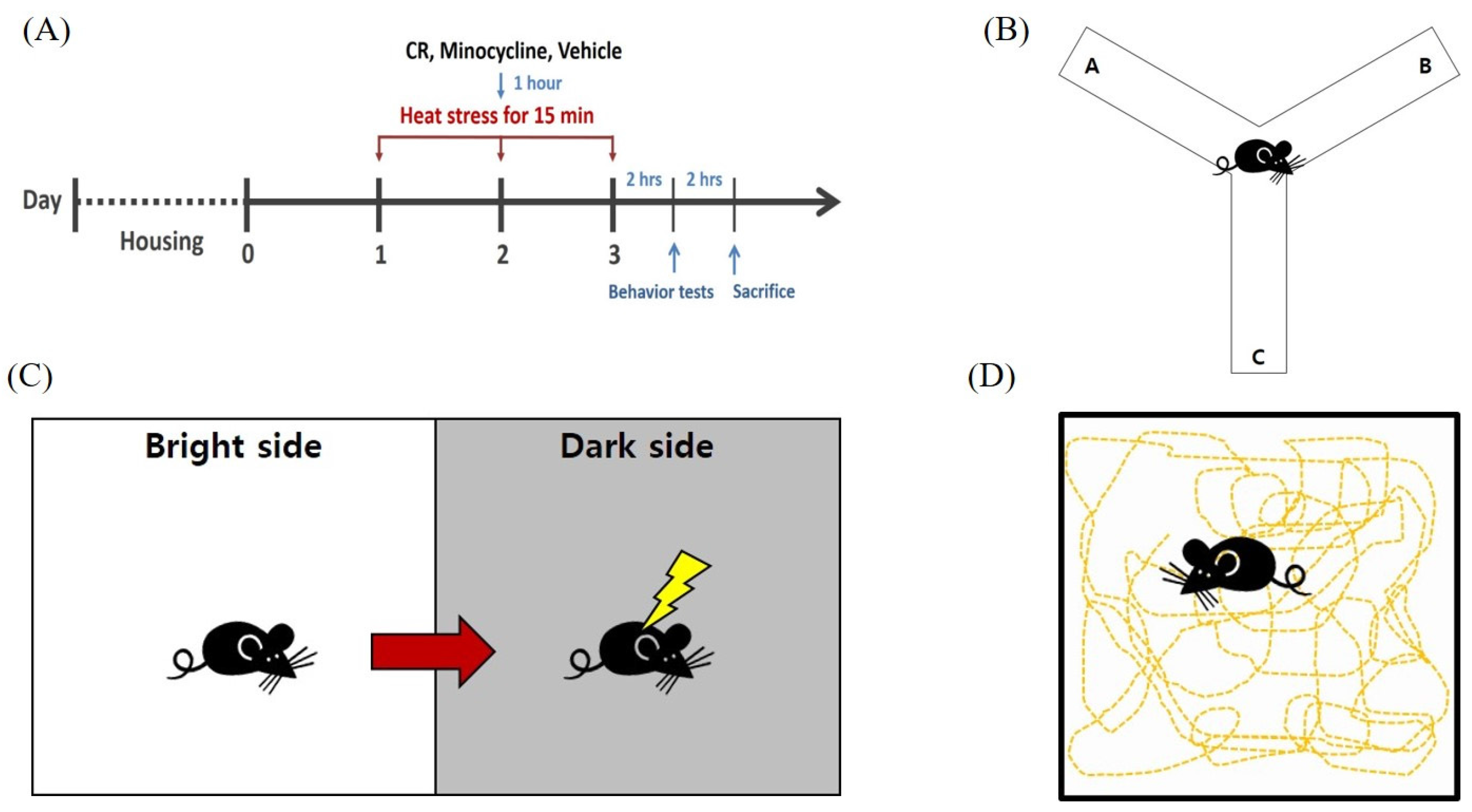
2.3. Heat Exposure and Drug Administration
2.4. Measurement of Cortisol Levels, TNF-α, IL-1β, IL-9, IL-13 and PGE2
2.5. Western Blotting
2.6. Behavior Tests
2.6.1. Passive Avoidance Test
2.6.2. Y Maze Task
2.6.3. Open-Field Test
2.7. Immunohistochemistry and Immunofluorescence
2.8. Statistical Analysis
3. Results
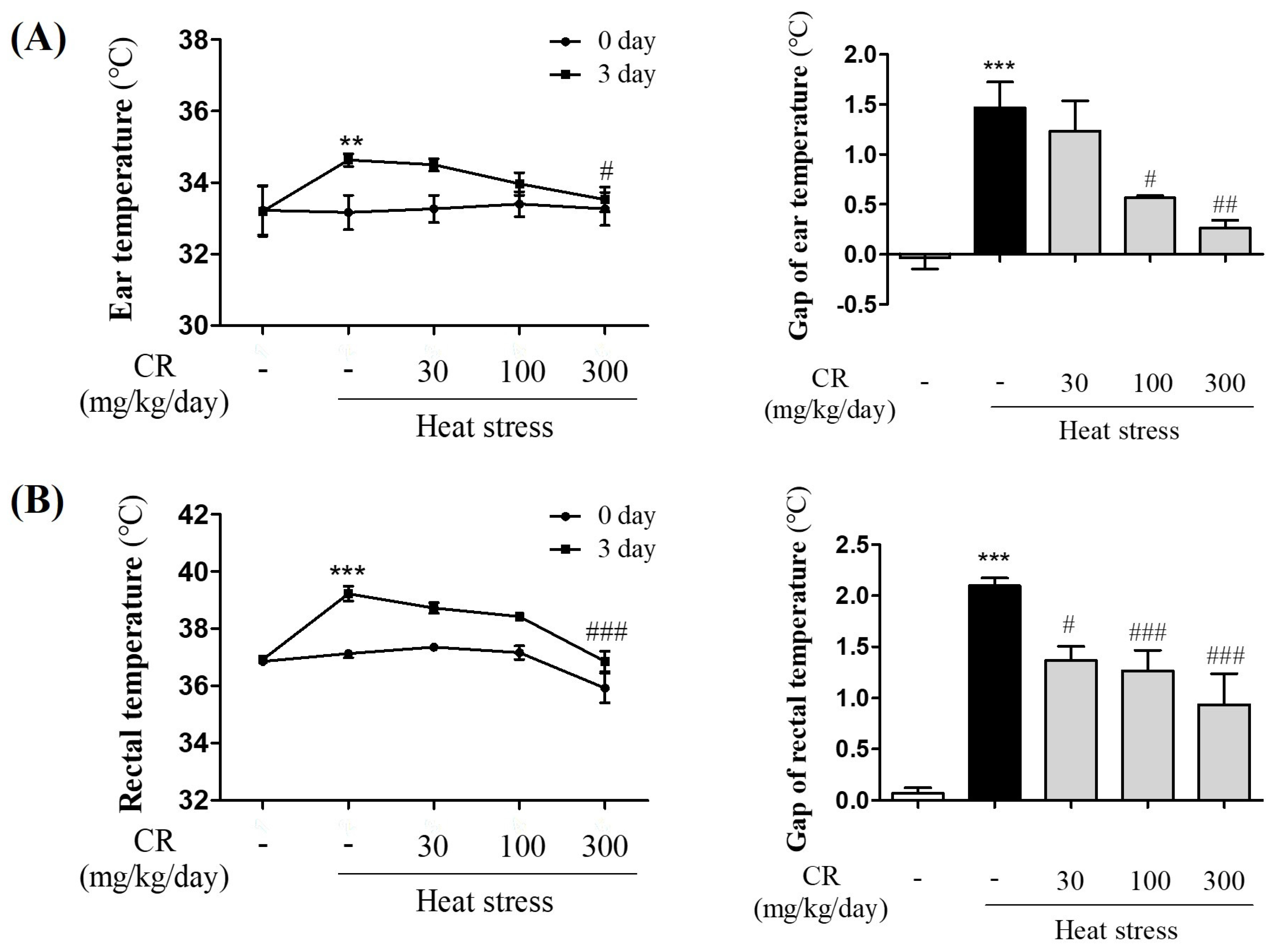
3.1. Inhibitory Effects of CR on Increased Body Temperature Induced by Heat Exposure
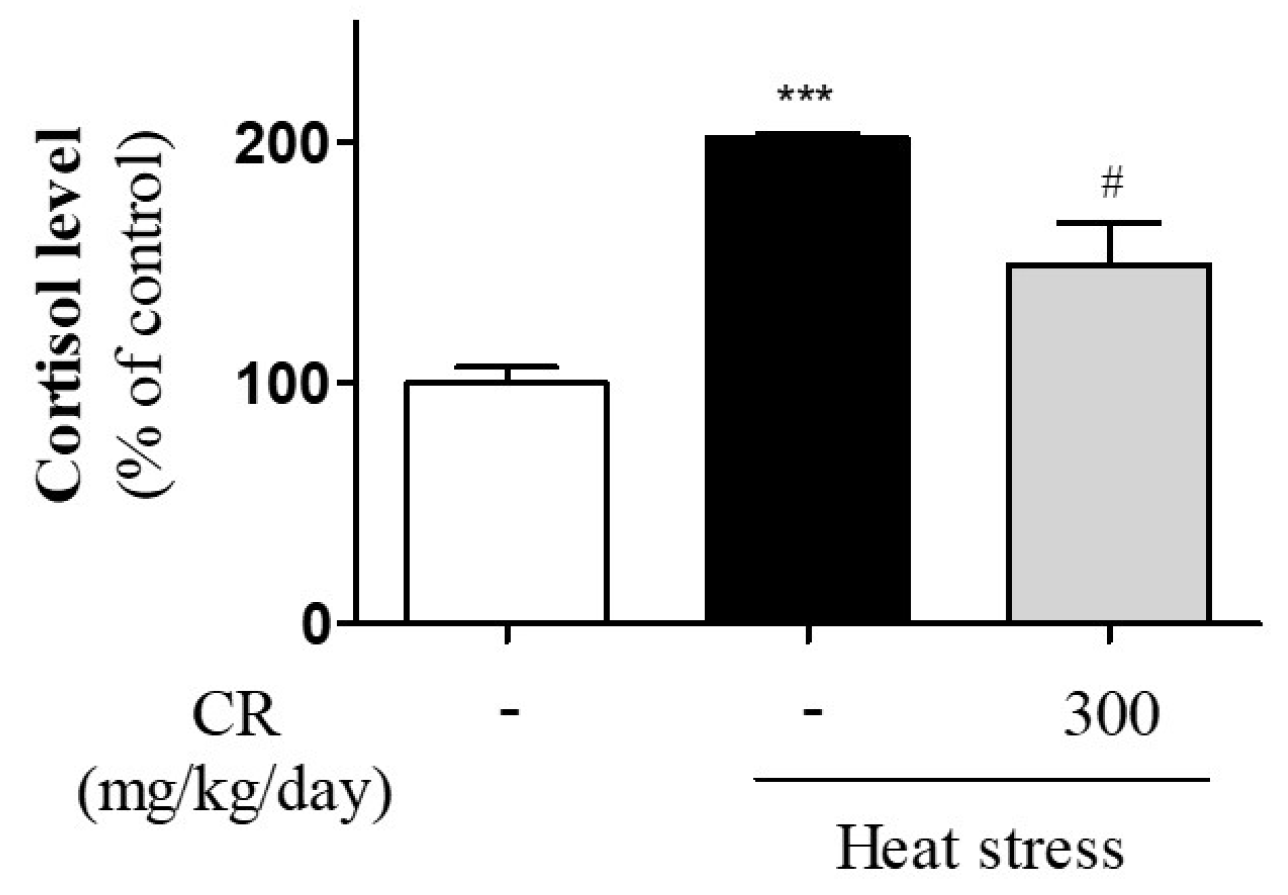
3.2. Inhibitory Effects of CR on Heat Stress-Induced Secretion of Cortisol
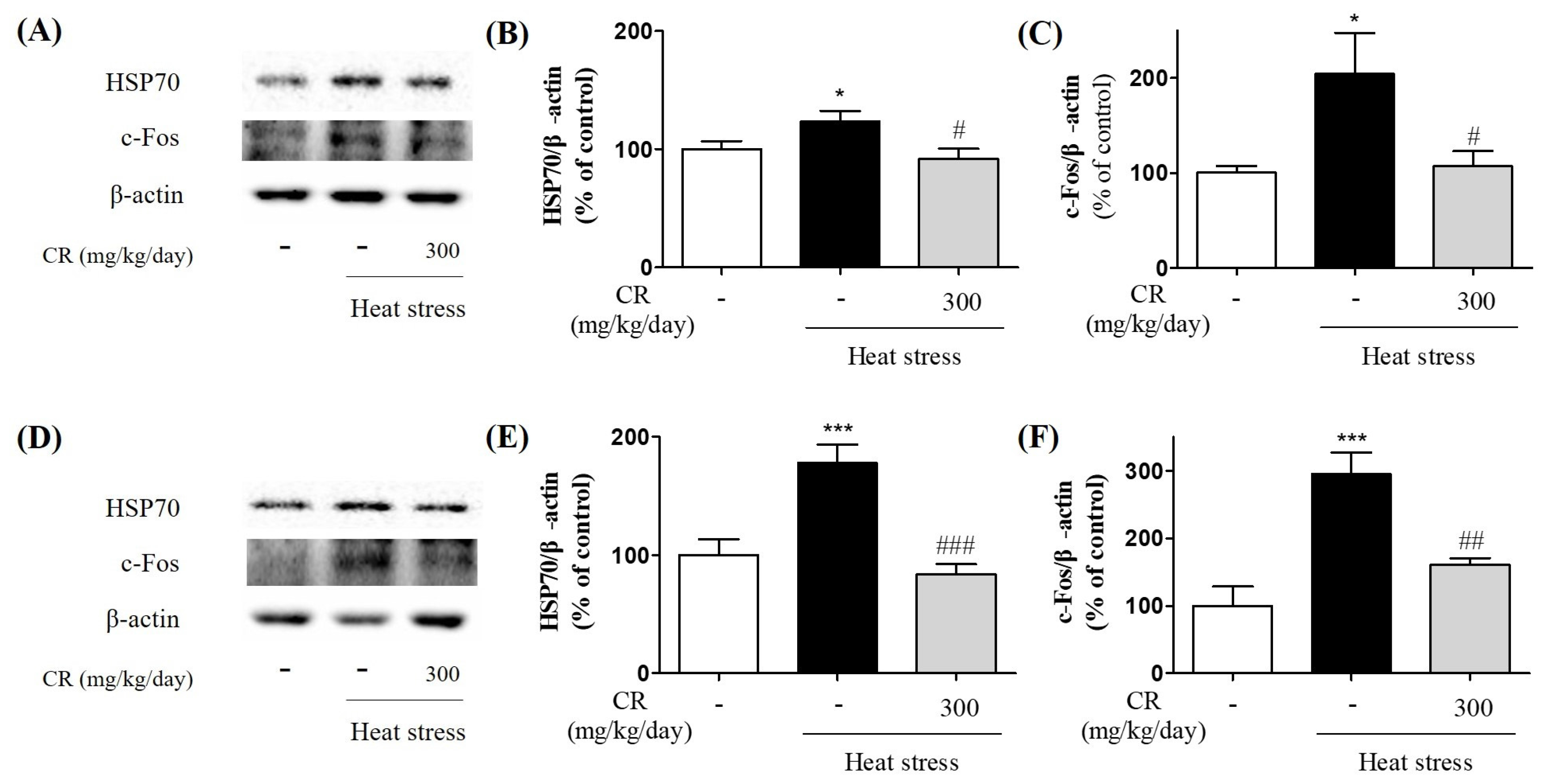
3.3. Inhibitory Effects of CR on Heat Stress-Induced Biochemical Changes in the Brain
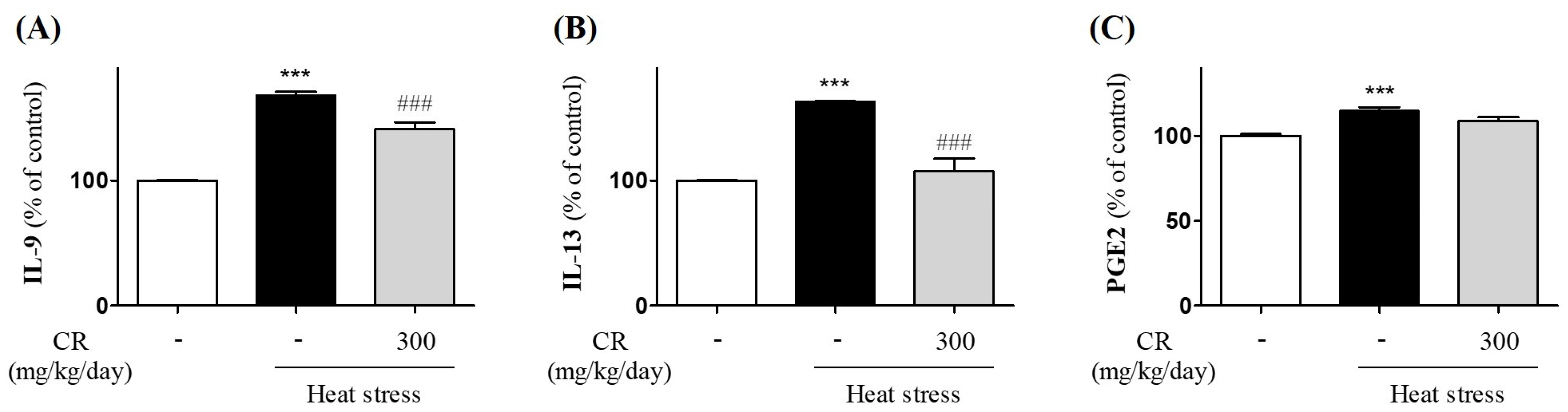
3.4. Attenuating Effects of CR on Heat Stress-Induced Production of Inflammatory Mediators
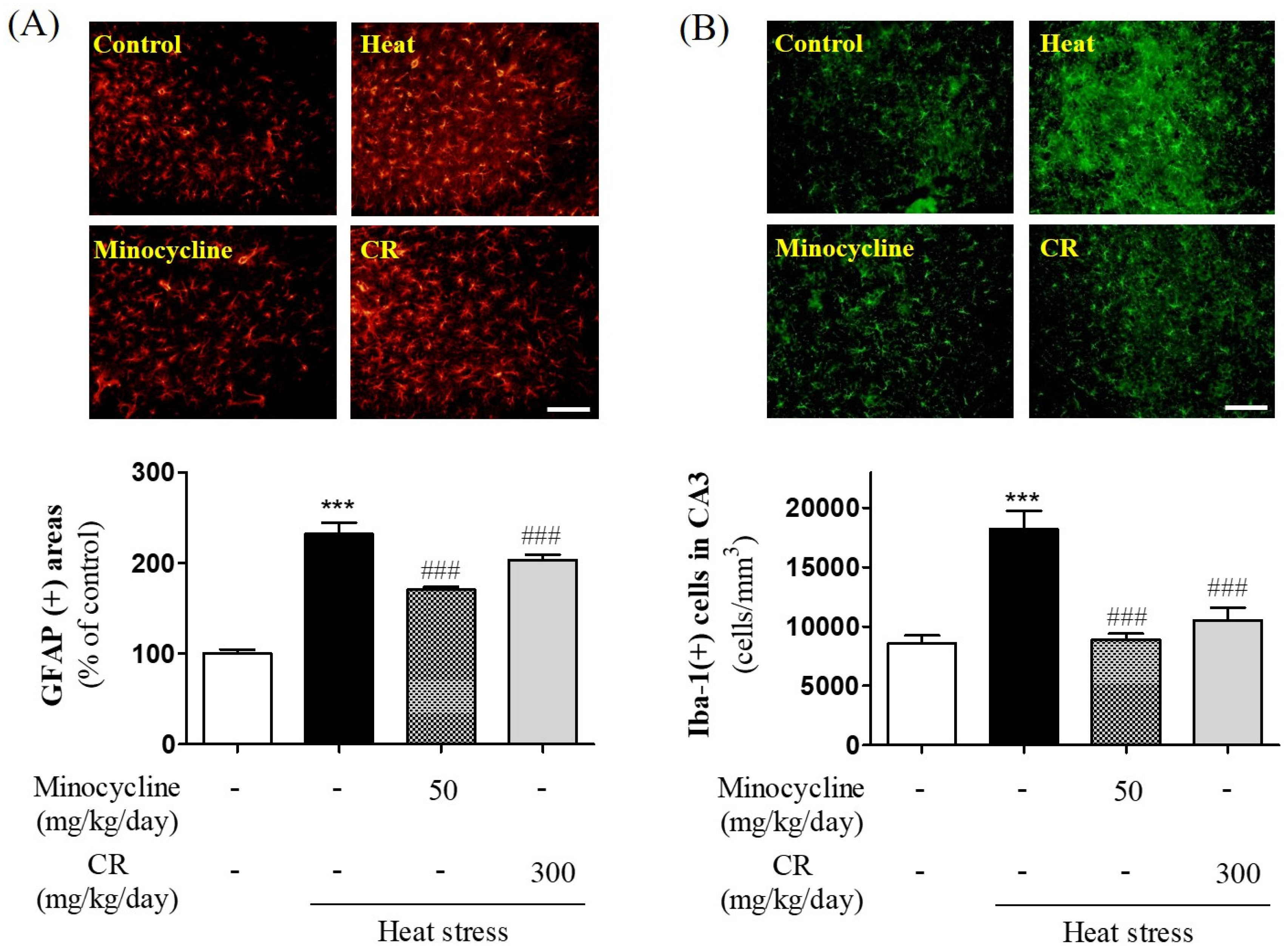
3.5. Suppressive Effects of CR on Heat Stress-Induced Gliosis in Hippocampus
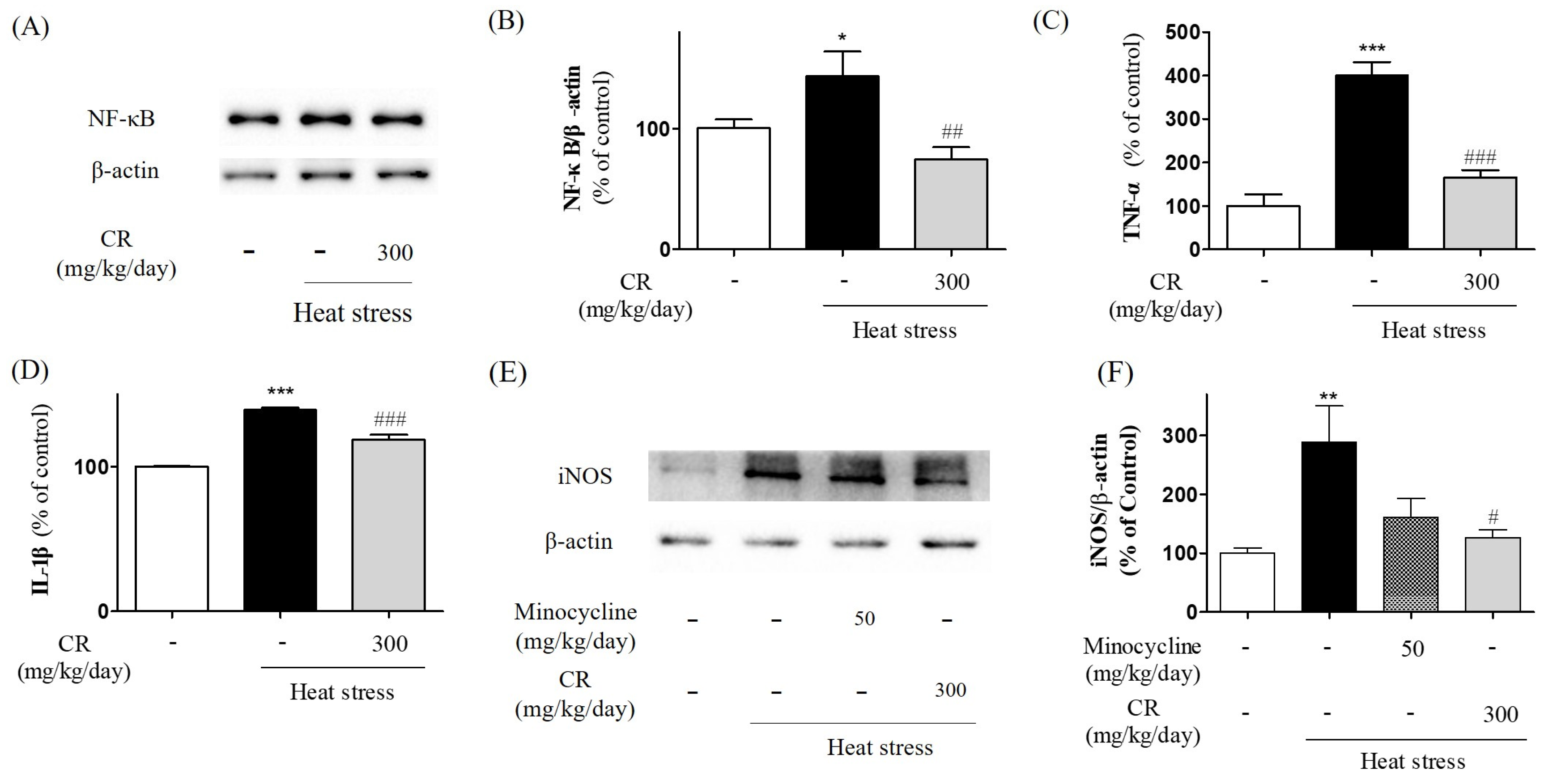
3.6. Inhibiting Effects of CR on Heat Stress-Induced NF-κB Activation and Production of Inflammatory Mediators
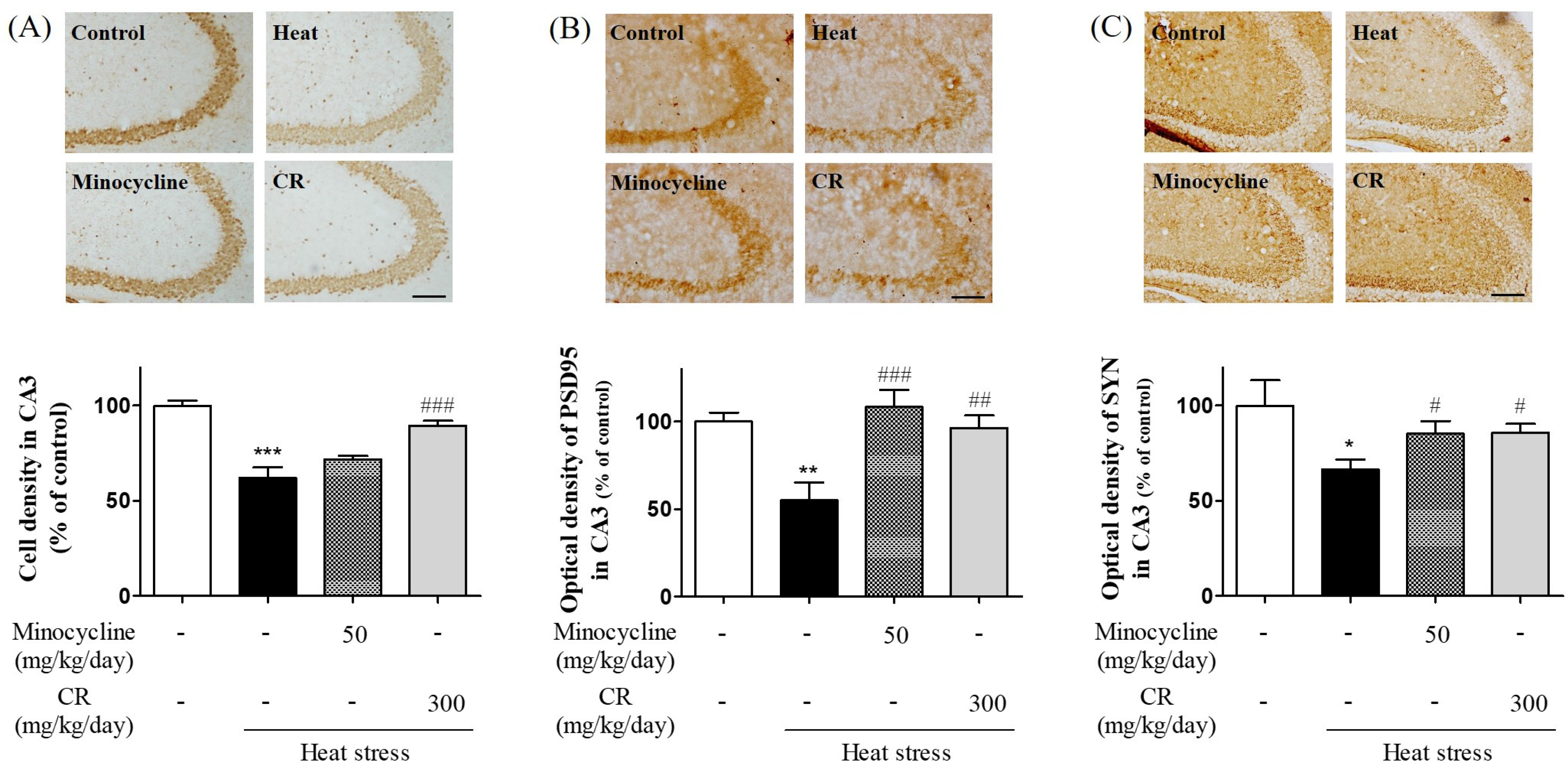
3.7. Atternuating Effects of CR on Heat Stress-Induced Neurodegeneration in Hippocampus
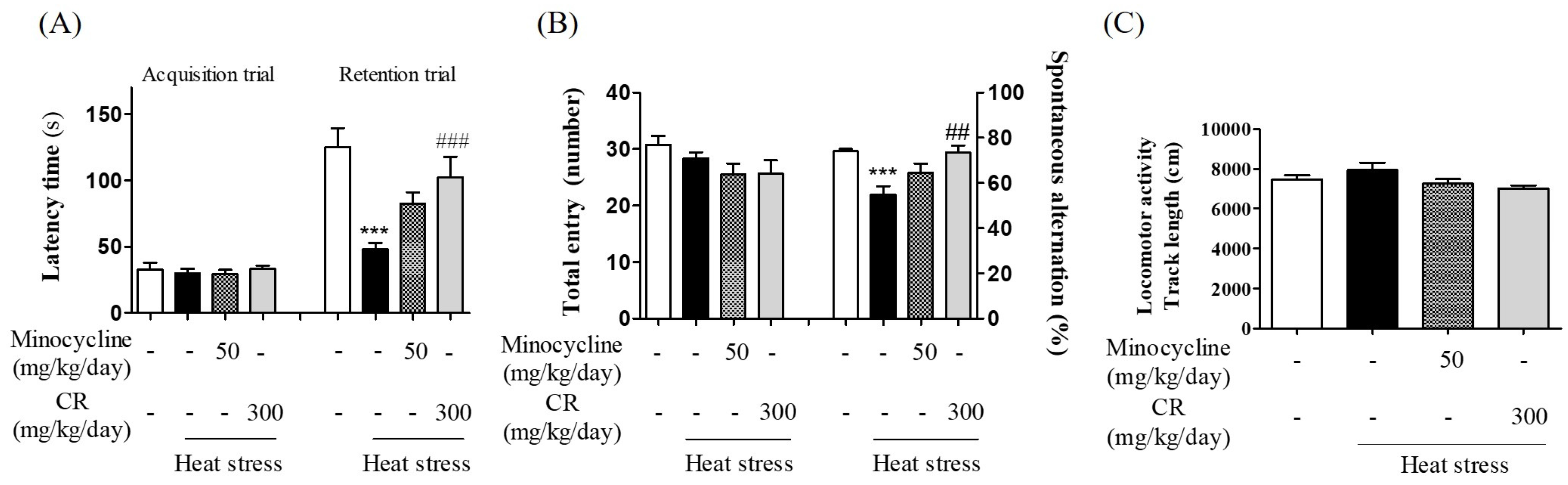
3.8. Inhibitory Effects of CR against Hyperthermia-Mediated Memory Impairment
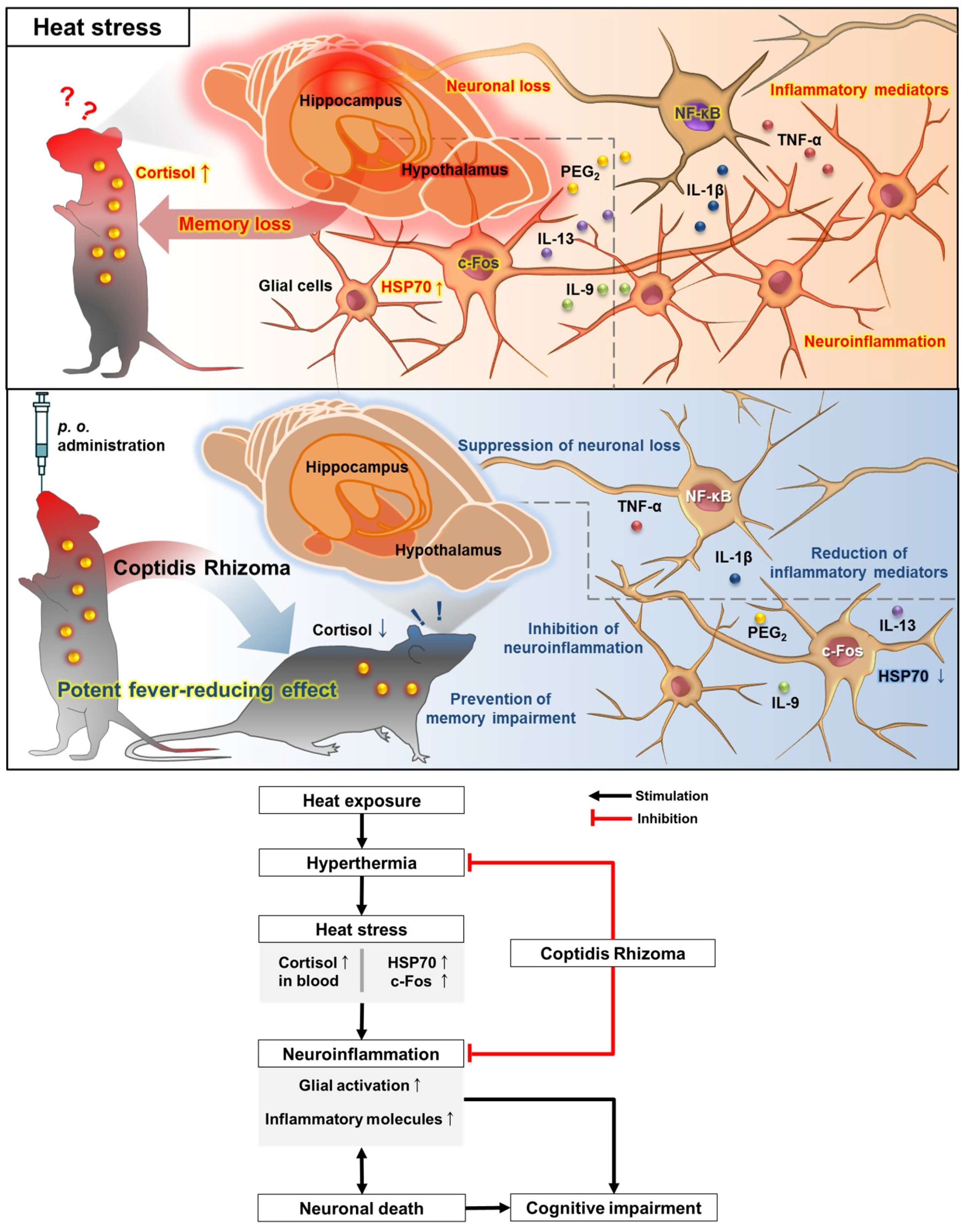
4. Discussion
Acknowledgments
Author Contributions
Conflict of Interest
Abbreviations
| CR | Coptidis Rhizoma |
| IL | Interleukin |
| HPA | Hypothalamic-pituitary-adrenal |
| HSP70 | Heat shock protein 70 |
| NF-κB | nuclear factor kappa β |
| TNF-α | tumor necrosis factor alpha |
| PGE2 | prostaglandin E2 |
| iNOS | inducible nitric oxide synthase |
| COX-2 | cyclooxygenase 2 |
| GR | glucocorticoid receptor |
| ELISA | Enzyme-linked immunosorbent assay |
| GFAP | Glial fibrillary acidic protein |
References
- Hoffman-Goetz, L.; Keir, R. Body temperature responses of aged mice to ambient temperature and humidity stress. J. Gerontol. 1984, 39, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.S.; Horowitz, M.; Meiri, U.; Laor, A.; Pandolf, K.B. The physiological strain index applied to heat-stressed rats. J. Appl. Physiol. 1999, 86, 895–901. [Google Scholar] [PubMed]
- Quinteiro-Filho, W.M.; Rodrigues, M.V.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sa, L.R.; Ferreira, A.J.; Palermo-Neto, J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: Role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012, 90, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Andreasson, K.I.; Kaufmann, W.E.; Barnes, C.A.; Worley, P.F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: Regulation by synaptic activity and glucocorticoids. Neuron 1993, 11, 371–386. [Google Scholar] [CrossRef]
- Lee, W.; Moon, M.; Kim, H.G.; Lee, T.H.; Oh, M.S. Heat stress-induced memory impairment is associated with neuroinflammation in mice. J. Neuroinflamm. 2015, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K. An approach to estimate EEG power spectrum as an index of heat stress using backpropagation artificial neural network. Med. Eng. Phys. 2007, 29, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Gaoua, N. Cognitive function in hot environments: A question of methodology. Scand. J. Med. Sci. Sports 2010, 20, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hancock, P.A.; Vasmatzidis, I. Effects of heat stress on cognitive performance: The current state of knowledge. Int. J. Hyperth. 2003, 19, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khan, R.; Khan, H.; Ullah, B.; Pervez, S. Antipyretic and antinociceptive potential of extract/fractions of potentilla evestita and its isolated compound, acacetin. BMC Complement. Altern. Med. 2014, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Saeed, M.; Khan, H. Antipyretic, analgesic and anti-inflammatory activity of viola betonicifolia whole plant. BMC Complement. Altern. Med. 2012, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.N.; Do, M.H.; Her, Y.R.; Lee, Y.R.; Kang, T.H. The effects of panax ginseng and panax quinquefolius on thermoregulation in animal models. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.O.; Lee, M.; Park, Y.; Kim, D.; Cho, K.H.; Kim, S.Y.; Lee, E.H. Effects of ginsenoside RB1 on the stress-induced changes of BDNF and HSP70 expression in rat hippocampus. Environ. Toxicol. Pharmacol. 2014, 38, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in kancheepuram district of tamil nadu, india. J. Ethnobiol. Ethnomed. 2006, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M. Traditional Chinese Pharmacology; Traditional Chinese Medicine Press: Beijing, China, 2007. [Google Scholar]
- Jiangsu New Medical College. Zhongyao Dacidian (Encyclopaedia of Chinese Traditional Medicine); The People’s Publishing Company: Zhenjiang, China, 1986; pp. 2307–2310. [Google Scholar]
- Khodavandi, A.; Nazira, A.B.T.; Poh, W.C.; Phelim, Y.V.C.; Alizadeh, F.; Harmal, N.S.; Chong, P.P. Antifungal activity of rhizome coptidis and alpinia galangal against candida species. J. Pure Appl. Microbiol. 2013, 7, 1725–1730. [Google Scholar]
- Huang, K.C. The Pharmacology of Chinese Herbs; CCRC Press Inc.: Birmingham, UK, 1999; pp. 381–382. [Google Scholar]
- Liu, S.; Lu, F.; Wang, X.; Sun, W.; Chen, P.; Dong, W. Metabolomic study of a rat fever model induced with 2,4-dinitrophenol and the therapeutic effects of a crude drug derived from coptis chinensis. Am. J. Chin. Med. 2011, 39, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.Y.; Kong, X.Y.; Li, X.M.; Zhu, H.W.; Su, X.H.; Lin, N. Effect of traditional chinese medicines with different properties on thermoregulation and temperature-sensitive transient receptor potentialion channel protein of rats with yeast-induced fever. Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2014, 39, 3813–3818. [Google Scholar]
- Jiang, J.F.; Wang, Y.G.; Hu, J.; Lei, F.; Kheir, M.M.; Wang, X.P.; Chai, Y.S.; Yuan, Z.Y.; Lu, X.; Xing, D.M.; et al. Novel effect of berberine on thermoregulation in mice model induced by hot and cold environmental stimulation. PLoS ONE 2013, 8, e54234. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wan, H.; Su, X.; Zhang, C.; Yang, Y.; Li, X.; Yao, L.; Lin, N. Rheum palmatum L. and coptis chinensis Franch., exert antipyretic effect on yeast-induced pyrexia rats involving regulation of TRPV1 and TRPM8 expression. J. Ethnopharmacol. 2014, 153, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, N.; Miyamoto, K.; Hazama, S.; Yoshino, S.; Yoshimura, K.; Okita, K.; Fukumoto, T.; Yamamoto, S.; Tangoku, A.; Oka, M. Anticachectic effects of Coptidis Rhizoma, an anti-inflammatory herb, on esophageal cancer cells that produce interleukin 6. Cancer Lett. 2000, 158, 35–41. [Google Scholar] [CrossRef]
- Hsiang, C.Y.; Wu, S.L.; Cheng, S.E.; Ho, T.Y. Acetaldehyde-induced interleukin-1beta and tumor necrosis factor-alpha production is inhibited by berberine through nuclear factor-kappab signaling pathway in HEPG2 cells. J. Biomed. Sci. 2005, 12, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, M.H.; Cho, I.H.; Kim, J.H.; Hong, J.; Lee, T.H.; Yang, W.M. Inhibitory effect of coptis chinensis on inflammation in lps-induced endotoxemia. J. Ethnopharmacol. 2013, 149, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Kim, H.G.; Choi, J.G.; Oh, H.; Lee, P.K.; Ha, S.K.; Kim, S.Y.; Park, Y.; Huh, Y.; Oh, M.S. 6-shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem. Biophys. Res. Commun. 2014, 449, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Moon, M.; Choi, J.G.; Park, G.; Kim, A.J.; Hur, J.; Lee, K.T.; Oh, M.S. Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo. Neurotoxicology 2014, 40, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Harikai, N.; Tomogane, K.; Miyamoto, M.; Shimada, K.; Onodera, S.; Tashiro, S. Dynamic responses to acute heat stress between 34 degrees C and 38.5 degrees C, and characteristics of heat stress response in mice. Biol. Pharm. Bull. 2003, 26, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C.; Tipton, C.M.; Seals, D.R. Thermal adjustments to nonexertional heat stress in mature and senescent fischer 344 rats. J. Appl. Physiol. 1990, 68, 1337–1342. [Google Scholar] [PubMed]
- Moran, D.; Shapiro, Y.; Meiri, U.; Laor, A.; Horowitz, M. Heat acclimation: Cardiovascular response to hot/dry and hot/wet heat loads in rats. J. Basic Clin. Physiol. Pharmacol. 1996, 7, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Li, J.J.; Wang, D.Q.; Li, G.Q.; Wang, G.L.; Lu, L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of muscovy and pekin ducks: Evidence for differential thermal sensitivities. Cell. Stress Chaperones 2014, 19, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Cwynar, P.; Kolacz, R.; Czerski, A. Effect of heat stress on physiological parameters and blood composition in polish merino rams. Berl. Munch. Tierarztl. Wochenschr. 2014, 127, 177–182. [Google Scholar] [PubMed]
- Wang, L.I.; Liu, F.; Luo, Y.; Zhu, L.; Li, G. Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats. Biomed. Rep. 2015, 3, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Manjari, R.; Yadav, M.; Ramesh, K.; Uniyal, S.; Rastogi, S.K.; Sejian, V.; Hyder, I. Hsp70 as a marker of heat and humidity stress in tarai buffalo. Trop. Anim. Health Prod. 2015, 47, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Romanovsky, A.A.; Scammell, T.E. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 2012, 15, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Elmquist, J.K.; Griffin, J.D.; Saper, C.B. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J. Neurosci. 1996, 16, 6246–6254. [Google Scholar]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor nf-kappab pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Salles, A.; Romano, A.; Freudenthal, R. Synaptic nf-kappa B pathway in neuronal plasticity and memory. J. Physiol. Paris 2014, 108, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Kellom, M.; Kim, H.W.; Rapoport, S.I.; Reese, E.A. Neuroinflammation and synaptic loss. Neurochem. Res. 2012, 37, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.; Cumiskey, D.; O’Connor, J.J. Actions of tnf-alpha on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 2005, 90, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. Thermal biology of the laboratory rat. Physiol. Behav. 1990, 47, 963–991. [Google Scholar] [CrossRef]
- Carter, C.S.; DeVries, A.C. Stress and soothing: An endocrine perspective. In Soothing and Stress; Psychology Press: Hove, UK, 1999; pp. 3–18. [Google Scholar]
- Akil, H.; Campeau, S.; Cullinan, W.E.; Lechan, R.M.; Toni, R.; Watson, S.J.; Moore, R.Y. Neuroendocrine systems I: Overview-thyroid and adrenal axes. In Fundamental Neuroscience; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1127–1150. [Google Scholar]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, U.; Hamalainen, E. Determination of cortisol in serum, saliva and urine. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; O’Halloran, D.J.; Shanahan, F. The stress response and the hypothalamic-pituitary-adrenal axis: From molecule to melancholia. QJM 2000, 93, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Busillo, J.M.; Cidlowski, J.A. The five RS of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Jung, H.J.; Kim, M.H.; Kang, C.; Jung, W.S.; Cho, K.H.; Lee, E.H. Chunghyuldan attenuates brain microglial inflammatory response. Can. J. Physiol. Pharmacol. 2009, 87, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S. Physiology and pathophysiology of prostanoid receptors. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Lin, Y.F.; Liu, T.T.; Wang, J.Y. Heme oxygenase-1 aggravates heat stress-induced neuronal injury and decreases autophagy in cerebellar purkinje cells of rats. Exp. Biol. Med. 2013, 238, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Belay, H.T.; Brown, I.R. Spatial analysis of cell death and hsp70 induction in brain, thymus, and bone marrow of the hyperthermic rat. Cell Stress Chaperones 2003, 8, 395–404. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Mendlovich, S.; Riwkes, S.; Braw, Y.; Levkovitch-Verbin, H.; Gal, G.; Fennig, S.; Treves, I.; Kron, S. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 2010, 71, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K.; Terwel, D.; Kummer, M.P. Neuroinflammatory processes in alzheimer’s disease. J. Neural Transm. 2010, 117, 919–947. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Franciosi, S.; Sattayaprasert, P.; Kim, S.U.; McLarnon, J.G. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia 2004, 48, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.P.; Che, C.T.; Liu, K.; Lin, Z.X. Evaluation of the anti-proliferative properties of selected psoriasis-treating chinese medicines on cultured hacat cells. J. Ethnopharmacol. 2006, 108, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Remppis, A.; Bea, F.; Greten, H.J.; Buttler, A.; Wang, H.; Zhou, Q.; Preusch, M.R.; Enk, R.; Ehehalt, R.; Katus, H.; et al. Rhizoma coptidis inhibits LPS-induced mcp-1/CCL2 production in murine macrophages via an AP-1 and nfkappab-dependent pathway. Mediat. Inflamm. 2010, 2010, 194896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Piao, X.L.; Piao, X.S.; Lu, T.; Wang, D.; Kim, S.W. Preventive effect of coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem. Toxicol. 2011, 49, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Chen, I.J.; Lo, Y.C. Effects of San-Huang-Xie-Xin-Tang on U46619-induced increase in pulmonary arterial blood pressure. J. Ethnopharmacol. 2008, 117, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Chen, P.Y.; Shih, T.C.; Ou, C.Y.; Jhuo, M.D.; Huang, Y.Y.; Cheng, C.H.; Wu, Y.C.; Chung, J.G. Computational pharmaceutical analysis of anti-alzheimer’s chinese medicine Coptidis Rhizoma alkaloids. Mol. Med. Rep. 2012, 5, 142–147. [Google Scholar] [PubMed]
- Pilcher, J.J.; Nadler, E.; Busch, C. Effects of hot and cold temperature exposure on performance: A meta-analytic review. Ergonomics 2002, 45, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Woo, J.J.; Jung, M.J.; Hwang, G.S.; Choi, J.S. Kaempferol glycosides with antioxidant activity from brassica juncea. Arch. Pharm. Res. 2009, 32, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, S.H.; Yang, W.M. Mechanisms of action of phytochemicals from medicinal herbs in the treatment of alzheimer’s disease. Planta Med. 2014, 80, 1249–1258. [Google Scholar] [PubMed]
- Kim, M.H.; Leem, Y.H. Chronic exercise improves repeated restraint stress-induced anxiety and depression through 5HT1A receptor and camp signaling in hippocampus. J. Exerc. Nutr. Biochem. 2014, 18, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, T.; Otto, B.; Klatschke, K.; Schumacher, U.; Tao, Y.; Leung, A.K.; Efferth, T.; Schroder, S. Coptis chinensis franch. Exhibits neuroprotective properties against oxidative stress in human neuroblastoma cells. J. Ethnopharmacol. 2014, 155, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.T.; Belani, L.K.; Das, S.; Jaafar, M.Z. Treatment of dementia with herbs: A short review. Clin. Ter. 2013, 164, 43–46. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, M.; Huh, E.; Lee, W.; Song, E.J.; Hwang, D.-S.; Lee, T.H.; Oh, M.S. Coptidis Rhizoma Prevents Heat Stress-Induced Brain Damage and Cognitive Impairment in Mice. Nutrients 2017, 9, 1057. https://doi.org/10.3390/nu9101057
Moon M, Huh E, Lee W, Song EJ, Hwang D-S, Lee TH, Oh MS. Coptidis Rhizoma Prevents Heat Stress-Induced Brain Damage and Cognitive Impairment in Mice. Nutrients. 2017; 9(10):1057. https://doi.org/10.3390/nu9101057
Chicago/Turabian StyleMoon, Minho, Eugene Huh, Wonil Lee, Eun Ji Song, Deok-Sang Hwang, Tae Hee Lee, and Myung Sook Oh. 2017. "Coptidis Rhizoma Prevents Heat Stress-Induced Brain Damage and Cognitive Impairment in Mice" Nutrients 9, no. 10: 1057. https://doi.org/10.3390/nu9101057
APA StyleMoon, M., Huh, E., Lee, W., Song, E. J., Hwang, D.-S., Lee, T. H., & Oh, M. S. (2017). Coptidis Rhizoma Prevents Heat Stress-Induced Brain Damage and Cognitive Impairment in Mice. Nutrients, 9(10), 1057. https://doi.org/10.3390/nu9101057





