Intestinal Production of Anti-Tissue Transglutaminase 2 Antibodies in Patients with Diagnosis Other Than Celiac Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Detection of Mucosal Deposits of anti-TG2 IgA Antibodies
2.3. Biopsy Specimens and Organ Culture
2.4. Measurement of Anti-TG2 IgA Antibodies Secreted into Culture Supernatants
2.5. Phage Display Library
2.6. Immunohistochemistry
2.7. Statistics
2.8. Ethical Approval
3. Results
3.1. Intestinal Anti-TG2 Antibodies Are Also Produced in Non-CD Patients
3.2. Phage Display Technology Confirms the Intestinal Production of Anti-TG2 Antibodies in Non-CD Patients
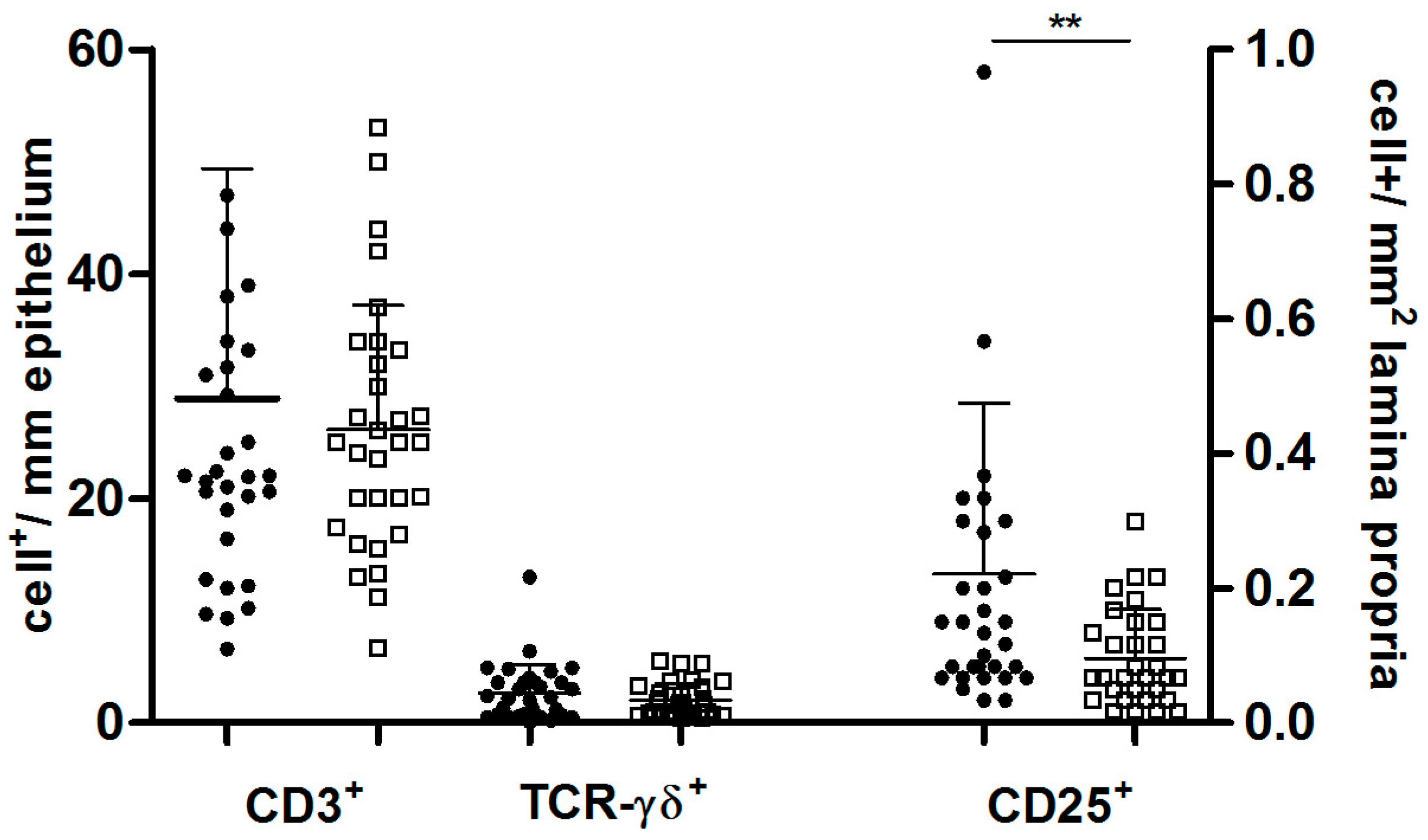
3.3. Positive Non-CD Patients Presented Signs of Activated Cell-Mediated Mucosal Immunity
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. ESPGHAN Working Group on Coeliac Disease Diagnosis, on behalf of the ESPGHAN Gastroenterology Committee, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S. ESPGHAN Working Group on Coeliac Disease Diagnosis. Accuracy of diagnostic antibody tests for coeliac disease in children: Summary of an evidence report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Marzari, R.; Sblattero, D.; Florian, F.; Tongiorgi, E.; Not, T.; Tommasini, A.; Ventura, A.; Bradbury, A. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J. Immunol. 2001, 166, 4170–4176. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabo, I.R.; Halttunen, T.; Szalai, Z.; Laurila, K.; Király, R.; Kovács, J.B.; Fésüs, L.; Mäki, M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004, 53, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Salmi, T.T.; Collin, P.; Korponay-Szabó, I.R.; Laurila, K.; Partanen, J.; Huhtala, H.; Király, R.; Lorand, L.; Reunala, T.; Mäki, M.; et al. Endomysial antibody-negative coeliac disease: Clinical characteristics and intestinal autoantibody deposits. Gut 2006, 55, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Peräaho, M.; Collin, P.; Partanen, J.; Woolley, N.; Kaartinen, T.; Nuutinen, T.; Halttunen, T.; Mäki, M.; Korponay-Szabo, I. Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: A prospective and randomized clinical study. Scand. J. Gastroenterol. 2005, 40, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Salmi, T.T.; Collin, P.; Jarvinen, O.; Haimila, K.; Partanen, J.; Laurila, K.; Korponay-Szabo, I.R.; Huhtala, H.; Reunala, T.; Mäki, M.; et al. Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming celiac disease. Aliment. Pharmacol. Ther. 2006, 24, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Tosco, A.; Salvati, M.V.; Auricchio, R.; Maglio, M.; Borrelli, M.; Coruzzo, A.; Paparo, F.; Boffardi, M.; Esposito, A.; D’Adamo, G.; et al. Natural history of potential celiac disease in children. Clin. Gastroenterol. Hepatol. 2011, 9, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Tosco, A.; Aitoro, R.; Auricchio, A.; Ponticelli, D.; Miele, E.; Paparo, F.; Greco, L.; Troncone, R.; Maglio, M. Intestinal anti-tissue transglutaminase antibodies in potential coeliac disease. Clin. Exp. Immunol. 2013, 171, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2012, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, O.; Collin, P.; Korponay-Szabo, I.; Salmi, T.; Iltanen, S.; Haimila, K.; Partanen, J.; Mäki, M.; Kaukinen, K. Gluten-dependent small bowel mucosal transglutaminase 2-specific IgA deposits in overt and mild enteropathy coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, O.; Collin, P.; Lindfors, K.; Laurila, K.; Mäki, M.; Kaukinen, K. Usefulness of small-bowel mucosal transglutaminase-2 specific autoantibody deposits in the diagnosis and follow-up of celiac disease. J. Clin. Gastroenterol. 2010, 44, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Tosco, A.; Auricchio, R.; Paparo, F.; Colicchio, B.; Miele, E.; Rapacciuolo, L.; Troncone, R. Intestinal deposits of anti-tissue transglutaminase IgA in childhood celiac disease. Dig. Liver Dis. 2011, 43, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Walker-Smith, J.A.; Guandalini, S.; Schmitz, J.; Shmerling, D.H.; Visacorpi, J.K. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Revised criteria for the diagnosis of celiac disease. Arch. Dis. Child. 1990, 65, 909–911. [Google Scholar]
- Tosco, A.; Maglio, M.; Paparo, F.; Rapacciuolo, L.; Sannino, A.; Miele, E.; Barone, M.V.; Auricchio, R.; Troncone, R. Immunoglobulin A anti-tissuetransglutaminase antibody deposits in the small intestinal mucosa of children with no villous atrophy. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kurppa, K.; Ashorn, M.; Iltanen, S.; Koskinen, L.L.; Saavalainen, P.; Koskinen, O.; Mäki, M.; Kaukinen, K. Celiac disease without villous atrophy in children: A prospective study. J. Pediatr. 2010, 157, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Sblattero, D.; Bradbury, A. Exploiting recombination in single bacteria to make large phage antibody libraries. Nat. Biotechnol. 2000, 18, 75–80. [Google Scholar] [PubMed]
- Available online: http://www.imgt.org/IMGT_vquest/share/textes/ (accessed on 1989).
- Paparo, F.; Petrone, E.; Tosco, A.; Maglio, M.; Borrelli, M.; Salvati, V.M.; Miele, E.; Greco, L.; Auricchio, S.; Troncone, R. Clinical, HLA and small bowel immunohistochemical features of children with positive serum antiendomysium antibodies and architecturally normal small intestinal mucosa. Am. J. Gastroenterol. 2005, 100, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Tinto, N.; Cola, A.; Piscopo, C.; Capuano, M.; Galatola, M.; Greco, L.; Sacchetti, L. High Frequency of Haplotype HLA-DQ7 in Celiac Disease Patients from South Italy: Retrospective Evaluation of 5535 Subjects at Risk of Celiac Disease. PLoS ONE 2015, 10, e0138324. [Google Scholar] [CrossRef] [PubMed]
- Setty, M.; Discepolo, V.; Abadie, V.; Kamhawi, S.; Mayassi, T.; Kent, A.; Ciszewski, C.; Maglio, M.; Kistner, E.; Bhagat, G.; et al. Distinct and Synergistic Contributions of Epithelial Stress and Adaptive Immunity to Functions of Intraepithelial Killer Cells and Active Celiac Disease. Gastroenterology 2015, 149, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Troncone, R.; Jabri, B. Coeliac disease and gluten sensitivity. J. Intern. Med. 2011, 269, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Not, T.; Ziberna, F.; Vatta, S.; Quaglia, S.; Martelossi, S.; Villanacci, V.; Marzari, R.; Florian, F.; Vecchiet, M.; Sulic, A.M.; et al. Cryptic genetic gluten intolerance revealed by intestinal antitransglutaminase antibodies and response to gluten-free diet. Gut 2011, 60, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Di Niro, R.; Mesin, L.; Zheng, N.Y.; Stamnaes, J.; Morrissey, M.; Lee, J.H.; Huang, M.; Iversen, R.; Du Pré, M.F.; Qiao, S.W.; et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat. Med. 2012, 18, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Florian, F.; Vecchiet, M.; Auricchio, R.; Paparo, F.; Spadaro, R.; Zanzi, D.; Rapacciuolo, L.; Franzese, A.; Sblattero, D.; et al. Majority of children with type 1 diabetes produce and deposit anti-tissue transglutaminase antibodies in the small intestine. Diabetes 2009, 58, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, S.; De Leo, L.; Ziberna, F.; Vatta, S.; Villanacci, V.; Granzotto, M.; Petix, V.; Martelossi, S.; Di Leo, G.; Torelli, L.; et al. Intestinal-mucosa anti-transglutaminase antibody assays to test for genetic gluten intolerance. Cell. Mol. Immunol. 2014, 11, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Holm, K.; Maki, M.; Savilahti, E.; Auricchio, R.; Paparo, F.; Spadaro, R. Intraepithelial gamma delta T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet 1992, 339, 1500–1503. [Google Scholar] [CrossRef]
- Tortora, R.; Capone, P.; Imperatore, N.; De Stefano, G.; Gerbino, N.; Leo, M.; Caporaso, N.; Rispo, A. Predictive value of “Marsh 1” type histology in subjects with suspected cealic disease. Scand. J. Gastroenterol. 2014, 49, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Jinga, M.; Balaban, D.V.; Peride, I.; Niculae, A.; DuŢescu, I.M.; Vasilescu, F.; Mäki, M.; Popp, A.M. Crypt hyperplastic enteropathy in distal duodenum in Helicobacter pylori infection—Report of two cases without evidence of celiac disease. Rom. J. Morphol. Embryol. 2017, 58, 685–688. [Google Scholar] [PubMed]
- Auricchio, R.; Paparo, F.; Maglio, M.; Franzese, A.; Lombardi, F.; Valerio, G.; Nardone, G.; Percopo, S.; Greco, L.; Troncone, R. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes 2004, 53, 1680–1683. [Google Scholar] [CrossRef] [PubMed]

| Patient | Deposits Anti-TG2 | Anti-TG2 into Supernatants | Anti-TG2 VH | HLA |
|---|---|---|---|---|
| n = 1 Non-CD | Neg | + | VH5 | ND |
| n = 2 Non-CD | Neg | + | VH5 | ND |
| n = 3 Non-CD | + | Neg | VH5 | ND |
| n = 4 Non-CD | + | Neg | VH5 | DQ2/DQ8 negative (DQ7DR5) |
| n = 5 Non-CD | + | Neg | VH5 | ND |
| n = 6 Non-CD | Neg | Neg | No | DQ2 positive |
| n = 7 Non-CD | + | Neg | VH5, VH3 | DQ2/DQ8 positive |
| n = 8 Non-CD | + | Neg | VH5, VH3 | DQ2/DQ8 positive |
| n = 9 Non-CD | Neg | Neg | No | DQ2 positive |
| n = 10 Non-CD | Neg | Neg | No | DQ2 positive |
| Marker | Positive No-CD Patients | Negative No-CD Patients | Χ2 Fisher’s Exact Test |
|---|---|---|---|
| CD25+ cells > cutoff | 24/32 (75.0%) | 14/31 (54.1%) | p = 0.02 |
| CD3+ cells > cutoff | 7/32 (21.8%) | 5/31 (16.0%) | p = 0.5 |
| TCR-γδ+ cells > cutoff | 11/32 (34.4%) | 6/31 (19.3%) | p = 0.2 |
| TCR-γδ+/CD3+ ratio > cutoff | 13/32 (40.6%) | 9/31 (29.0%) | p = 0.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maglio, M.; Ziberna, F.; Aitoro, R.; Discepolo, V.; Lania, G.; Bassi, V.; Miele, E.; Not, T.; Troncone, R.; Auricchio, R. Intestinal Production of Anti-Tissue Transglutaminase 2 Antibodies in Patients with Diagnosis Other Than Celiac Disease. Nutrients 2017, 9, 1050. https://doi.org/10.3390/nu9101050
Maglio M, Ziberna F, Aitoro R, Discepolo V, Lania G, Bassi V, Miele E, Not T, Troncone R, Auricchio R. Intestinal Production of Anti-Tissue Transglutaminase 2 Antibodies in Patients with Diagnosis Other Than Celiac Disease. Nutrients. 2017; 9(10):1050. https://doi.org/10.3390/nu9101050
Chicago/Turabian StyleMaglio, Mariantonia, Fabiana Ziberna, Rosita Aitoro, Valentina Discepolo, Giuliana Lania, Virginia Bassi, Erasmo Miele, Tarcisio Not, Riccardo Troncone, and Renata Auricchio. 2017. "Intestinal Production of Anti-Tissue Transglutaminase 2 Antibodies in Patients with Diagnosis Other Than Celiac Disease" Nutrients 9, no. 10: 1050. https://doi.org/10.3390/nu9101050
APA StyleMaglio, M., Ziberna, F., Aitoro, R., Discepolo, V., Lania, G., Bassi, V., Miele, E., Not, T., Troncone, R., & Auricchio, R. (2017). Intestinal Production of Anti-Tissue Transglutaminase 2 Antibodies in Patients with Diagnosis Other Than Celiac Disease. Nutrients, 9(10), 1050. https://doi.org/10.3390/nu9101050




