Hepcidin is a Better Predictor of Iron Stores in Premenopausal Women than Blood Loss or Dietary Intake
Abstract
:1. Introduction
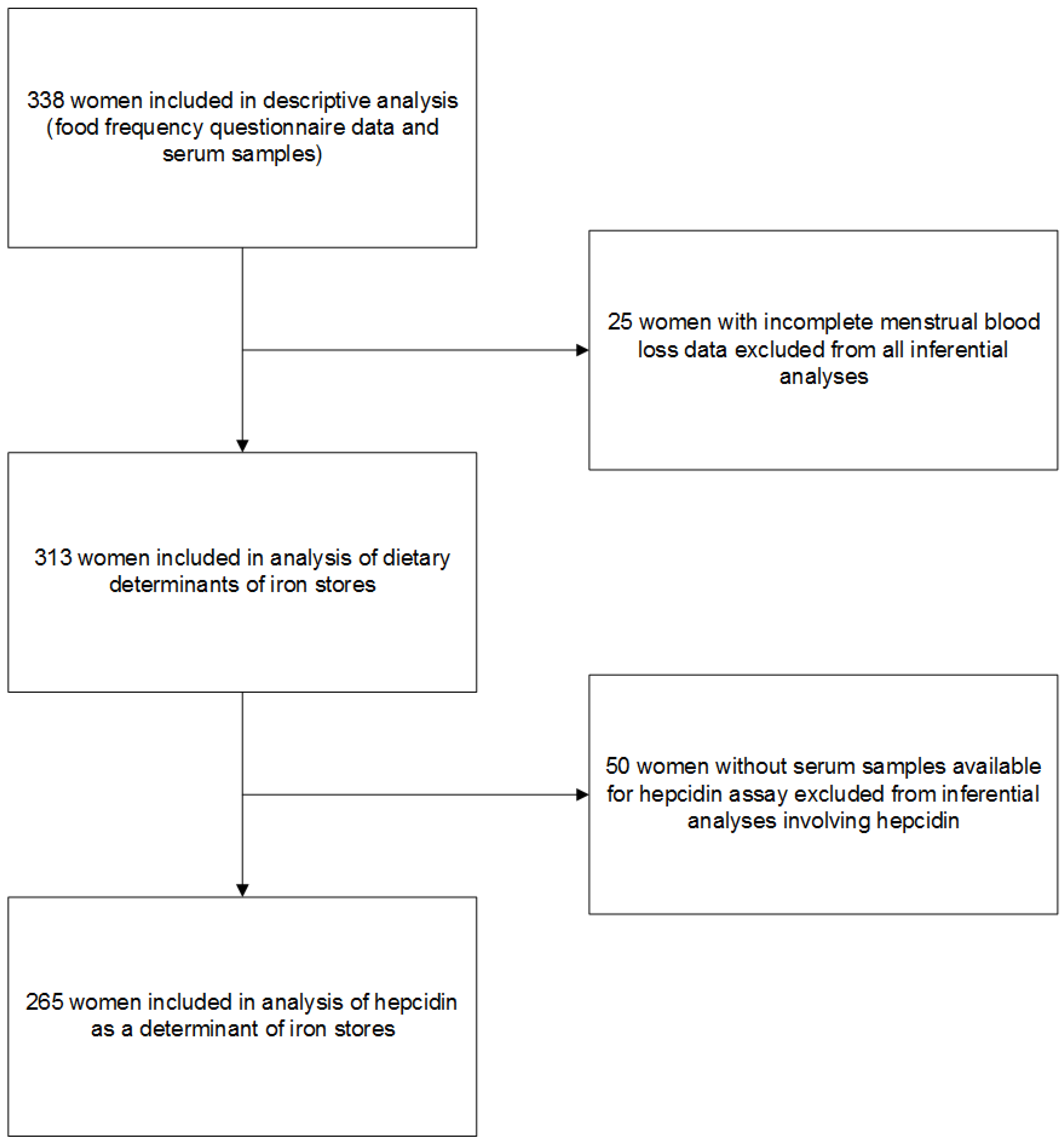
2. Materials and Methods
2.1. Study Design and Participants
2.2. Assessment of Usual Dietary Intake
2.3. Assessment of Supplemental Iron Intake
2.4. Assessment of Blood Loss
2.5. Biochemical Analyses
2.6. Assessment of Covariates
2.7. Statistical Analysis
3. Results
3.1. Predictors of Iron Stores
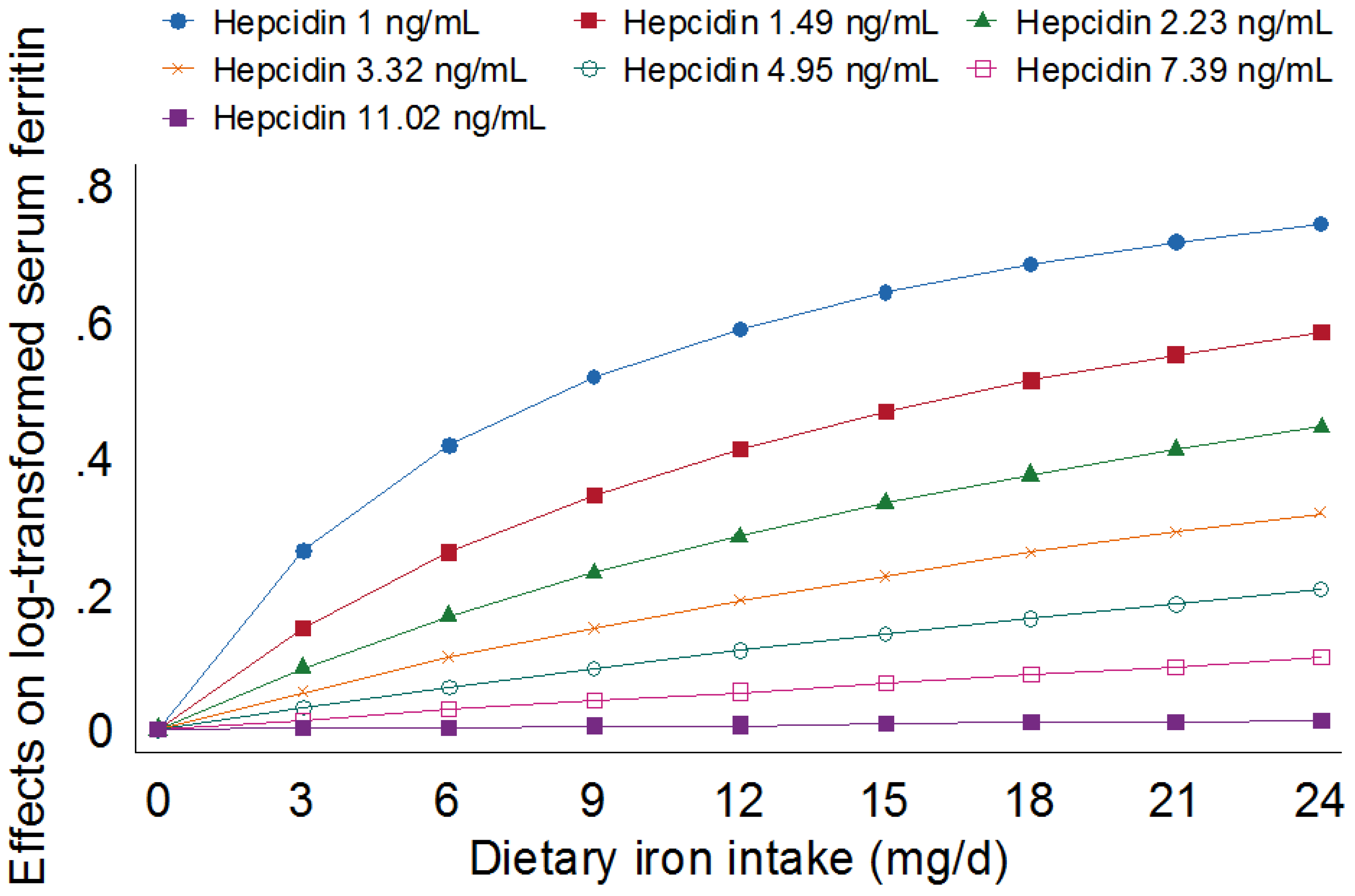
3.2. Association between Iron Intake and Iron Stores Independent of Hepcidin
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Turning the Tide of Malnutrition: Responding to the Challenge of the 21st Century. 2000. Available online: http://www.who.int/mip2001/files/2232/NHDbrochure.pdf (accessed on 24 August 2012).
- World Health Organization. Serum Ferritin Concentrations of the Assessment of Iron Status and Iron Deficiency in Populations. 2011. Available online: http://www.who.int/vmnis/indicators/ferritin/en/ (accessed on 22 September 2014).
- Pasricha, S.R.; Low, M.; Thompson, J.; Farrell, A.; De-Regil, L.M. Iron supplementation benefits physical performance in women of reproductive age: A systematic review and meta-analysis. J. Nutr. 2014, 144, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolb, L.E.; Beard, J.L. Iron treatment normalizes cognitive functioning in young women. Am. J. Clin Nutr. 2007, 85, 778–787. [Google Scholar] [PubMed]
- Leonard, A.J.; Chalmers, K.A.; Collins, C.E.; Patterson, A.J. A study of the effects of latent iron deficiency on measures of cognition: A pilot randomised controlled trial of iron supplementation in young women. Nutrients 2014, 6, 2419–2435. [Google Scholar] [CrossRef] [PubMed]
- Steinbicker, A.U.; Muckenthaler, M.U. Out of balance—Systemic iron homeostasis in iron-related disorders. Nutrients 2013, 5, 3034–3061. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L. Bioavailability of dietary iron in man. Ann. Rev. Nutr. 1981, 1, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Collings, R.; Harvey, L.J.; Hooper, L.; Hurst, R.; Brown, T.J.; Ansett, J.; King, M.; Fairweather-Tait, S.J. The absorption of iron from whole diets: A systematic review. Am. J. Clin. Nutr. 2013, 98, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Brune, M.; Rossander, L. Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [PubMed]
- Hallberg, L.; Rossander, L.; Skanberg, A. Phytates and the inhibitory effect of bran on iron absorption in man. Am. J. Clin. Nutr. 1987, 45, 988–996. [Google Scholar] [PubMed]
- Hallberg, L.; Brune, M.; Erlandsson, M.; Sandberg, A.; Rossander-Hulten, L. Calcium: Effect of different amounts on nonheme- and heme-iron absorption in humans. Am. J. Clin. Nutr. 1991, 53, 112–119. [Google Scholar] [PubMed]
- Disler, P.B.; Lynch, S.R.; Charlton, R.W.; Torrance, J.D.; Bothwell, T.H.; Walker, R.B.; Mayet, F. The effect of tea on iron absorption. Gut 1975, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Morck, T.A.; Lynch, S.R.; Cook, J.D. Inhibition of food iron absorption by coffee. Am. J. Clin. Nutr. 1983, 37, 416–420. [Google Scholar] [PubMed]
- Casgrain, A.; Collings, R.; Harvey, L.J.; Hooper, L.; Fairweather-Tait, S.J. Effect of iron intake on iron status: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Roe, M.A.; Collings, R.; Dainty, J.R.; Swinkels, D.W.; Fairweather-Tait, S.J. Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am. J. Clin. Nutr. 2009, 89, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Glahn, R.P.; Ariza-Nieto, M.; Inglis, J.; Olbina, G.; Westerman, M.; O’Brien, K.O. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am. J. Clin. Nutr. 2009, 89, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Troesch, B.; Biebinger, R.; Egli, I.; Zeder, C.; Hurrell, R.F. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am. J. Clin. Nutr. 2009, 90, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, D.; De Smet, S.; Ulens, M. The near equivalence of haem and non-haem iron bioavailability and the need for reconsidering dietary iron recommendations. Eur. J. Clin. Nutr. 2014, 68, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B. Blood donor iron status: Are we bleeding them dry? Curr. Opin. Hematol. 2013, 20, 533–539. [Google Scholar] [CrossRef]
- Hallberg, L.; Rossander-Hulten, L. Iron requirements in menstruating women. Am. J. Clin. Nutr. 1991, 54, 1047–1058. [Google Scholar] [PubMed]
- Mast, A.E.; Schlumpf, K.S.; Wright, D.J.; Johnson, B.; Glynn, S.A.; Busch, M.P.; Olbina, G.; Westerman, M.; Nemeth, E.; Ganz, T. Hepcidin level predicts hemoglobin concentration in individuals undergoing repeated phlebotomy. Haematologica 2013, 98, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and disorders of iron metabolism. Ann. Rev. Med. 2011, 62, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Tussing-Humphreys, L.M.; Nemeth, E.; Fantuzzi, G.; Freels, S.; Guzman, G.; Holterman, A.X.; Braunschweig, C. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity 2010, 18, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Tussing-Humphreys, L.; Pusatcioglu, C.; Nemeth, E.; Braunschweig, C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: Introducing hepcidin. J. Acad. Nutr. Diet. 2012, 112, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pynaert, I.; De Bacquer, D.; Matthys, C.; Delanghe, J.; Temmerman, M.; De Backer, G.; De Henauw, S. Determinants of ferritin and soluble transferrin receptors as iron status parameters in young adult women. Public Health Nutr. 2009, 12, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Rigas, A.S.; Sorensen, C.J.; Pedersen, O.B.; Petersen, M.S.; Thorner, L.W.; Kotze, S.; Sorensen, E.; Magnussen, K.; Rostgaard, K.; Erikstrup, C.; et al. Predictors of iron levels in 14,737 Danish blood donors: Results from the Danish Blood Donor Study. Transfusion 2014, 54, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Booth, A.; Szymlek-Gay, E.A.; Gibson, R.S.; Bailey, K.B.; Irving, D.; Nowson, C.; Riddell, L. Associations between dietary iron and zinc intakes, and between biochemical iron and zinc status in women. Nutrients 2015, 7, 2983–2999. [Google Scholar] [CrossRef] [PubMed]
- Cancer Council Victoria. Dietary Questionnaire for Epidemiological Studies Version 2 (DQES v2). 2014. Available online: http://www.cancervic.org.au/downloads/cec/FFQs/Jan_2015_Update/DQES_guide_22jan15.pdf (accessed on 13 March 2015).
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia New Zealand. Nutrient Tables for Use in Australia (NUTTAB 2010). Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/nutrientables/pages/default.aspx (accessed on 6 February 2015).
- Food Standards Australia New Zealand. Australian Food, Supplement and Nutrient Database (AUSNUT 2007). Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/Pages/ausnut2007.aspx (accessed on 6 February 2015).
- Hartley, N.K. Phytate and Zinc Intakes of New Zealand Toddlers Aged 12–24 Months. PhD. Thesis, University of Otago, Dunedin, New Zealand, 2014. [Google Scholar]
- Food Standards Australia New Zealand. AUSNUT 2011-13 Food and Dietary Supplement Classification System. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/ausnutdatafiles/Pages/foodclassification.aspx (accessed on 19 February 2015).
- Australian Bureau of Statistics. Australian Health Survey: Users’ Guide, 2011-13. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4363.0.55.001Chapter6502011-13 (accessed on 19 May 2015).
- Heath, A.L.; Skeaff, C.M.; Gibson, R.S. Validation of a questionnaire method for estimating extent of menstrual blood loss in young adult women. J. Trace Elem. Med. Biol. 1998, 12, 231–235. [Google Scholar] [CrossRef]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition. Available online: http://whqlibdoc.who.int/publications/2004/9241546123.pdf (accessed on 24 August 2012).
- Vittinghoff, E.; Glidden, D.; Shiboski, S.; McCulloch, C. Regression Methods in Biostatistics, 2nd ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Ganz, T.; Olbina, G.; Girelli, D.; Nemeth, E.; Westerman, M. Immunoassay for human serum hepcidin. Blood 2008, 112, 4292–4297. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Kroot, J.J.; van Herwaarden, A.E.; Tjalsma, H.; Jansen, R.T.; Hendriks, J.C.; Swinkels, D.W. Second round robin for plasma hepcidin methods: First steps toward harmonization. Am. J. Hematol. 2012, 87, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Wu, A.; An, P.; Du, X.; Wu, Q.; Shen, X.; Wang, F. Black soyabean seed coat extract regulates iron metabolism by inhibiting the expression of hepcidin. Br. J. Nutr. 2014, 111, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Conlon, C.A.; Kruger, R.; Coad, J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: A review. Nutrients 2014, 6, 3747–3776. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Yoon, H.C.; Preziosi, P.; Viteri, F.; Valeix, P.; Fieux, B.; Briancon, S.; Malvy, D.; Roussel, A.M.; Favier, A.; et al. Determining factors in the iron status of adult women in the SU.VI.MAX study. Supplementation en Vitamines et Mineraux AntioXydants. Eur. J. Clin. Nutr. 1998, 52, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.L.; Skeaff, C.M.; Williams, S.; Gibson, R.S. The role of blood loss and diet in the aetiology of mild iron deficiency in premenopausal adult New Zealand women. Public Health Nutr. 2001, 4, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.J.; Chalmers, K.A.; Collins, C.E.; Patterson, A.J. The effect of nutrition knowledge and dietary iron intake on iron status in young women. Appetite 2014, 81, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, M olybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Beck, K.L.; Kruger, R.; Conlon, C.A.; Heath, A.L.; Matthys, C.; Coad, J.; Stonehouse, W. Suboptimal iron status and associated dietary patterns and practices in premenopausal women living in Auckland, New Zealand. Eur. J. Nutr. 2013, 52, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Profiles of Health, Australia, 2011-13—Overweight and Obesity. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4338.0~2011-13~Main%20Features~Overweight%20and%20obesity~10007 (accessed on 22 August 2016).
- Australian Bureau of Statistics. Australian Health Survey: First Results, 2011-12. Available online: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/4364.0.55.0012011-12?OpenDocument (accessed on 26 June 2015).
- Australian Bureau of Statistics. Education and Work, Australia, May 2014. Available online: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/6227.0May%202014?OpenDocument (accessed on 26 June 2015).
- Simetic, L.; Zibar, L. Laboratory use of hepcidin in renal transplant recipients. Biochem. Med. 2016, 26, 34–52. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 338) | |
|---|---|
| Demography and anthropometry | |
| Age (years) | 29.1 (28.3, 29.9) |
| Body mass index (kg/m2) | 24.0 (23.6, 24.4) |
| Underweight b | 11 (3%) |
| Normal weight b | 224 (67%) |
| Overweight b | 69 (21%) |
| Obese b | 29 (9%) |
| Tertiary education | 274 (81%) |
| Currently employed | 297 (89%) |
| Current smoker | 26 (7%) |
| Has children | 55 (16%) |
| Diet and supplements | |
| Dietary iron intake (mg/day) | 10.4 (10.0, 10.8) |
| Taking supplements containing iron | 71 (21%) |
| Supplemental iron intake among users (mg/day, n = 71) c | 7.2 (5.3, 9.8) |
| Total iron intake (diet + supplements) c | 11.4 (10.7, 12.0) |
| Dietary ascorbic acid intake (mg/day) | 121 (115, 127) |
| Phytate intake (mg/day) | 691 (653, 733) |
| Energy intake (MJ/day) | 7.7 (7.4, 7.9) |
| Blood loss | |
| Donated blood in previous 12 months | 159 (47%) |
| Amount donated (mL/year, n = 159) | 844 (769, 919) |
| Using oral contraception | 125 (37%) |
| Menstrual blood loss units per menses c | 30 (28, 33) |
| Menstrual blood loss units per year c | 82 (76, 89) |
| Experiences nosebleeds | 36 (11%) |
| Biochemistry | |
| Serum ferritin (µg/L) a,d | 19.1 (17.3, 21.0) |
| Venous haemoglobin (g/L, n = 147) | 135 (133, 136) |
| Capillary haemoglobin (g/L, n = 191) | 130 (129, 132) |
| Hepcidin (ng/mL) c | 5.84 (5.01, 6.79) |
| Food Group | Contribution to Dietary Iron Intake (% (95% CI)) |
|---|---|
| Cereals and cereal products | 30.1 (28.8, 31.5) |
| Breakfast cereals, ready to eat | 11.9 (10.5, 13.3) |
| Regular breads and bread rolls (plain/unfilled/untopped varieties) | 10.8 (10.1, 11.6) |
| Flours and other cereal grains and starches | 3.7 (3.2, 4.1) |
| Breakfast cereals, hot porridge style | 2.1(1.7, 2.5) |
| Pasta and pasta products (without sauce) | 1.6 (1.5, 1.7) |
| Cereal based products and dishes | 6.1 (5.7, 6.5) |
| Savoury biscuits | 2.0 (1.7, 2.2) |
| Cakes, muffins, scones, cake-type desserts | 1.4 (1.3, 1.6) |
| Pastries | 1.1 (1.0, 1.3) |
| Vegetable products and dishes | 16.1 (15.2, 16.9) |
| Leaf and stalk vegetables | 6.2 (5.7, 6.7) |
| Other fruiting vegetables | 2.5 (2.4, 2.7) |
| Cabbage, cauliflower and similar brassica vegetables | 2.3 (2.1, 2.6) |
| Peas and beans | 1.8 (1.6, 2.0) |
| Tomato and tomato products | 1.0 (1.0, 1.1) |
| Carrot and similar root vegetables | 1.0 (0.9, 1.1) |
| Meat, poultry and game products and dishes | 13.9 (12.8, 15) |
| Beef, sheep and pork, unprocessed | 8.7 (7.9, 9.5) |
| Poultry and feathered game | 2.7 (2.4, 2.9) |
| Processed meat | 1.9 (1.6, 2.2) |
| Fruit products and dishes | 8.8 (8.3, 9.3) |
| Tropical and subtropical fruit | 1.9 (1.7, 2.0) |
| Berry fruit | 1.9 (1.7, 2.0) |
| Other fruit | 1.8 (1.7, 2.0) |
| Stone fruit | 1.2 (1.0, 1.3) |
| Legume and pulse products and dishes | 3.4 (2.9, 3.9) |
| Seed and nut products and dishes | 3.2 (2.9, 3.6) |
| Non-alcoholic beverages | 3.2 (3.0, 3.5) |
| Confectionery and cereal/nut/fruit/seed bars | 2.6 (2.3, 2.9) |
| Chocolate and chocolate-based confectionary | 2.5 (2.2, 2.7) |
| Milk products and dishes | 2.2 (2.0, 2.3) |
| Miscellaneous | 2.1 (1.8, 2.4) |
| Egg products and dishes | 1.9 (1.7, 2.1) |
| Fish and seafood products and dishes | 1.7 (1.5, 1.9) |
| Dairy and meat substitutes | 1.6 (1.2, 2.0) |
| Alcoholic beverages | 1.3 (1.1, 1.5) |
| Savoury sauces and condiments | 1.0 (0.9, 1.2) |
| β | 95% CI | p | |
|---|---|---|---|
| Model A: Major food sources of dietary iron and intakes of dietary ascorbic acid and phytate Adj. R2 = 0.20, p < 0.001 | |||
| Blood donation (100 mL/year) | −0.077 | −0.095, −0.059 | <0.001 |
| Arbitrary menstrual blood loss units per year | −0.002 | −0.004, −0.001 | 0.001 |
| Red + white meat (g/day) b,c | 0.089 | 0.006, 0.171 | 0.035 |
| Cereals and cereal products (g/day) b,d,e | 0.090 | −0.080, 0.260 | 0.30 |
| Vegetable products and dishes (g/day) d | 0.001 | 0, 0.002 | 0.07 |
| Ascorbic acid (mg/day) | −0.001 | −0.003, 0.001 | 0.29 |
| Phytate (mg/day) a | −0.011 | −0.193, 0.172 | 0.91 |
| Model B: Dietary intakes of iron, ascorbic acid and phytate Adj. R2 = 0.20, p < 0.001 | |||
| Blood donation (100 mL/year) | −0.075 | −0.093, −0.057 | <0.001 |
| Arbitrary menstrual blood loss units per year | −0.003 | −0.004, −0.001 | <0.001 |
| Dietary iron intake (mg/day) | 0.047 | 0.011, 0.083 | 0.011 |
| Ascorbic acid (mg/day) | −0.001 | −0.003, 0.001 | 0.23 |
| Phytate (mg/day) b | −0.094 | −0.303, 0.115 | 0.38 |
| Model C: Total iron intake and dietary intakes of ascorbic acid and phytate Adj. R2 = 0.21, p < 0.001 | |||
| Blood donation (100 mL/year) | −0.076 | −0.095, −0.058 | <0.001 |
| Arbitrary menstrual blood loss units per year | −0.002 | −0.004, −0.001 | 0.001 |
| Total iron intake (incl. Supplements, mg/day) b | 0.299 | 0.091, 0.506 | 0.005 |
| Ascorbic acid (mg/day) | −0.001 | −0.003, 0.001 | 0.30 |
| Phytate (mg/day) b | −0.046 | −0.232, 0.140 | 0.63 |
| β | 95% CI | p | |
|---|---|---|---|
| Model A: Dietary iron intake Adj. R2 = 0.65, p < 0.001 | |||
| Blood donation (100 mL/year) | −0.029 | −0.043, −0.015 | <0.001 |
| Arbitrary menstrual loss units per year | −0.001 | −0.002, 0 | 0.036 |
| Dietary iron intake (mg/day) | 0.011 | −0.009, 0.031 | 0.27 |
| Timing of sampling (h) | −0.080 | −0.113, −0.047 | <0.001 |
| Hepcidin (ng/mL) b | 0.788 | 0.704, 0.872 | <0.001 |
| Model B: Total iron intake Adj. R2 = 0.66, p < 0.001 | |||
| Blood donation (100 mL/year) | −0.030 | −0.045, −0.016 | <0.001 |
| Arbitrary menstrual loss units per year | −0.001 | −0.002, 0 | 0.05 |
| Total iron intake (mg/day) b | 0.155 | 0.028, 0.281 | 0.017 |
| Timing of sampling (h) | −0.075 | −0.109, −0.042 | <0.001 |
| Hepcidin (ng/mL) b | 0.784 | 0.699, 0.868 | <0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.H.C.; Booth, A.O.; Nowson, C.A.; Szymlek-Gay, E.A.; Irving, D.O.; Riddell, L.J. Hepcidin is a Better Predictor of Iron Stores in Premenopausal Women than Blood Loss or Dietary Intake. Nutrients 2016, 8, 540. https://doi.org/10.3390/nu8090540
Lim KHC, Booth AO, Nowson CA, Szymlek-Gay EA, Irving DO, Riddell LJ. Hepcidin is a Better Predictor of Iron Stores in Premenopausal Women than Blood Loss or Dietary Intake. Nutrients. 2016; 8(9):540. https://doi.org/10.3390/nu8090540
Chicago/Turabian StyleLim, Karen H. C., Alison O. Booth, Caryl A. Nowson, Ewa A. Szymlek-Gay, David O. Irving, and Lynn J. Riddell. 2016. "Hepcidin is a Better Predictor of Iron Stores in Premenopausal Women than Blood Loss or Dietary Intake" Nutrients 8, no. 9: 540. https://doi.org/10.3390/nu8090540
APA StyleLim, K. H. C., Booth, A. O., Nowson, C. A., Szymlek-Gay, E. A., Irving, D. O., & Riddell, L. J. (2016). Hepcidin is a Better Predictor of Iron Stores in Premenopausal Women than Blood Loss or Dietary Intake. Nutrients, 8(9), 540. https://doi.org/10.3390/nu8090540





