Associations between Dietary Nutrient Intakes and Hepatic Lipid Contents in NAFLD Patients Quantified by 1H-MRS and Dual-Echo MRI
Abstract
:1. Introduction
2. Methods
2.1. Participants and Demographic Characteristics
2.2. Dietary Nutrients Intake Assessment
2.3. Anthropometric and Laboratory Measurements
2.4. Definition of NAFLD
2.5. Hepatic Lipid Contents Measurements
2.6. Statistical Analysis
3. Results
3.1. Characteristics and Anthropometric and Clinical Variables of Participants
3.2. Dietary Intakes of Participants
3.3. Associations of Dietary Nutrients and Hepatic Lipid Contents
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| H-MRS | 1H-magnetic resonance spectroscopy |
| Dual-echo MRI | Dual-echo in-phase and out-phase magnetic resonance spectroscopy imaging |
| NAFLD | nonalcoholic fatty liver disease |
| IDQC | internet-based diet and lifestyle questionnaire for Chinese |
| SFA | saturated fatty acid |
| PUFA | polyunsaturated fatty acid |
| HFF | hepatic fat fraction |
| IHL | intrahepatic lipid |
| MUFA | monounsaturated fatty acid |
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the american association for the study of liver diseases, american college of gastroenterology, and the american gastroenterological association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F. Hepatic steatosis as a marker of metabolic dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (nafld) with hepatocellular carcinoma (hcc) in the united states from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Bendsen, N.T.; Chabanova, E.; Thomsen, H.S.; Larsen, T.M.; Newman, J.W.; Stender, S.; Dyerberg, J.; Haugaard, S.B.; Astrup, A. Effect of trans fatty acid intake on abdominal and liver fat deposition and blood lipids: A randomized trial in overweight postmenopausal women. Nutr. Diabetes 2011, 1, e4. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Yamanouchi, T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2012, 23, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.D. Polyunsaturated fatty acid regulation of gene transcription: A molecular mechanism to improve the metabolic syndrome. J. Nutr. 2001, 131, 1129–1132. [Google Scholar] [CrossRef]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. N. Engl. J. Med. 1986, 314, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Pollesello, P.; Ricci, C.; Masutti, F.; Kvam, B.J.; Bercich, L.; Croce, L.S.; Grigolato, P.; Paoletti, S.; de Bernard, B.; et al. Proton mr spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J. Magn. Reson. Imaging 1995, 5, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; McCarthy, R.; Thakrar, K.; Finn, J.P.; Rao, S.M.; Koffron, A.J.; Abecassis, M.; Blei, A.T. Dual-echo, chemical shift gradient-echo magnetic resonance imaging to quantify hepatic steatosis: Implications for living liver donation. Liver Transplant. 2003, 9, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Adams, L.A. Non-alcoholic fatty liver disease. Crit. Rev. Clin. Lab. Sci. 2011, 48, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Miyati, T.; Minami, T.; Takeshita, Y.; Ryu, Y.; Matsuda, T.; Ohno, N.; Hamaguchi, T.; Kato, K.; Takamura, T.; et al. Quantitative analysis of hepatic fat fraction by single-breath-holding mr spectroscopy with t(2) correction: Phantom and clinical study with histologic assessment. Radiol. Phys. Technol. 2013, 6, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Martin, D.R.; Pineda, N.; Xu, Q.; Vos, M.; Anania, F.; Hu, X. Quantitative analysis of t2-correction in single-voxel magnetic resonance spectroscopy of hepatic lipid fraction. J. Magn. Reson. Imaging 2009, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J. Magn. Reson. Imaging 2011, 34, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.N.; Du, S.S.; Chen, Y.; Li, Z.; Zhang, Y.F.; Sun, C.H.; Jiang, Y.S. Development and validation of an internet-based food frequency questionnaire to assess the diets of a large chinese population. Asia Pac. J. Clin. Nutr. 2013, 25. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Y.; Liu, L.Y.; Chen, Y.; Zi, T.Q.; Du, S.S.; Jiang, Y.S.; Feng, R.N.; Sun, C.H. The ratio of dietary branched-chain amino acids is associated with a lower prevalence of obesity in young northern chinese adults: An internet-based cross-sectional study. Nutrients 2015, 7, 9573–9589. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Li, C.L.; Qi, J.Y.; Huang, L.N.; Shi, D.; Du, S.S.; Liu, L.Y.; Feng, R.N.; Sun, C.H. Relationships of dietary histidine and obesity in northern chinese adults, an internet-based cross-sectional study. Nutrients 2016, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Li, Y.C.; Feng, R.N.; Jiang, Y.S. Reliability and validity of internet-based food frequency questionnaire. J. Harbin Med. Univ. 2014, 5, 376–380. [Google Scholar]
- Yang, Y.; Wang, G.; Guo, X. China Food Composition Tables; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.N.; Du, S.S.; Wang, C.; Li, Y.C.; Liu, L.Y.; Guo, F.C.; Sun, C.H. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight chinese population. World J. Gastroenterol. 2014, 20, 17932–17940. [Google Scholar] [PubMed]
- Fishbein, M.H.; Gardner, K.G.; Potter, C.J.; Schmalbrock, P.; Smith, M.A. Introduction of fast mr imaging in the assessment of hepatic steatosis. Magn. Reson. Imaging 1997, 15, 287–293. [Google Scholar] [CrossRef]
- Hervey, G.R. Regulation of energy balance. Nature 1969, 222, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Godos, J.; Salomone, F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: A review of observational studies and intervention trials. Ther. Adv. Gastroenterol. 2016, 9, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, M.H.; Zyriax, B.C.; Jagemann, B.; Roth, E.; Windler, E.; Schulze Zur Wiesch, J.; Lohse, A.W.; Kluwe, J. Nonalcoholic fatty liver disease is associated with excessive calorie intake rather than a distinctive dietary pattern. Medicine 2016, 95, e3887. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; May, M.D.; Loveman, E.; Colquitt, J.L.; Rees, K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2016, 1, CD011472. [Google Scholar] [PubMed]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [PubMed]
- Angulo, P.; Lindor, K.D. Non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2002, 17, S186–S190. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, M.; Miller, C. A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr. Rev. 2001, 59, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Carbohydrate intake and nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Senechal, M.; MacIntosh, A.C.; Hay, J.; Wicklow, B.A.; Wittmeier, K.D.; Sellers, E.A.; Dean, H.J.; Ryner, L.; Berard, L.; et al. Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am. J. Clin. Nutr. 2014, 99, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, R.W.; Hammer, S.; Lamb, H.J.; Frolich, M.; Diamant, M.; Rijzewijk, L.J.; de Roos, A.; Romijn, J.A.; Smit, J.W. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J. Clin. Endocrinol. Metab. 2008, 93, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Faga, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E275–E281. [Google Scholar] [CrossRef] [PubMed]
- Capanni, M.; Calella, F.; Biagini, M.R.; Genise, S.; Raimondi, L.; Bedogni, G.; Svegliati-Baroni, G.; Sofi, F.; Milani, S.; Abbate, R.; et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: A pilot study. Aliment. Pharmacol. Ther. 2006, 23, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
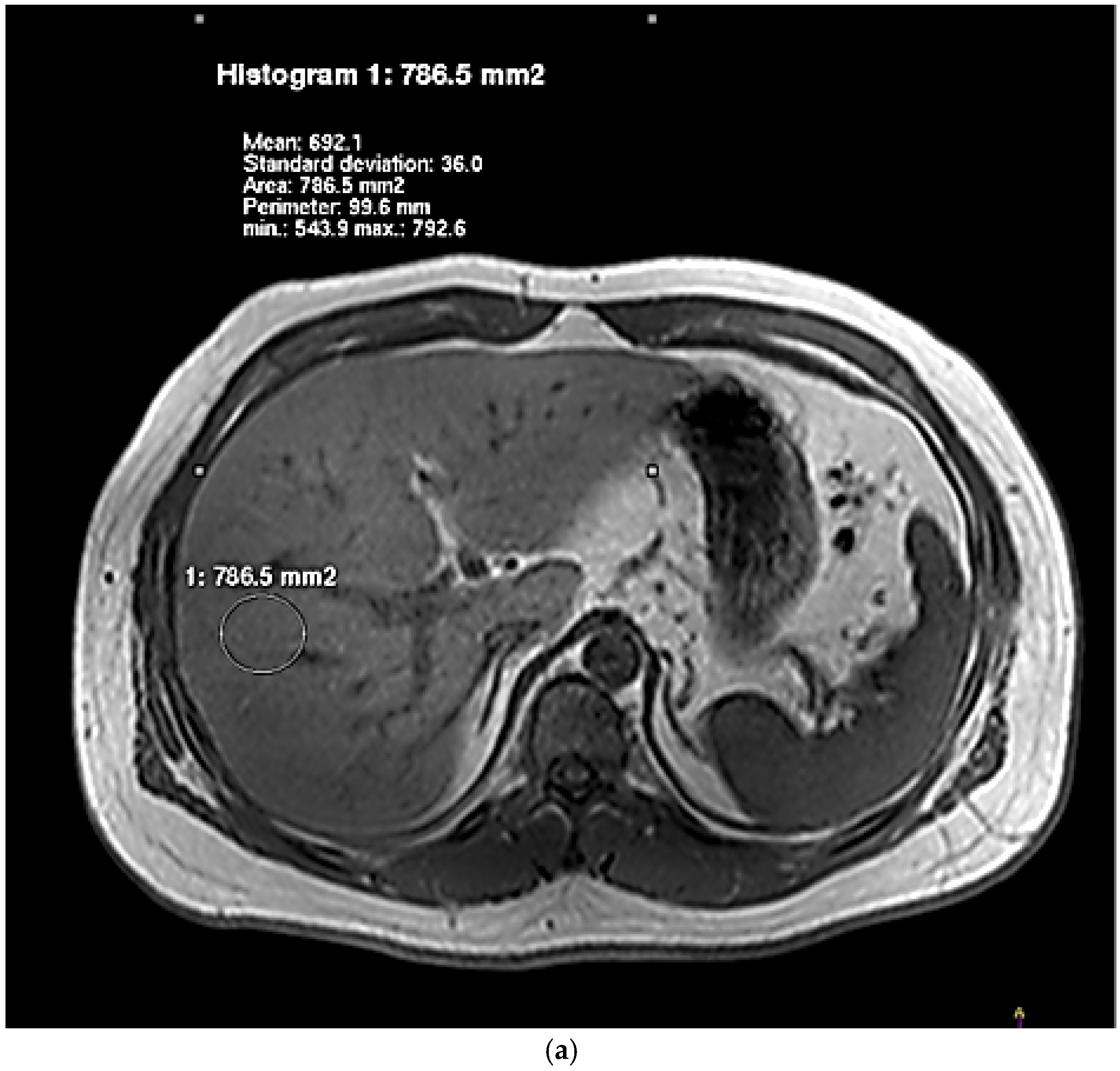
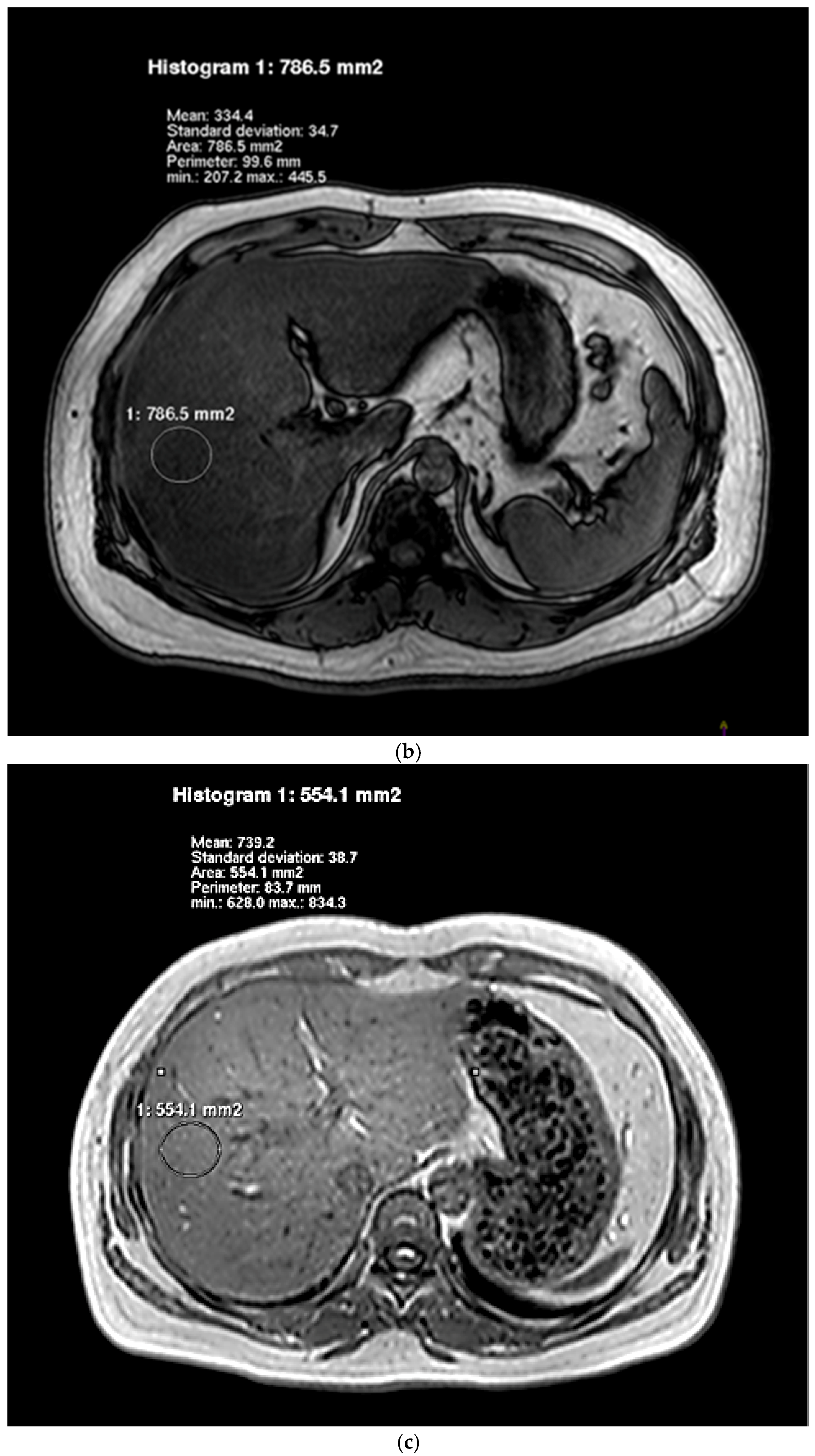
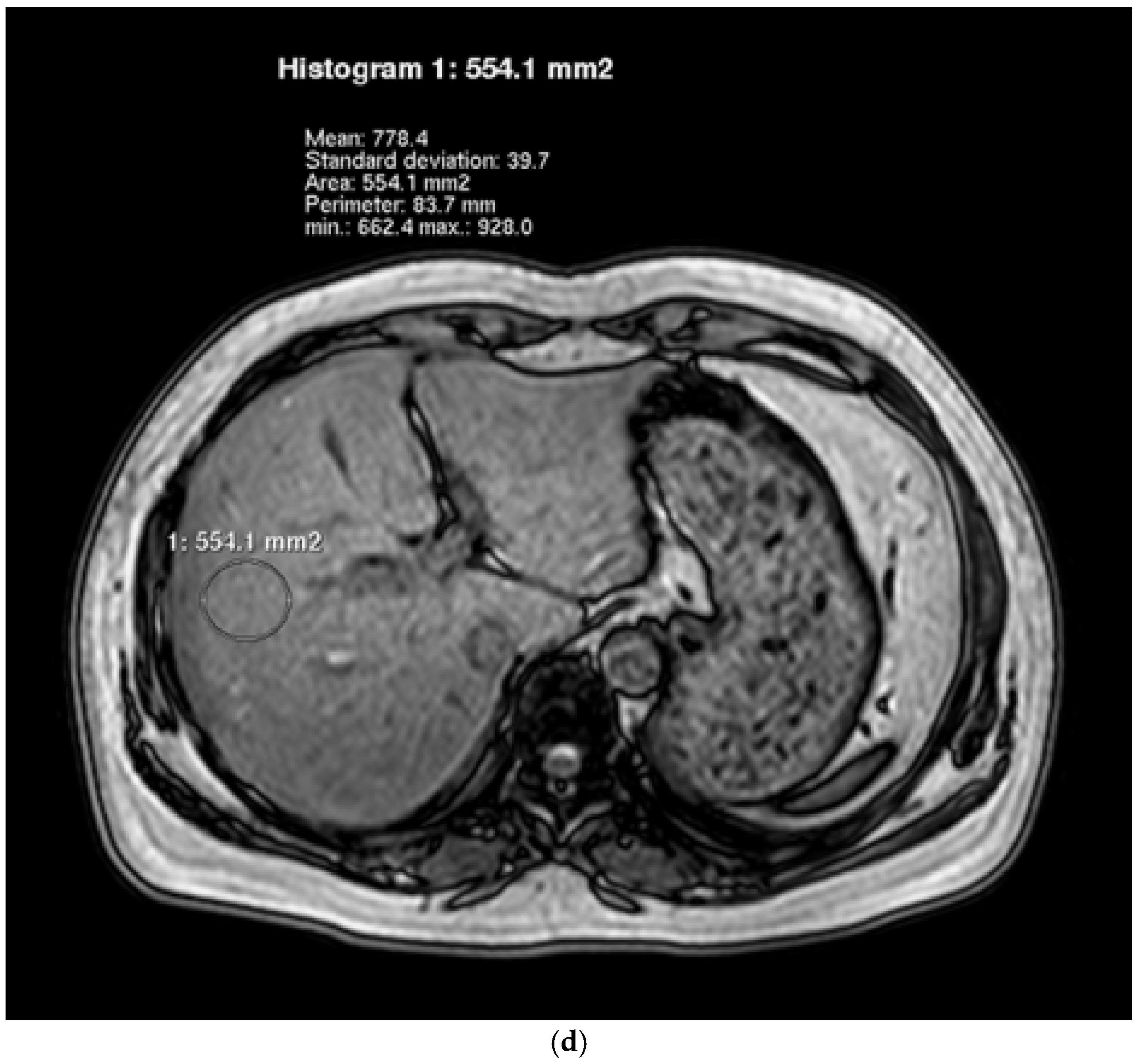
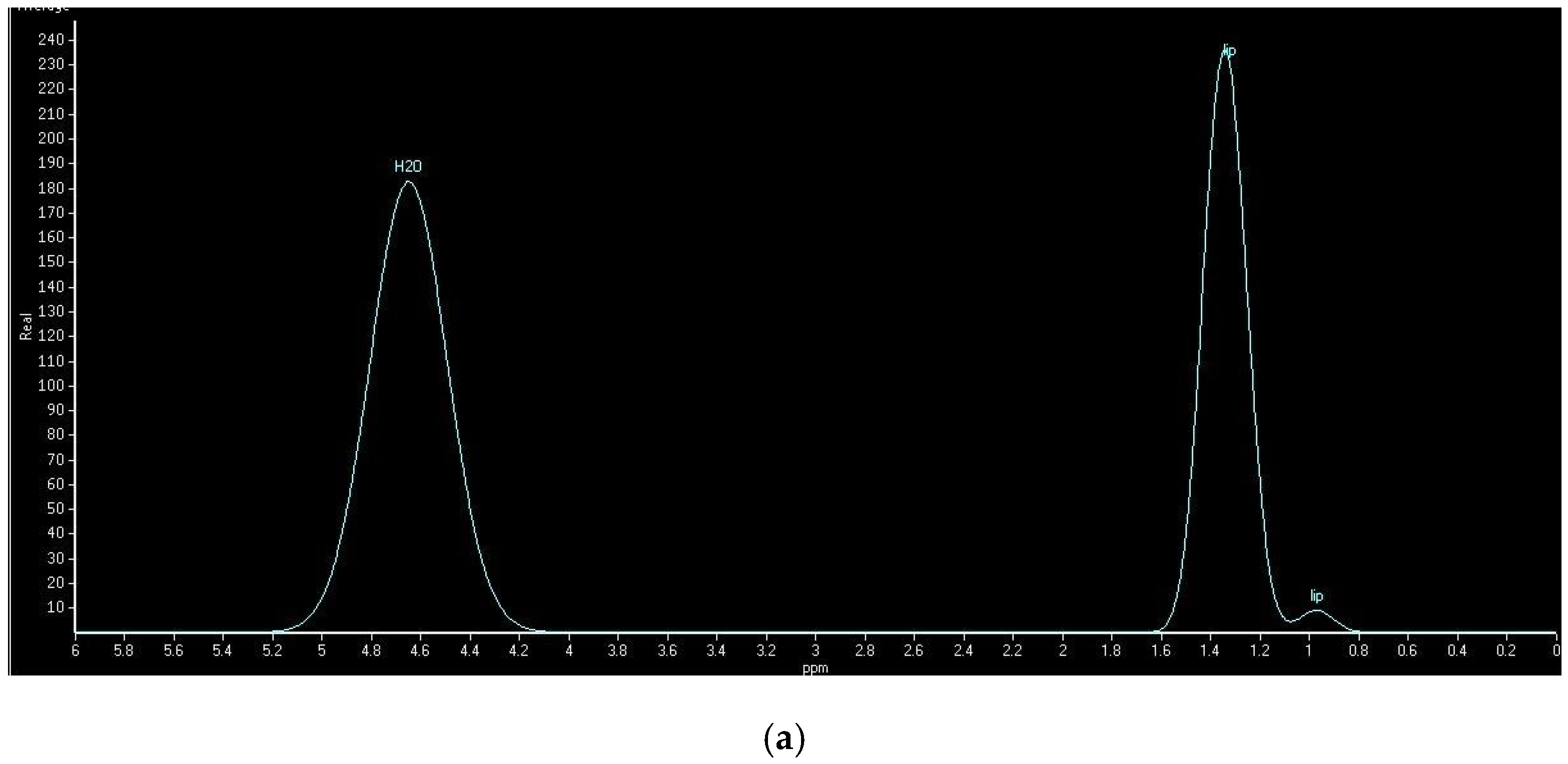
| Characteristics | Control (n = 17) | NAFLD (n = 19) | p Value |
|---|---|---|---|
| Age (years) | 48.76 ± 12.15 | 43.53 ± 10.94 | 0.18 |
| Menopause (%) | 35.29 | 26.32 | 0.82 |
| Physical activity at leisure time (%) | 0.24 | ||
| 0–30 min/week | 11.76 | 15.79 | |
| 31–60 min/week | 11.76 | 36.84 | |
| 61–120 min/week | 52.94 | 26.32 | |
| Above 120 min/week | 23.53 | 21.05 | |
| Physical activity at work (%) | 0.55 | ||
| Sedentary | 76.47 | 89.47 | |
| Moderate | 23.53 | 10.53 | |
| Heavy | 0 | 0 | |
| BMI (kg/m2) | 26.84 ± 4.30 | 35.76 ± 4.53 | <0.001 |
| BFR (%) | 35.63 ± 5.23 | 42.06 ± 3.55 | <0.001 |
| WC (cm) | 95.90 ± 2.14 | 103.36 ± 7.86 | 0.001 |
| SBP (mmHg) | 102.50 ± 15.19 | 120.63 ± 16.73 | <0.001 |
| DBP (mmHg) | 84.07 ± 15.78 | 92.89 ± 8.21 | 0.06 |
| FBG (mmol/L) | 5.17 ± 0.81 | 5.58 ± 1.13 | 0.22 |
| Insulin (mU/L) | 6.48 ± 0.69 | 18.23 ± 7.15 | <0.001 |
| HOMA-IR | 1.49 ± 0.30 | 4.65 ± 2.38 | <0.001 |
| TC (mmol/L) | 4.20 ± 0.39 | 5.15 ± 0.61 | <0.001 |
| TG (mmol/L) | 0.67 ± 0.20 | 1.69 ± 1.14 | <0.001 |
| HDL-C (mmol/L) | 1.64 ± 0.32 | 1.36 ± 0.16 | 0.002 |
| LDL-C (mmol/L) | 1.98 ± 0.34 | 3.03 ± 0.68 | <0.001 |
| ALT (U/L) | 15.71 ± 2.42 | 23.35 ± 11.94 | 0.02 |
| AST (U/L) | 18.06 ± 1.98 | 21.33 ± 6.95 | 0.07 |
| ALP (U/L) | 43.00 ± 13.22 | 46.05 ± 9.99 | 0.44 |
| BUN (mmol/L) | 4.79 ± 1.28 | 5.22 ± 1.56 | 0.39 |
| CRE (µmol/L) | 49.88 ± 8.13 | 55.89 ± 11.78 | 0.09 |
| UA (µmol/L) | 239.24 ± 20.37 | 339.91 ± 75.13 | <0.001 |
| HFF (%) | 5.22 ± 1.27 | 20.13 ± 1.12 | <0.001 |
| IHL (%) | 3.54 ± 1.81 | 23.42 ± 4.78 | <0.001 |
| Variables | Controls (n = 17) | NAFLD (n = 19) | p Value |
|---|---|---|---|
| Energy (Kcal) | 2423.74 ± 431.08 | 2901.00 ± 682.74 | 0.02 |
| Protein (g) | 79.17 ± 17.72 | 96.77 ± 22.59 | 0.01 |
| Fat (g) | 60.35 ± 16.33 | 86.56 ± 18.26 | <0.001 |
| Carbohydrate (g) § | 390.98 ± 83.33 | 433.73 ± 117.72 | 0.22 |
| Fiber (g) | 19.67 ± 5.47 | 21.45 ± 6.21 | 0.37 |
| SFA (g) | 6.13 ± 1.42 | 8.13 ± 2.31 | <0.001 |
| MUFA (g) § | 8.80 ± 3.84 | 10.78 ± 3.04 | 0.09 |
| PUFA (g) § | 11.2 ± 4.78 | 15.95 ± 5.24 | 0.01 |
| Variables in Model | HFF (%, n = 36) | IHL (%, n = 36) | ||
|---|---|---|---|---|
| β | p | β | p | |
| Carbohydrate | ||||
| Model 1 | 0.11 | 0.25 | 0.55 | 0.01 |
| Model 2 | 0.05 | 0.52 | 0.04 | 0.38 |
| Model 3 | 0.02 | 0.37 | 0.31 | 0.07 |
| Model 4 | 0.02 | 0.41 | 0.17 | 0.15 |
| Fiber | ||||
| Model 1 | −0.29 | 0.04 | −0.39 | 0.04 |
| Model 2 | −0.24 | 0.07 | −0.09 | 0.23 |
| Model 3 | −0.16 | 0.08 | −0.08 | 0.35 |
| Model 4 | −0.13 | 0.10 | −0.05 | 0.40 |
| Protein | ||||
| Model 1 | −0.36 | 0.01 | −0.65 | 0.001 |
| Model 2 | −0.03 | 0.56 | −0.38 | 0.06 |
| Model 3 | −0.01 | 0.39 | −0.13 | 0.11 |
| Model 4 | −0.01 | 0.41 | −0.11 | 0.14 |
| Total fat | ||||
| Model 1 | 0.58 | 0.005 | 0.76 | 0.003 |
| Model 2 | 0.31 | 0.04 | 0.74 | <0.001 |
| Model 3 | 0.34 | 0.03 | 0.49 | 0.002 |
| Model 4 | 0.36 | 0.03 | 0.42 | 0.01 |
| Total energy | ||||
| Model 5 | 0.35 | 0.01 | 0.47 | 0.004 |
| Model 6 | 0.34 | 0.02 | 0.49 | 0.01 |
| Model 7 | 0.24 | 0.02 | 0.38 | 0.02 |
| Variables in Model | HFF (%, n = 36) | IHL (%, n = 36) | ||
|---|---|---|---|---|
| β | p | β | p | |
| SFA | ||||
| Model 1 | 0.90 | 0.008 | 2.18 | 0.007 |
| Model 2 | 0.76 | 0.02 | 1.79 | 0.02 |
| Model 3 | 0.72 | 0.03 | 1.66 | 0.03 |
| Model 4 | 0.45 | 0.03 | 1.16 | 0.03 |
| MUFA | ||||
| Model 1 | −0.96 | 0.006 | −0.74 | 0.04 |
| Model 2 | −0.79 | 0.03 | −0.59 | 0.05 |
| Model 3 | −0.41 | 0.16 | −0.54 | 0.06 |
| Model 4 | −0.39 | 0.19 | −0.51 | 0.07 |
| PUFA | ||||
| Model 1 | −2.38 | 0.05 | −0.72 | 0.02 |
| Model 2 | −2.32 | 0.05 | −0.72 | 0.02 |
| Model 3 | −1.39 | 0.06 | −0.06 | 0.08 |
| Model 4 | −1.19 | 0.10 | −0.10 | 0.07 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zhang, K.; Chen, Y.; Li, Y.; Li, Y.; Fu, K.; Feng, R. Associations between Dietary Nutrient Intakes and Hepatic Lipid Contents in NAFLD Patients Quantified by 1H-MRS and Dual-Echo MRI. Nutrients 2016, 8, 527. https://doi.org/10.3390/nu8090527
Cheng Y, Zhang K, Chen Y, Li Y, Li Y, Fu K, Feng R. Associations between Dietary Nutrient Intakes and Hepatic Lipid Contents in NAFLD Patients Quantified by 1H-MRS and Dual-Echo MRI. Nutrients. 2016; 8(9):527. https://doi.org/10.3390/nu8090527
Chicago/Turabian StyleCheng, Yipeng, Kewei Zhang, Yang Chen, Yanchuan Li, Yuzheng Li, Kuang Fu, and Rennan Feng. 2016. "Associations between Dietary Nutrient Intakes and Hepatic Lipid Contents in NAFLD Patients Quantified by 1H-MRS and Dual-Echo MRI" Nutrients 8, no. 9: 527. https://doi.org/10.3390/nu8090527
APA StyleCheng, Y., Zhang, K., Chen, Y., Li, Y., Li, Y., Fu, K., & Feng, R. (2016). Associations between Dietary Nutrient Intakes and Hepatic Lipid Contents in NAFLD Patients Quantified by 1H-MRS and Dual-Echo MRI. Nutrients, 8(9), 527. https://doi.org/10.3390/nu8090527




