Effect of High Fat Dietary Intake during Maternal Gestation on Offspring Ovarian Health in a Pig Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Measurement of Body Weight and Back Fat Thickness
2.3. Sample Collection
2.4. Gene Expression
2.5. Oxidative Stress Biomarkers
2.6. Statistical Analysis
3. Results
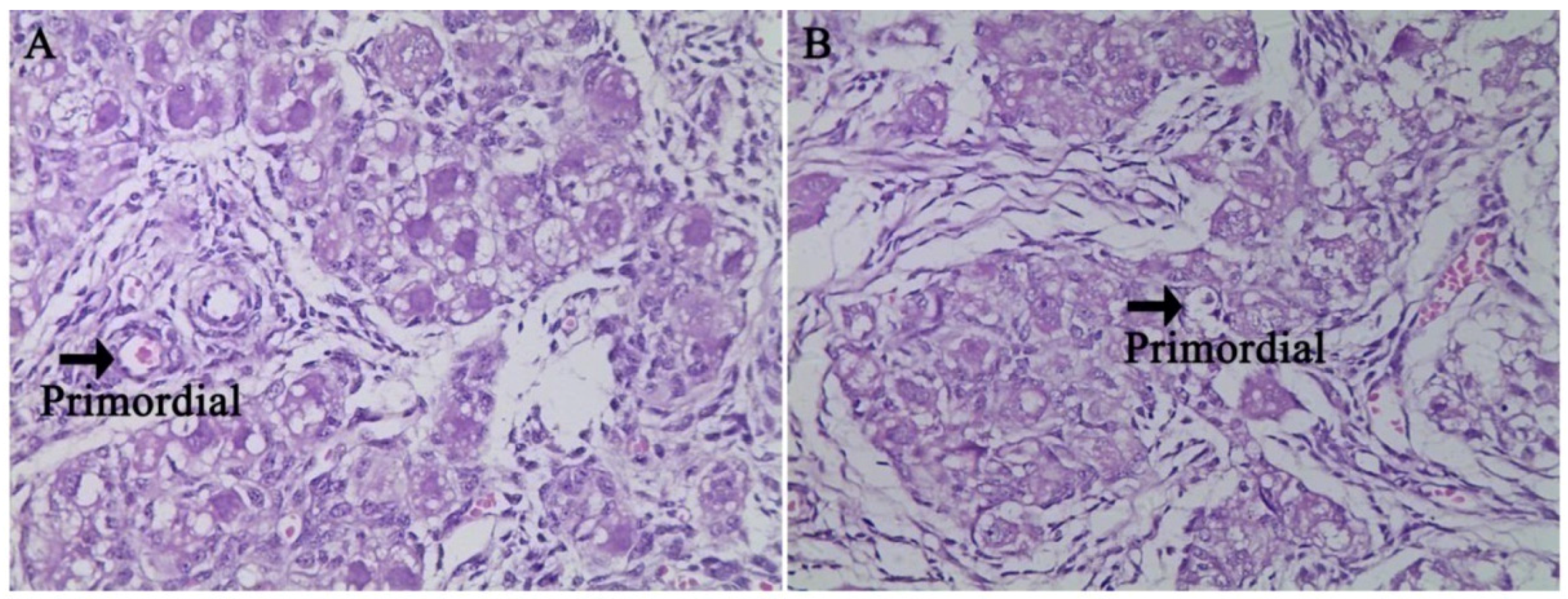
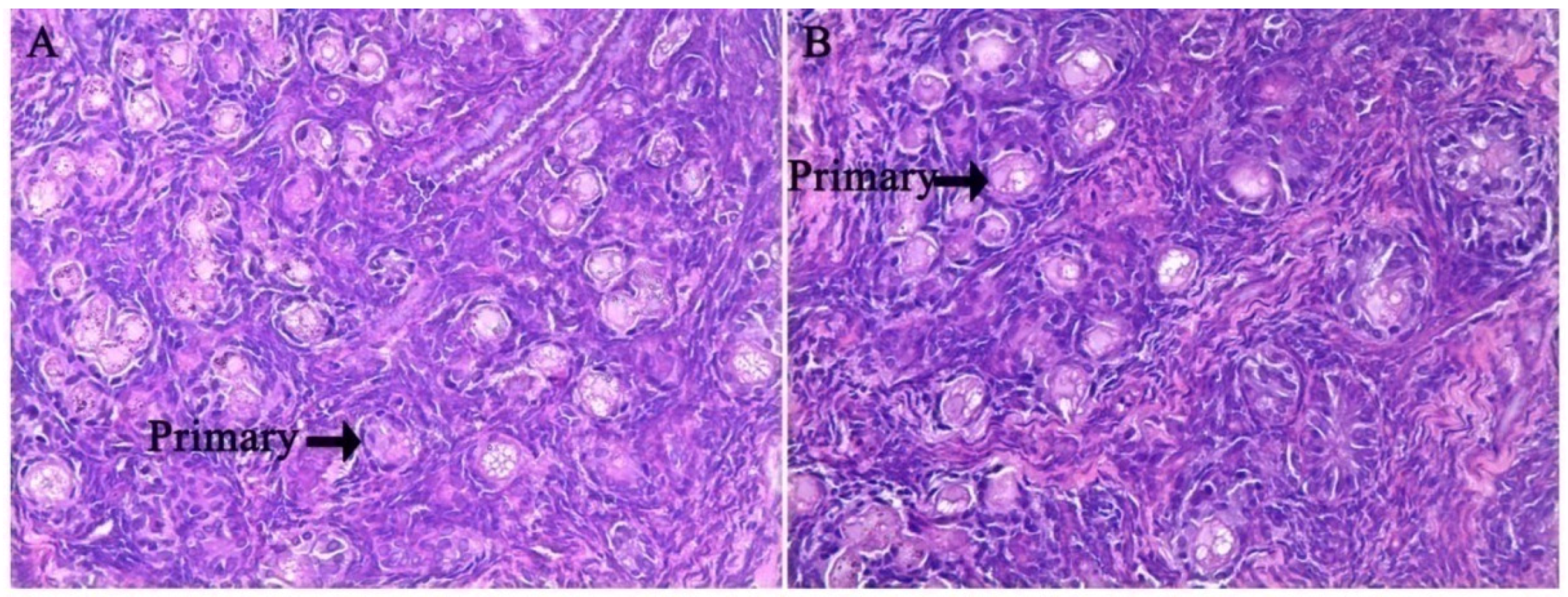
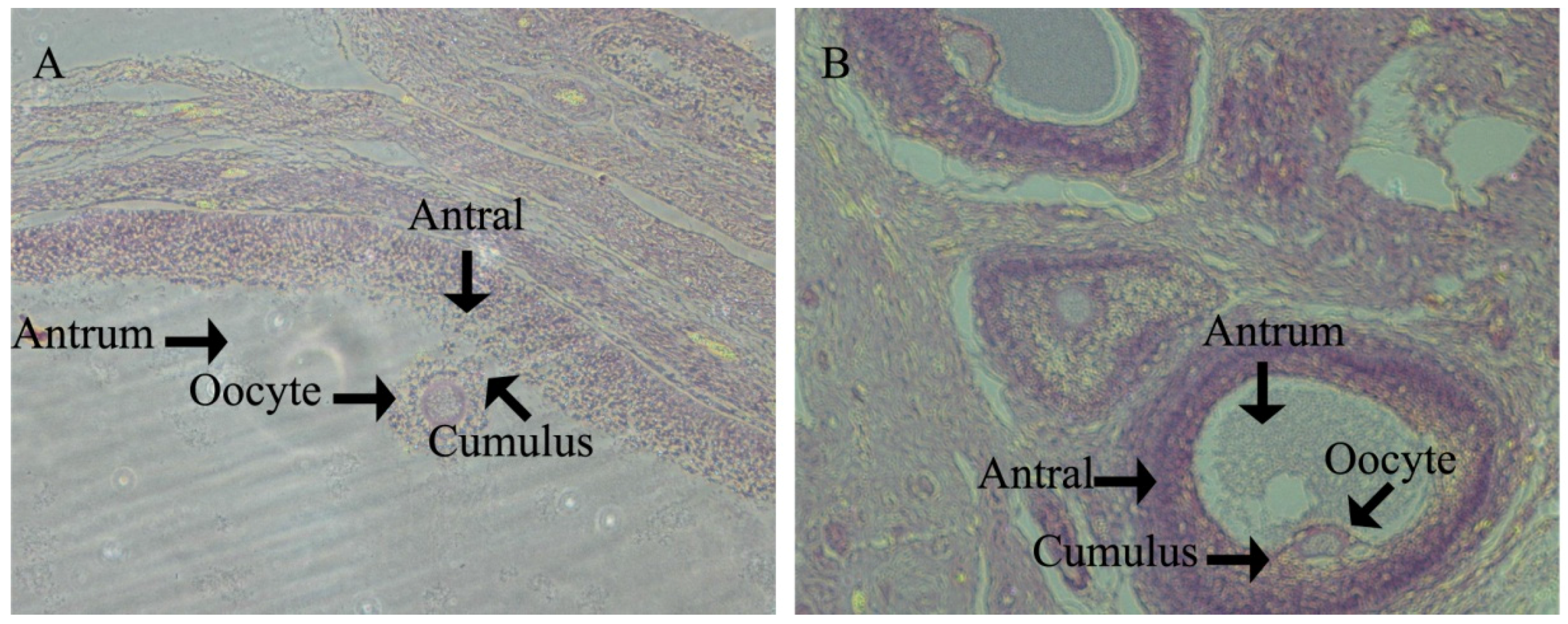
3.1. Histological Characteristics in the Ovary of Offspring
3.2. Reproductive and Growth Performance of Offspring Gilts
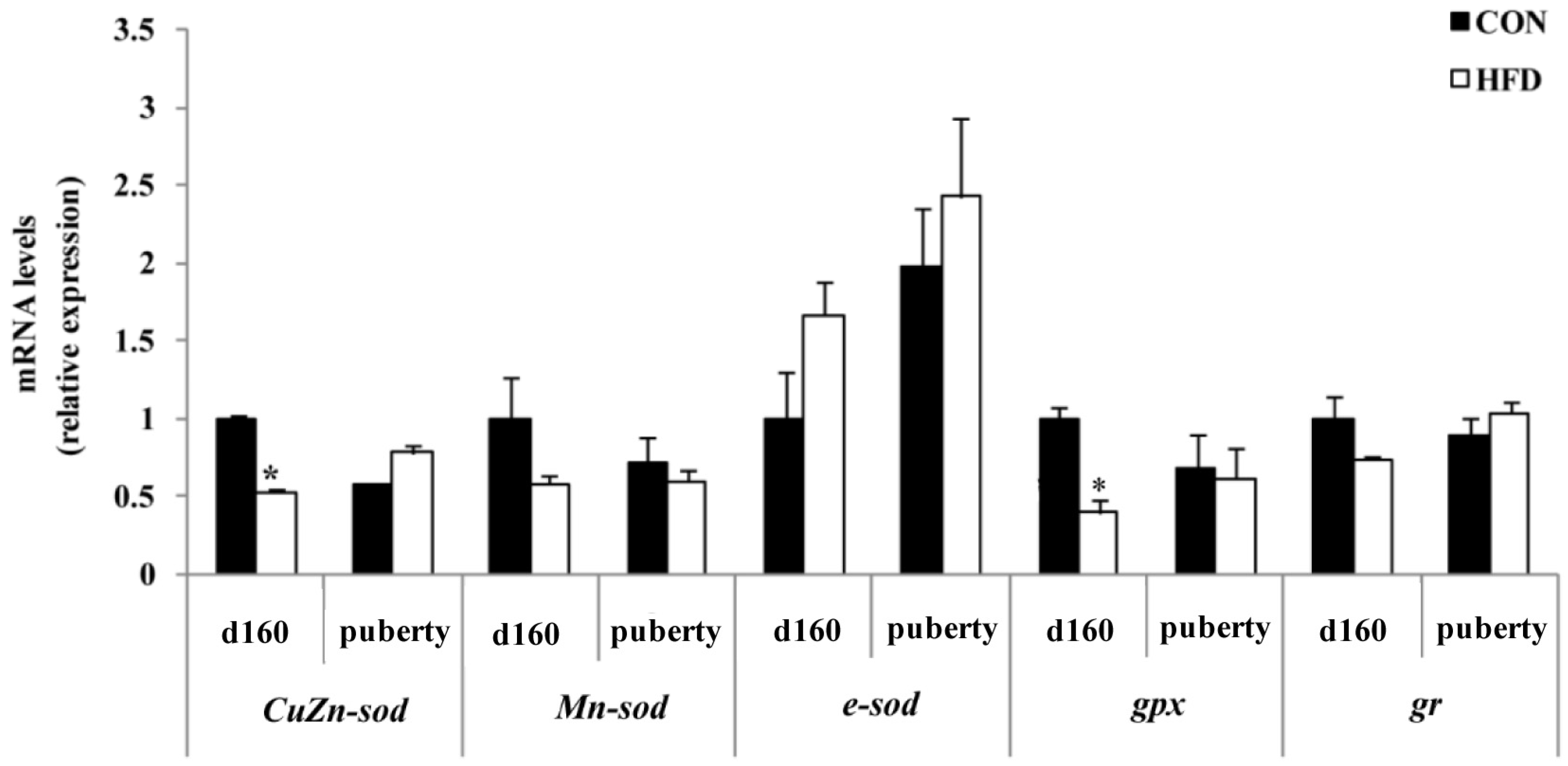
3.3. Ovarian Oxidative Stress Status
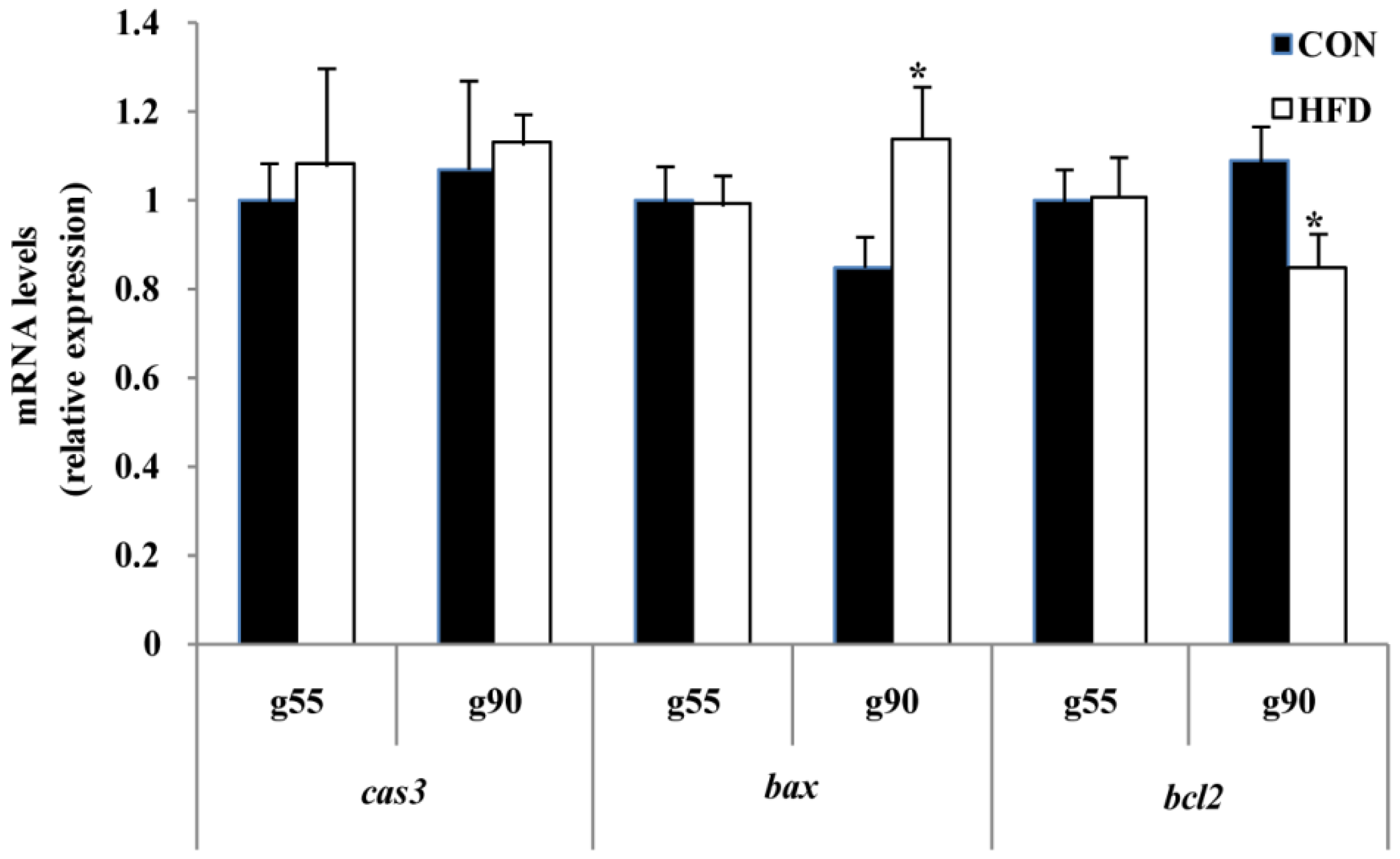
3.4. Ovarian Development and Apoptosis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wirfält, E.; Drake, I.; Wallström, P. What do review papers conclude about food and dietary patterns? Food Nutr. Res. 2013, 57, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.; Robinson, S.; Barker, D.J.P.; Osmond, C.; Cox, V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. Bmj. Lin. Res. 1996, 312, 410. [Google Scholar] [CrossRef]
- Moffett, A.; Hiby, S.E.; Sharkey, A.M. The role of the maternal immune system in the regulation of human birthweight. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.S.; Zhou, Y.; Che, L.Q.; Lin, Y.; Fang, Z.F.; Wu, D. Nutrient restriction induces failure of reproductive function and molecular changes in hypothalamus-pituitary-gonadal axis in postpubertal gilts. Mol. Biol. Rep. 2014, 41, 4733–4742. [Google Scholar] [CrossRef] [PubMed]
- Hułas-Stasiak, M.; Dobrowolski, P.; Tomaszewska, E.; Kostro, K. Maternal acrylamide treatment reduces ovarian follicle number in newborn Guinea pig offspring. Reprod. Toxicol. 2013, 42, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Roy, M.; Audet, I.; Palin, M.; Guay, F.; Lapointe, J.; Matte, J.J. Interaction between vitamin B6 and source of selenium on the response of the selenium-dependent glutathione peroxidase system to oxidative stress induced by oestrus in pubertal pig. J. Trace Elem. Med. Biol. 2015, 32, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Ramezanzadeh, F.; Nasr-Esfahani, M.H.; Yaraghi, A.A.S.; Ahmadi, M. Does dietary fat intake influence oocyte competence and embryo quality by inducing oxidative stress in follicular fluid? Iran. J. Reprod. Med. 2013, 11, 1005–1012. [Google Scholar] [PubMed]
- Che, L.Q.; Xuan, Y.; Hu, L.; Liu, Y.; Xu, Q.; Fang, Z.F.; Lin, Y.; Xu, S.Y.; Wu, D.; Zhang, K.Y.; Chen, D.W. Effect of postnatal nutrition restriction on the oxidative status of neonates with intrauterine growth restriction in a pig model. Neonatology 2015, 107, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, X.; Hu, Y.S.; Wu, Y.; Wang, Q.Z.; Dong, X.C. Evaluation of in vivo antioxidant activities of Ganodermalucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- Che, L.; Yang, Z.G.; Xu, M.M.; Zhang, Z.Y.; Liu, P.L.; Xu, S.Y.; Fang, Z.F.; Lin, Y.; Xu, S.Y.; Wu, D. Dietary energy intake affects fetal survival and development during early and middle pregnancy in Large White and Meishan gilts. Anim. Nutr. 2015, 1, 152–159. [Google Scholar] [CrossRef]
- Mossa, F.; Walsh, S.; Ireland, J.J.; Evans, A.C.O. Early nutritional programming and progeny performance: Is reproductive success already set at birth. Anim. Front. 2015, 5, 18–24. [Google Scholar] [CrossRef]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; Mcconnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; Sugino, N. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, J.L.; Barker, D.J.P.; Osmond, C.; Egger, P.; Phillips, D.I.; Fraser, R.B. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet 1997, 350, 1131–1135. [Google Scholar] [CrossRef]
- Grieger, J.A.; Clifton, V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 2014, 7, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Murabayashi, N.; Sugiyama, T.; Zhang, L.; Kamimoto, Y.; Umekawa, T.; Ma, N.; Saqawa, N. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Sichuan Agricultural University, Chengdu, China. Gilts with similar bodyweights were allocated to two fat levels diet during gestation, collected placental samples at day 55 or 90 of gestation to analyse nutrient transport. 2016. [Google Scholar]
- Godfrey, K.M.; Barker, D.J.P. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344s–1352s. [Google Scholar] [PubMed]
- Roongsitthichai, A.; Tummaruk, P. Importance of backfat thickness to reproductive performance in female pigs. Thai J. Vet. Med. 2004, 44, 171–178. [Google Scholar]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Rozeboom, D. Replacement Gilt and Boar Nutrient Recommendations and Feeding Management; National Swine Nutrition Guide; US Pork Center of Excellence: Ames, IA, USA, 2010; pp. 97–107. [Google Scholar]
- Byskov, A.G.; Høyer, P.E.; Björkman, N.; Mørk, A.B.; Olsen, B.; Grinsted, J. Ultrastructure of germ cells and adjacent somatic cells correlated to initiation of meiosis in the fetal pig. Anat. Embryol. 1986, 175, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Pepling, M. Oocyte Development before and during folliculogenesis. Oocyte Physiol. Dev. Domest. Anim. 2013. [Google Scholar] [CrossRef]
- Da Silva-Buttkus, P.; Marcelli, G.; Franks, S.; Stark, J.; Hardy, K. Inferring biological mechanisms from spatial analysis: Prediction of a local inhibitor in the ovary. Proc. Natl. Acad. Sci. USA 2009, 106, 456–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.L.; Fu, X.F.; Wang, L.Q.; Wang, J.J.; Ma, H.G.; Cheng, S.F.; Hou, Z.M.; Ma, J.M.; Quan, G.B.; Shen, W.; et al. Primordial follicle assembly was regulated by notch signaling pathway in the mice. Mol. Biol. Rep. 2014, 41, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Ballow, D.J.; Xin, Y.; Rajkovic, A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol. Reprod. 2008, 79, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Ji, S.; Hao, C.; Mu, Y.; Hao, J. Sohlh2 inhibits ovarian cancer cell proliferation by upregulation of p21 and downregulation of cyclin D1. Carcinogenesis 2014, 35, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Gagliardi, A.; Campanella, G.; Landi, C.; Capaldo, A.; Carleo, A.; Armini, A.; de Leo, V.; Piomboni, P.; Focarelli, R.; et al. A methodological and functional proteomic approach of human follicular fluid en route for oocyte quality evaluation. J. Proteom. 2013, 90, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K.; Bhujwala, R.A. Elevated serum α-fetoprotein and impaired immune response in malnutrition. Int. Arch. Allergy Immunol. 1997, 53, 180–185. [Google Scholar] [CrossRef]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- Lee, A.S. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 2004, 71, 289–297. [Google Scholar] [PubMed]
- Kwiatkowski, D.J. Functions of gelsolin: Motility, signaling, apoptosis, cancer. Curr. Opin. Cell Biol. 1999, 11, 103–108. [Google Scholar] [CrossRef]
- Kogure, K.; Nakamura, K.; Ikeda, S.; Kitahara, Y.; Iwamune, M.; Mineqishi, T. Glucose-regulated protein, 78-kilodalton is a modulator of luteinizing hormone receptor expression in luteinizing granulosa cells in rats. Biol. Reprod. 2013, 88, 116. [Google Scholar] [CrossRef] [PubMed]
- Vargas, T.; Antequera, D.; Ugalde, C.; Spuch, C.; Carro, E. Gelsolin Restores Aβ-Induced Alterations in Choroid Plexus Epithelium. J. Biomed. Biotechnol. 2010, 2010, 805405. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Che, L.; Wang, D.Y.; Zhang, P.; Lin, Y.; Fang, Z.F.; Che, L.Q.; Li, J.; Chen, D.W.; Wu, D.; et al. Proteomic analysis of fetal ovary reveals that ovarian developmental potential is greater in Meishan pigs than in Yorkshire pigs. PLoS ONE 2015, 10, e0135514. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lin, F.; Liu, H. Changes in the messenger RNA expression levels of Bcl-2 family members and caspase-8 and -3 in porcine ovarian follicles during follicular atresia. Anim. Sci. J. 2013, 84, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Boss, J.M. C/EBPβ regulates TNF induced MnSOD expression and protection against apoptosis. Apoptosis 2006, 11, 1837–1849. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Lai, R.J.; Finegold, M.; Hsueh, A.J. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology 1996, 137, 4837–4843. [Google Scholar] [PubMed]
- Ratts, V.S.; Flaws, J.A.; Kolp, R.; Tilly, J.L. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 1995, 136, 3665–3668. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Drwal, E.; Wróbel, A.; Gregoraszczuk, E.Ł. Resistin is a survival factor for porcine ovarian follicular cells. Reproduction 2015, 150, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Nandedkar, T.D.; Dharma, S.J. Expression of bclxs and c-myc in atretic follicles of mouse ovary. Reprod. Biomed. Online 2001, 3, 221–225. [Google Scholar] [CrossRef]
- Kugu, K.; Ratts, V.S.; Piquette, G.N.; Tilly, K.I.; Tao, X.J.; Martimbeau, S.; Aberdeen, G.W.; Krajewski, S.; Reed, J.C.; Pepe, G.J.; et al. Analysis of apoptosis and expression of bcl-2 gene family members in the human and baboon ovary. Cell Death Differ. 1998, 5, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Slot, K.A.; Voorendt, M.; de Boer-Brouwer, M.; van Vuqt, H.H.; Teerds, K.J. Estrous cycle dependent changes in expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and pro- and active caspase-3 in the rat ovary. J. Endocrinol. 2006, 188, 179–192. [Google Scholar] [CrossRef] [PubMed]




| Ingredient (%) | Pregnancy | Lactation | |
|---|---|---|---|
| HFD | CON | ||
| Corn | 45.00 | 45.00 | 62.12 |
| Soybean meal | 13.60 | 13.60 | 23.24 |
| Fish meal | - | - | 3.00 |
| Wheat bran | 27.80 | 27.80 | 5.00 |
| Soy oil | 9.10 | 4.50 | 2.50 |
| Wheat fiber | - | 2.54 | - |
| Soybean fiber | - | 1.10 | - |
| Corn fiber | - | 0.96 | - |
| Salt | 0.40 | 0.40 | 0.40 |
| Choline chloride | 0.14 | 0.14 | 0.15 |
| Calcium carbonate | 1.24 | 1.24 | 1.10 |
| Dicalcium phosphate | 1.99 | 1.99 | 1.27 |
| Sodium bicarbonate | - | - | 0.40 |
| Lysine | 0.10 | 0.10 | 0.23 |
| Threonine | 0.10 | 0.10 | 0.03 |
| Tryptophan | - | - | 0.01 |
| Premix | 0.53 * | 0.53 * | 0.55 & |
| Total | 100.00 | 100.00 | 100.00 |
| Nutritional compositions, % | |||
| Digestible energy, MJ/kg | 3.40 | 3.00 | 3.26 |
| Crude fat | 11.78 | 7.27 | - |
| Crude fiber | 3.41 | 4.97 | - |
| Crude protein | 13.49 | 13.92 | 17.67 |
| Lysine | 0.69 | 0.69 | 1.07 |
| sulfur-containing amino acid | 0.35 | 0.35 | 0.55 |
| Threonine | 0.46 | 0.46 | 0.73 |
| Ca | 0.96 | 0.96 | 0.90 |
| Total P | 0.79 | 0.79 | 0.68 |
| Ingredient (%) | 5–8 Weeks | 9–13 Weeks | 14–18 Weeks | 19 Weeks–Puberty |
|---|---|---|---|---|
| Corn | 26.70 | 68.30 | 70.50 | 74.80 |
| Extruded corn | 27.70 | - | - | - |
| Extruded soybean | 13.00 | - | - | - |
| Soybean meal | 15.90 | 24.00 | 24.74 | 21.30 |
| Soy oil | 0.90 | 2.00 | 2.00 | 1.50 |
| Fish meal | 4.50 | 3.00 | - | - |
| Sucrose | 2 | - | - | - |
| Whey | 6 | - | - | - |
| Lysine | 0.38 | 0.40 | 0.26 | 0.20 |
| Methionine | 0.14 | 0.07 | - | - |
| Threonine | 0.12 | - | - | - |
| Calcium carbonate | 0.78 | 0.90 | 0.75 | 0.78 |
| Calcium hydrophosphate | 0.83 | 0.58 | 1.00 | 0.67 |
| Choline chloride | 0.10 | 0.10 | 0.10 | 0.10 |
| Zinc oxide | 0.3 | - | - | - |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Premix * | 0.35 | 0.35 | 0.35 | 0.35 |
| Total | 100 | 100 | 100 | 100 |
| Nutritional compositions, % | ||||
| Digestible energy, MJ/kg | 3.47 | 3.34 | 3.34 | 3.34 |
| Crude protein | 19.54 | 17.87 | 16.29 | 15.11 |
| Ca | 0.81 | 0.67 | 0.60 | 0.52 |
| Total P | 0.62 | 0.53 | 0.52 | 0.45 |
| Available phosphorus | 0.45 | 0.35 | 0.33 | 0.27 |
| Lysine | 1.34 | 1.21 | 0.97 | 0.85 |
| sulfur-containing amino acid | 0.76 | 0.65 | 0.53 | 0.50 |
| Threonine | 0.89 | 0.73 | 0.67 | 0.56 |
| Gene | Primers | Sequence | Accession Number | Product Size (bp) |
|---|---|---|---|---|
| gpX | Forward | 5′-GCTCGGTGTATGCCTTCTCT-3′ | NM_214201.1 | 103 |
| Reverse | 5′-AGCGACGCTACGTTCTCAAT-3′ | |||
| Gr | Forward | 5′-CTACGTGAGCCGACTGAACA-3′ | AY368271.1 | 146 |
| Reverse | 5′-TCAGGATGTGAGGAGCTGTG-3′ | |||
| CuZn-sod | Forward | 5′-GAGACCTGGGCAATGTGACT-3′ | GU944822.1 | 139 |
| Reverse | 5′-CTGCCCAAGTCATCTGGTTT-3′ | |||
| Mn-sod | Forward | 5′-TGGAGGCCACATCAATCATA-3′ | NM_214127.2 | 136 |
| Reverse | 5′-AGCGGTCAACTTCTCCTTGA-3′ | |||
| e-sod | Forward | 5′-ACGCTGCTCTGTGCTTACCT-3′ | NM_001078688.1 | 142 |
| Reverse | 5′-TCAACTCCTGCCAGATCTCC-3′ | |||
| Aat | Forward | 5’-CTCGGGTGTCACAGAGGAAC-3’ | X88780.1 | 132 |
| Reverse | 5’-GTGGCCTCTGTCCCTTTCTC-3’ | |||
| Gen | Forward | 5’-CACTACTGGCTGGGCAATGA-3’ | X13871.1 | 87 |
| Reverse | 5’-ATCCTGATGCCACTCCTCCT-3’ | |||
| Afp | Forward | 5’-GCACGAAGAGATGCCCATAAAC-3’ | NM214317.1 | 116 |
| Reverse | 5’-ATGTCTCATCCAACACCAAGCT-3’ | |||
| grp78 | Forward | 5’-AACCAAGGACGCTGGAACTATT-3’ | X92446.1 | 181 |
| Reverse | 5’-AACACCAGGATGTTCTTCTCCC-3’ | |||
| lhx8 | Forward | 5’-GCTGTCTCCACCCATGTTAGAA-3’ | FJ587986.1 | 92 |
| Reverse | 5’-CATCCATGTAACTATGCAGCGC-3’ | |||
| Nobox | Forward | 5’-CCAAGAAGACCACTATCCGGAC-3’ | FJ587509.1 | 150 |
| Reverse | 5’-GTGTCCTTGTTCTCCTTCCCAT-3’ | |||
| sohlh2 | Forward | 5’-TTGAACGTGTACTCTGTCCCTG-3’ | XM_013980443.1 | 147 |
| Reverse | 5’-AGCATGGAAGGAATTGAGAGCA-3’ | |||
| bcl2 | Forward | 5’-CTTGGAGGGGACACTCTTCTTC-3’ | XM_003121700 | 140 |
| Reverse | 5’-TTTCTCATCACTGTCCTTCGGG-3’ | |||
| Bax | Forward | 5’-TTCAGGGTTTCATCCAGGATCG-3’ | XM_003127290 | 157 |
| Reverse | 5’-ATCCTCTGCAGCTCCATGTTAC-3’ | |||
| cas3 | Forward | 5’-TGGCGTGTCAGAAAATACCAGT-3’ | NM_214131.1 | 94 |
| Reverse | 5’-GATCCGTCCTTTGAATTTCGCC-3’ | |||
| β-actin | Forward | 5’-GGCCGCACCACTGGCATTGTCAT-3’ | DQ845171.1 | 104 |
| Reverse | 5’-AGGTCCAGACGCAGGATGGCG-3’ |
| Item | CON | HFD | p-Value |
|---|---|---|---|
| Body weight at Day 55 of gestation (g) | 92.59 ± 2.21 | 86.07 ± 3.56 | 0.195 |
| Body weight at Day 90 of gestation (g) | 673.34 ± 57.88 | 632.32 ± 102.17 | 0.739 |
| Body weight at birth (kg) | 1.17 ± 0.69 | 1.20 ± 0.15 | 0.859 |
| Body weight at Day 160 of offspring (kg) | 75.75 ± 0.85 | 84.13 ± 3.73 | 0.071 |
| Body weight upon puberty of offspring (kg) | 110.8 ± 2.41 | 110.8 ± 2.14 | 1 |
| Item | d160 | p-Value | Puberty | p-Value | ||
|---|---|---|---|---|---|---|
| CON | HFD | CON | HFD | |||
| Small follicles numbers (1–3 mm) | 13.33 ± 1.20 | 14.00 ± 3.05 | 0.849 | 29.33 ± 1.76 | 16.33 ± 4.10 | <0.05 |
| Large follicles numbers (≥4 mm) | 16.67 ± 1.76 | 9.33 ± 0.67 | <0.05 | 16.67 ± 0.67 | 14.00 ± 1.00 | 0.091 |
| Ovary weight (g) | 10.18 ± 0.70 | 9.70 ± 0.05 | 0.530 | 16.96 ± 1.07 | 11.25 ± 2.17 | 0.078 |
| Liver weight (g) | 1403.4 ± 37.00 | 1241.75 ± 52.78 | <0.05 | 1768.5 ± 43.12 | 1667.33 ± 84.78 | 0.299 |
| Spleen weight (g) | 158.53 ± 8.80 | 158.33 ± 17.18 | 0.186 | 275 ± 33.29 | 201.5 ± 9.95 | 0.059 |
| Back fat thickness (mm) | 14.6 ± 0.05 | 18.2 ± 0.06 | <0.05 | 19.6 ± 0.03 | 23.9 ± 0.11 | <0.05 |
| Age at puberty (day) | - | - | - | 185.00 ± 2.04 | 189.50 ± 3.86 | 0.370 |
| Item | g55 | p-Value | g90 | p-Value | d160 | p-Value | Puberty | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HFD | CON | HFD | CON | HFD | CON | HFD | |||||
| MDA, nmol/mg | 6.98 ± 0.14 | 7.55 ± 0.47 | 0.315 | 7.47 ± 0.57 | 9.57 ± 0.48 | <0.05 | 6.98 ± 0.22 | 6.42 ± 0.07 | 0.078 | 6.57 ± 0.85 | 4.95 ± 0.25 | 0.143 |
| T-SOD, U/mg | 55.50 ± 0.69 | 62.42 ± 4.11 | 0.173 | 67.72 ± 2.77 | 57.55 ± 1.08 | <0.01 | 33.06 ± 1.87 | 25.51 ± 1.34 | <0.05 | 30.58 ± 0.47 | 33.07 ± 1.46 | 0.18 |
| CuZn-SOD, U/mg | 47.17 ± 3.41 | 58.16 ± 4.40 | 0.119 | 65.52 ± 3.95 | 53.91 ± 1.67 | <0.05 | 23.61 ± 4.60 | 27.61 ± 4.39 | 0.563 | 25.04 ± 0.59 | 15.22 ± 1.84 | 0.143 |
| GR, U/g | 22.18 ± 1.70 | 20.29 ± 0.97 | 0.389 | 21.00 ± 0.45 | 19.27 ± 0.64 | 0.05 | 11.39 ± 1.15 | 13.84 ± 1.47 | 0.258 | 14.70 ± 0.72 | 15.85 ± 0.52 | 0.265 |
| GPx, U | 50.45 ± 4.50 | 55.00 ± 1.73 | 0.400 | 115 ± 3.19 | 95 ± 2.32 | <0.01 | 127.91 ± 9.51 | 90.49 ± 2.03 | <0.05 | 100 ± 9.97 | 146 ± 15.27 | 0.066 |
| T-AOC, U/mg | 11.72 ± 0.52 | 8.69 ± 0.76 | <0.05 | 9.44 ± 0.59 | 6.86 ± 0.20 | <0.05 | 5.29 ± 0.22 | 3.28 ± 0.29 | <0.05 | 6.89 ± 0.22 | 5.81 ± 0.20 | <0.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Che, L.; Yang, Z.; Zhang, P.; Shi, J.; Li, J.; Lin, Y.; Fang, Z.; Che, L.; Feng, B.; et al. Effect of High Fat Dietary Intake during Maternal Gestation on Offspring Ovarian Health in a Pig Model. Nutrients 2016, 8, 498. https://doi.org/10.3390/nu8080498
Xu M, Che L, Yang Z, Zhang P, Shi J, Li J, Lin Y, Fang Z, Che L, Feng B, et al. Effect of High Fat Dietary Intake during Maternal Gestation on Offspring Ovarian Health in a Pig Model. Nutrients. 2016; 8(8):498. https://doi.org/10.3390/nu8080498
Chicago/Turabian StyleXu, Mengmeng, Long Che, Zhenguo Yang, Pan Zhang, Jiankai Shi, Jian Li, Yan Lin, Zhengfeng Fang, Lianqiang Che, Bin Feng, and et al. 2016. "Effect of High Fat Dietary Intake during Maternal Gestation on Offspring Ovarian Health in a Pig Model" Nutrients 8, no. 8: 498. https://doi.org/10.3390/nu8080498
APA StyleXu, M., Che, L., Yang, Z., Zhang, P., Shi, J., Li, J., Lin, Y., Fang, Z., Che, L., Feng, B., Wu, D., & Xu, S. (2016). Effect of High Fat Dietary Intake during Maternal Gestation on Offspring Ovarian Health in a Pig Model. Nutrients, 8(8), 498. https://doi.org/10.3390/nu8080498







