Breakfast Macronutrient Composition Influences Thermic Effect of Feeding and Fat Oxidation in Young Women Who Habitually Skip Breakfast
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
2.3. Test Breakfasts
2.4. Anthropometric Measurements
2.5. Energy Expenditure and Substrate Oxidation
2.6. Appetite and Palatability Ratings
2.7. Blood Glucose Measurements
2.8. Dietary Assessment
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
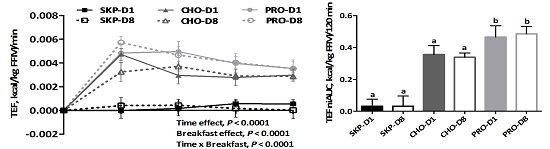
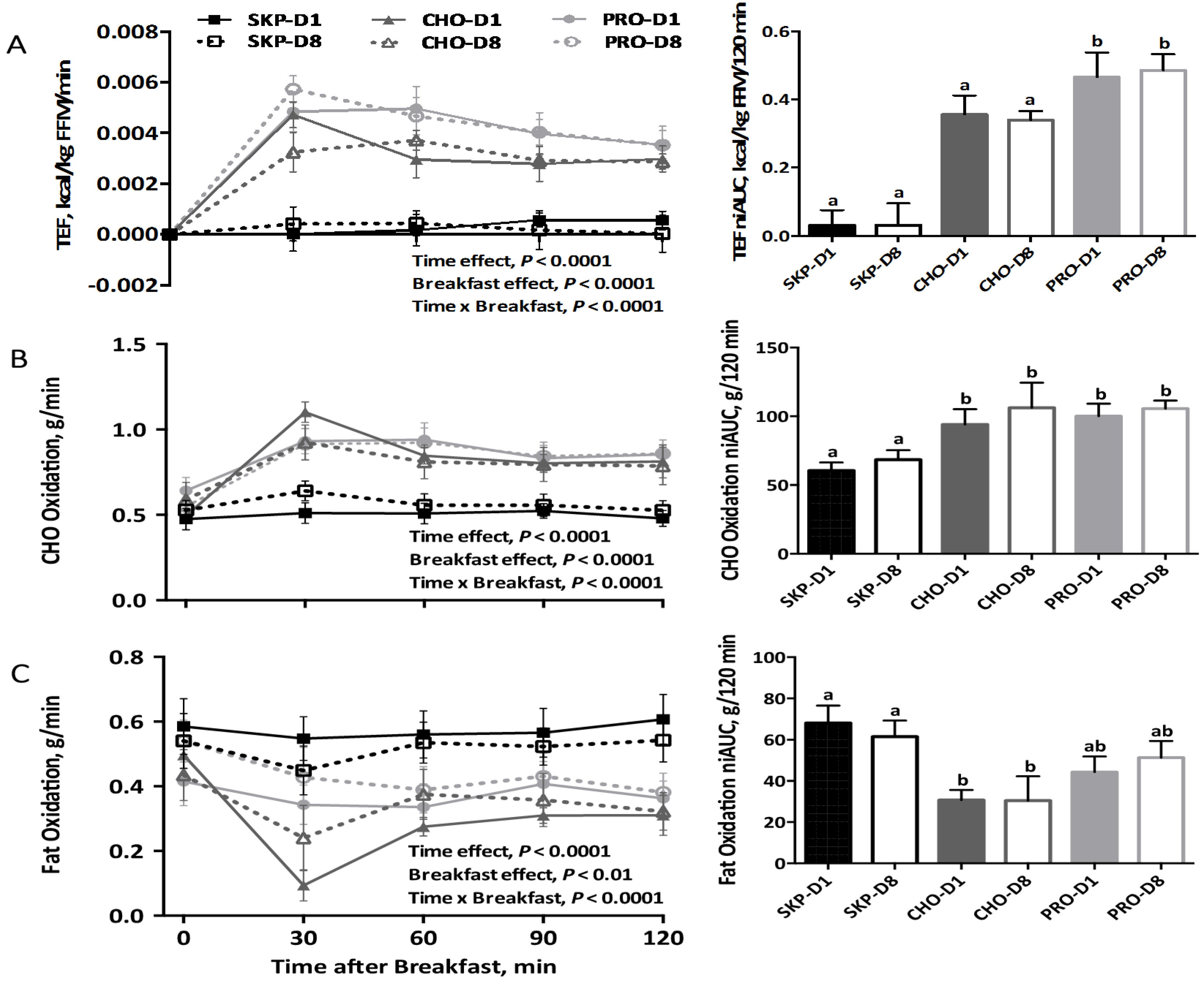
3.2. Energy Expenditure and Substrate Oxidation
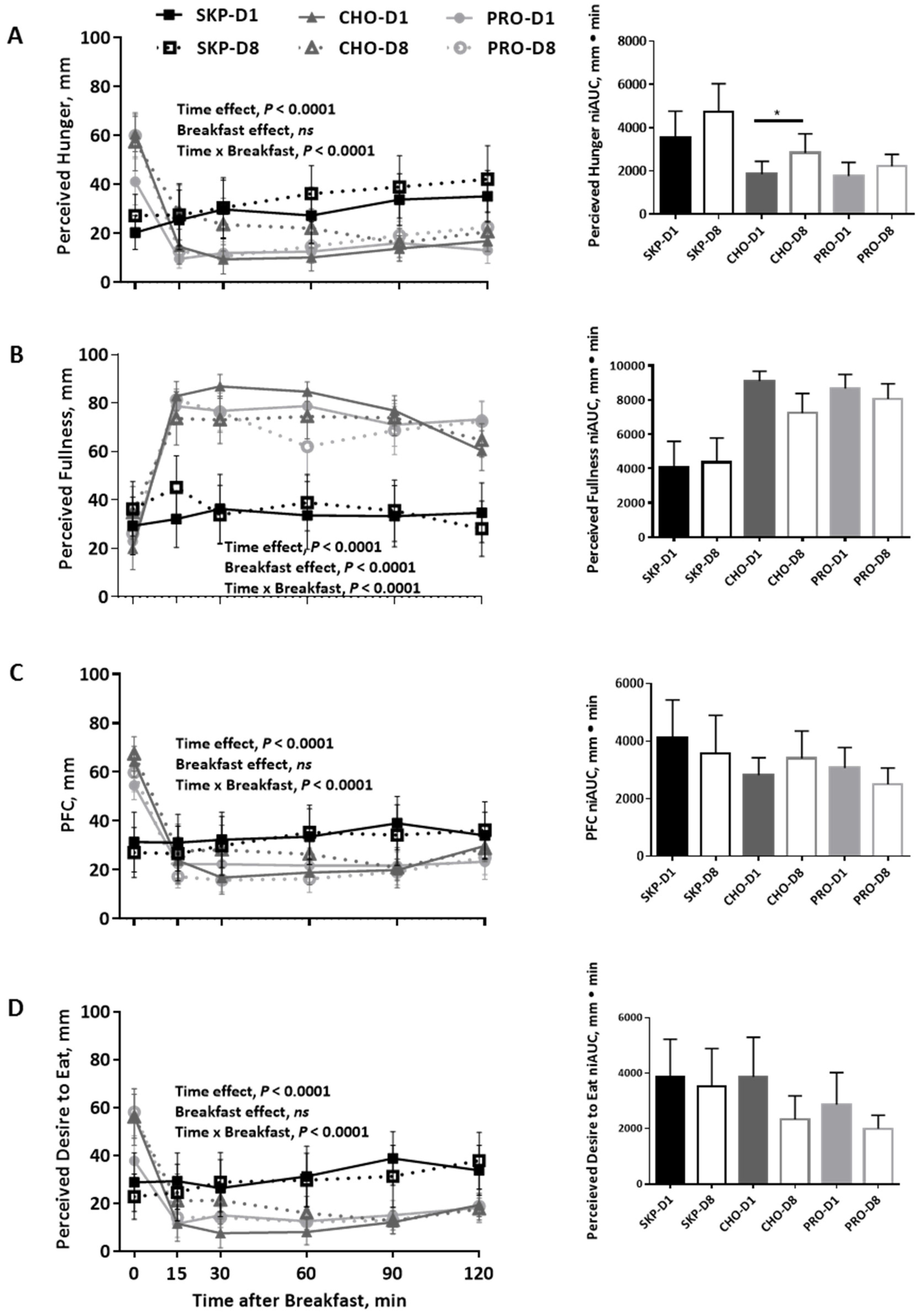
3.3. Appetite and Palatability Ratings
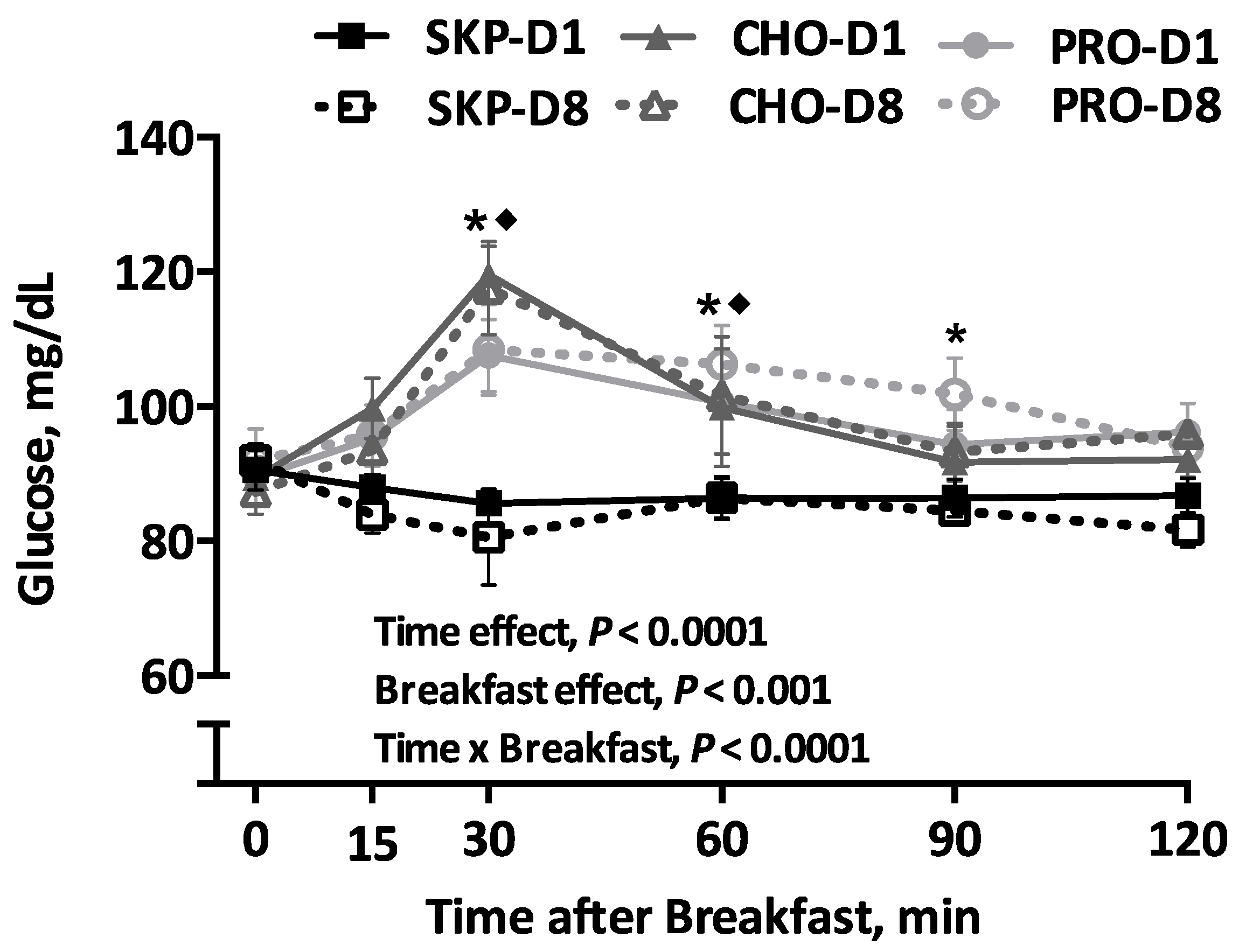
3.4. Blood Glucose
3.5. Ad Libitum Dietary Assessment
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beydoun, M.A.; Liang, L.; Caballero, B.; Kumanyika, S.K. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity 2008, 16, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Insulin resistance: The link between obesity and cardiovascular disease. Med. Clin. N. Am. 2011, 95, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.M.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephrol. Dial. Transplant. 2011, 26, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hutchesson, M.J.; Hulst, J.; Collins, C.E. Weight management interventions targeting young women: A systematic review. J. Acad. Nutr. Diet. 2013, 113, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh-Taskar, P.; Nicklas, T.A.; Radcliffe, J.D.; O’Neil, C.E.; Liu, Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr. 2013, 16, 2073–2082. [Google Scholar] [PubMed]
- Timlin, M.T.; Pereira, M.A. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr. Rev. 2007, 65, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.; Brown, M.M.B.; Allison, D.B. Belief beyond the evidence: Using the proposed effect of breakfast on obesity to show 2 practices that distort scientific evidence. Am. J. Clin. Nutr. 2013, 98, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.J.; James, L.J. The effect of breakfast on appetite regulation, energy balance and exercise performance. Proc. Nutr. Soc. 2015, 75, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.M. The time of day and the proportions of macronutrients eaten are related to total daily food intake. Br. J. Nutr. 2007, 98, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- McCrory, M.A. Meal skipping and variables related to energy balance in adults: A brief review, with emphasis on the breakfast meal. Physiol. Behav. 2014, 134, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.B.; Reynolds, L.J.; Douglas, S.M.; Kearney, M.L.; Hoertel, H.A.; Shafer, R.S.; Thyfault, J.P.; Leidy, H.J. A pilot study examining the effects of consuming a high-protein vs normal-protein breakfast on free-living glycemic control in overweight/obese ‘breakfast skipping’ adolescents. Int. J. Obes. 2015, 39, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Rains, T.M.; Leidy, H.J.; Sanoshy, K.D.; Lawless, A.L.; Maki, K.C. A randomized, controlled, crossover trial to assess the acute appetitive and metabolic effects of sausage and egg-based convenience breakfast meals in overweight premenopausal women. Nutr. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Heden, T.D.; Liu, Y.; Nyhoff, L.M.; Thyfault, J.P.; Leidy, H.J.; Kanaley, J.A. A high-protein breakfast induces greater insulin and glucose-dependent insulinotropic peptide responses to a subsequent lunch meal in individuals with type 2 diabetes. J. Nutr. 2015, 145, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Alwattar, A.Y.; JThyfault, P.; Leidy, H.J. The effect of breakfast type and frequency of consumption on glycemic response in overweight/obese late adolescent girls. Eur. J. Clin. Nutr. 2015, 69, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, H.A.; Will, M.J.; Leidy, H.J. A randomized crossover, pilot study examining the effects of a normal protein vs. high protein breakfast on food cravings and reward signals in overweight/obese “breakfast skipping”, late-adolescent girls. Nutr. J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Racki, E.M. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in ‘breakfast-skipping’ adolescents. Int. J. Obes. 2010, 34, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Hoertel, H.A.; Douglas, S.M.; Higgins, K.A.; Shafer, R.S. A high-protein breakfast prevents body fat gain, through reductions in daily intake and hunger, in “Breakfast skipping” adolescents. Obesity 2015, 23, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh-Taskar, P.R.; Radcliffe, J.D.; Liu, Y.; Nicklas, T.A. Do breakfast skipping and breakfast type affect energy intake, nutrient intake, nutrient adequacy, and diet quality in young adults? NHANES 1999–2002. J. Am. Coll. Nutr. 2010, 29, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Gray, M.; Binns, A. Breakfasts higher in protein increase postprandial energy expenditure, increase fat oxidation, and reduce hunger in overweight children from 8 to 12 years of age. J. Nutr. 2015, 145, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.B.; York, D.A.; Wasilewska, A.; Portman, J. A study of the thermic responses to a meal and to a sympathomimetic drug (ephedrine) in relation to energy balance in man. Br. J. Nutr. 1982, 47, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Day, C.S.; Swan, P.D. Postprandial thermogenesis is increased 100% on a high-protein, low-fat diet versus a high-carbohydrate, low-fat diet in healthy, young women. J. Am. Coll. Nutr. 2002, 21, 55–61. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Claps, N.; Heshka, S.; Heymsfield, S.B.; Kosteli, A. Greater resting energy expenditure and lower respiratory quotient after 1 week of supplementation with milk relative to supplementation with a sugar-only beverage in children. Metabolism 2007, 56, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Ormsbee, M.J.; Gentile, C.L.; Nindi, B.C.; Brestoff, J.R.; Ruby, M. Increased protein intake and meal frequency reduces abdominal fat during energy balance and energy deficit. Obesity 2013, 21, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Vander Wal, J.S.; Gupta, A.; Kholsa, P.; Dhurandhar, N.V. Egg breakfast enhances weight loss. Int. J. Obes. 2008, 32, 1545–1551. [Google Scholar]
- Ratliff, J.; Leite, J.O.; de Ogburn, R.; Puglisis, M.J.; VanHeest, J.; Fernandez, M.L. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 h in adult men. Nutr. Res. 2010, 30, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Saeed, A.; Jordan, K.; Hoover, H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 734–741. [Google Scholar] [PubMed]
- Rabinovitz, H.R.; Boaz, M.; Ganz, T.; Jakubowicz, D.; Matas, Z.; Madar, Z.; Wainstein, J. Big breakfast rich in protein and fat improves glycemic control in type 2 diabetics. Obesity 2014, 22, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Shiue, H.; Sather, C.; Erickson, D.J.; Baum, J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J. Nutr. 2003, 133, 405–410. [Google Scholar] [PubMed]
- Acheson, K.J.; Bloondel-Lubrano, A.; Oguey-Araymon, S.; Beaumont, M.; Emady-Azar, S.; Ammon-Zufferey, C.; Monnard, I.; Pinaud, S.; Nielsen-Moennoz, C. Protein choices targeting thermogenesis and metabolism. Am. J. Clin. Nutr. 2011, 93, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A.; Watras, A.C.; O’Brien, M.J.; Luke, A.; Dobratz, J.R.; Earthman, C.P.; Schoeller, D.A. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J. Am. Diet. Assoc. 2009, 109, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.E.; Byrd-Bredbenner, C.; Hayes, D.; Jana, L.; Klinger, S.E.; Stephenson-Martin, S. The role of breakfast in health: Definition and criteria for a quality breakfast. J. Acad. Nutr. Diet. 2014, 114, S8–S26. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Anthony, T.G.; Rasmussen, B.B.; Adams, S.H.; Lynch, C.J.; Brinkworth, G.D.; Davis, T.A. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. Protein quantity and quality at levels above the RDA improves adult weight loss. J. Am. Coll. Nutr. 2004, 23, 631–636. [Google Scholar] [CrossRef]
- Layman, D.K.; Baum, J.I. Dietary protein impact on glycemic control during weight loss. J. Nutr. 2004, 134, 968–973. [Google Scholar]
- Millward, D.J.; Layman, D.K.; Tome, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576–1581. [Google Scholar]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein—Its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbach-Waelen, A.; Veldhorst, M.A.; Nieuwenhuizen, A.G.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Comparison of 2 diets with either 25% or 10% of energy as casein on energy expenditure, substrate balance, and appetite profile. Am. J. Clin. Nutr. 2009, 89, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Westerterp, K.R.; Engelen, M.P.K.J.; Brummer, R.M.; Deutz, N.E.P.; Westerterp-Plantenga, M.S. Comparison of the effects of a high- and normal-casein breakfast on satiety, ‘satiety’ hormones, plasma amino acids and subsequent energy intake. Br. J. Nutr. 2009, 101, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.A.; Gonnissen, H.K.; Gatta-Cherifi, B.; Janssens, P.L.; Westerterp-Plantenga, M.S. Maintenance of energy expenditure on high-protein vs. high-carbohydrate diets at a constant body weight may prevent a positive energy balance. Clin. Nutr. 2015, 34, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.A.; Richardson, J.D.; Chowdhury, E.A.; Holman, G.D.; Tsintzas, K.; Thompson, D. The causal role of breakfast in energy balance and health: A randomized controlled trial in lean adults. Am. J. Clin. Nutr. 2014, 100, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, E.A.; Richardson, J.D.; Holman, G.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. The causal role of breakfast in energy balance and health: A randomized controlled trial in obese adults. Am. J. Clin. Nutr. 2016, 103, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Ortinau, L.C.; Douglas, S.M.; Hoertel, H.A. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping”, late-adolescent girls. Am. J. Clin. Nutr. 2013, 97, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, E.; Luscombe, N.D.; Noakes, M.; Wittert, G.; Argyiou, E.; Clifton, P.M. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am. J. Clin. Nutr. 2003, 78, 31–39. [Google Scholar] [PubMed]
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sorensen, T.I.; Speakman, J.R.; Jeansonne, M.; Allison, D.B. Energy balance measurement: When something is not better than nothing. Int. J. Obes. 2015, 39, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
| CHO | PRO | |
|---|---|---|
| Energy content, kJ (kcal) | 1469 (351) | 1464 (350) |
| Total protein, g | 10 | 30 |
| Total carbohydrate, g | 59 | 39 |
| Total sugars, g | 27 | 9 |
| Fiber, g | 1 | 2 |
| Total fat, g | 8 | 8 |
| Breakfast Appearance, mm 2 | 69 ± 4 | 64 ± 5 |
| Breakfast Palatability, mm 2 | 75 ± 3 | 68 ± 5 |
| SKP | CHO | PRO | |
|---|---|---|---|
| Participants, n | 8 | 8 | 8 |
| Age, year | 27.1 ± 1.8 a | 21.9 ± 0.9 b | 23.3 ± 1.3 a,b |
| Height, cm | 168.4 ± 2.1 | 162.1 ± 4.1 | 164.9 ± 2.2 |
| Weight, kg | 78.9 ± 6.3 | 67.0 ± 7.0 | 72.6 ± 6.3 |
| BMI | 27.8 ± 2.2 | 26.0 ± 1.9 | 26.6 ± 2.1 |
| Fat Mass, % | 45.3 ± 1.6 | 37.4 ± 3.1 | 40.5 ± 3.4 |
| Fat Free Mass, kg | 45.8 ± 3.4 | 43.6 ± 2.3 | 44.5 ± 1.5 |
| Ethnicity | |||
| Caucasian | 5 | 3 | 6 |
| Hispanic | 1 | 1 | 1 |
| Black | 1 | 1 | 1 |
| Asian | 2 | ||
| Indian | 1 | 1 |
| SKP | CHO | PRO | |
|---|---|---|---|
| Energy Intake | |||
| Total, kJ | 8406 ± 808 | 6707 ± 531 | 8941 ± 1460 |
| Total, kcal | 2009 ± 193 | 1603 ± 127 | 2137± 349 |
| Protein, g | 88 ± 13 a,b | 57 ± 6 b | 99 ± 8 a |
| Carbohydrate, g | 234 ± 22 | 231 ± 19 | 246 ± 40 |
| Fat, g | 75 ± 9 | 54 ± 5 | 81 ± 17 |
| Macronutrient Intake 2 | |||
| Protein, g | 18 | 14 | 19 |
| Carbohydrate, g | 47 | 57 | 46 |
| Fat, g | 35 | 29 | 35 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, B.L.; Dunn, A.; Johnson, D.; Adams, J.D.; Baum, J.I. Breakfast Macronutrient Composition Influences Thermic Effect of Feeding and Fat Oxidation in Young Women Who Habitually Skip Breakfast. Nutrients 2016, 8, 490. https://doi.org/10.3390/nu8080490
Neumann BL, Dunn A, Johnson D, Adams JD, Baum JI. Breakfast Macronutrient Composition Influences Thermic Effect of Feeding and Fat Oxidation in Young Women Who Habitually Skip Breakfast. Nutrients. 2016; 8(8):490. https://doi.org/10.3390/nu8080490
Chicago/Turabian StyleNeumann, Brianna L., Amy Dunn, Dallas Johnson, J. D. Adams, and Jamie I. Baum. 2016. "Breakfast Macronutrient Composition Influences Thermic Effect of Feeding and Fat Oxidation in Young Women Who Habitually Skip Breakfast" Nutrients 8, no. 8: 490. https://doi.org/10.3390/nu8080490
APA StyleNeumann, B. L., Dunn, A., Johnson, D., Adams, J. D., & Baum, J. I. (2016). Breakfast Macronutrient Composition Influences Thermic Effect of Feeding and Fat Oxidation in Young Women Who Habitually Skip Breakfast. Nutrients, 8(8), 490. https://doi.org/10.3390/nu8080490