1. Introduction
Undernutrition is a global health problem associated with increased morbidity and mortality in children [
1,
2]. Long-term undernutrition results in impaired health and physical and cognitive development, and lower productivity of populations. In the context of economic and demographic transition, several developing countries also encounter nutritional transitions with the increasing prevalence of overweight and obesity. This aspect of malnutrition has consequences on health through its association with non-communicable diseases [
3]. Therefore, developing countries where problems of under- and overnutrition coexist suffer a so-called double burden of malnutrition.
Several efforts have been made to reduce malnutrition, resulting in a global decrease of undernutrition over the past decades [
4]. Nevertheless, despite these efforts, improving nutritional status remains a challenge. Several actions were identified as efficient in preventing malnutrition. These actions concern both direct and indirect determinants, e.g., access to health services or increased food security as well as basic causes of malnutrition like poverty. Several interventions were implemented locally with proven beneficial effects on nutritional status. However, these actions need to be scaled up to reach the populations that need them most. Indeed, the magnitude of progress in the coverage of intervention influences the extent to which inequity between wealth groups can be reduced; the more coverage increases, the more inequity has a chance to decrease [
5]. The prevalence of stunting and wasting among children under five has declined globally but still remains prevalent in South Asia and Africa [
4]; these global improvements may hide disparities between child nutritional outcomes and improvements unevenly affecting populations according to their living area and socioeconomic characteristics. The poorest people living in rural areas are often the most at risk for undernutrition [
4,
6,
7]. Gender inequality is also reported, even if its effects are less severe than economic inequality, with boys often being more affected by stunting and mortality before five years old than girls [
4]. The picture of global trends and inequalities is different for overweight and obesity. Black
et al. [
4] reported a 54% increase in the prevalence of overweight in children under five years old from 1990 to 2011. Socioeconomic inequalities are less pronounced than for undernutrition, and overweight tends to affect the richest population groups more than the poorest.
Although a lot of developing countries succeeded in reducing undernutrition, most of them failed to significantly reduce the inequalities,
i.e., the differences in the prevalence of nutrition problems between subgroups of the population differing by their socioeconomic or demographic characteristics [
8]. Yet the analysis of inequalities trends could help to define more efficient policies to reduce inequalities and to reach the most vulnerable subgroups of population.
In Cambodia, the great economic growth over the past two decades was not beneficial in the same manner to every subgroup of the population [
9]. For example, the analysis of Kinnon
et al. [
9] showed that in Cambodia in 2010 the risk of neonatal death was 85% lower for infants belonging to the wealthier class compared to the lower class. These authors also reported that, of the 24 countries studied, Cambodia is one of the rare countries where wealth and educational inequalities in neonatal mortality rates increased.
The aims of the present study were to analyze the trends in nutritional status (anthropometry and anemia) of children under five years in Cambodia and to assess the effect of inequality (age, gender, mother’s education, living area, and wealth index) on nutrition in this population. To focus on the most recent situation of the country we also modeled the contribution of different socioeconomic factors and living areas in the prevalence of childhood undernutrition in 2014.
3. Results
Table 1 presents the characteristics of children from the four surveys. The male/female ratio was, as expected, close to 50/50 in each survey. The rural/urban ratio was approximately 6/1 in 2000 and decreased to 2.8/1 in the 2014 survey. The percentage of mothers without education decreased from 3/10 to approximately 1/10 over time. The mean age of children was not significantly different over the four studies. In contrast, mean height and weight of all children and in males and females increased progressively and significantly over time. Consequently, height-for-age and weight-for-age indices improved significantly from 2000 to 2014, while weight-for-height
z-scores and BMI-for-age
z-scores did not change significantly over the four surveys despite an improvement between 2000 and 2005.
Concerning the nutritional indicators, stunting represented a public health problem in all surveys: very high in boys from 2000 to 2010 and high in 2014 according to the WHO classification [
17]; very high in girls in 2000 and high from 2005 to 2014 (
Table 2). Stunting was similarly prevalent in both sexes over time, except it was significantly higher in males in 2005. Stunting prevalence decreased significantly over the study period for both sexes. In each survey, the risk of being stunted was significantly higher in children whose mothers had no education than for those of mothers with secondary education or higher (the prevalence of stunting was intermediate in women with primary education). The prevalence of stunting was also significantly higher in children living in rural areas in all four surveys than in those living in urban areas. From 2005 to 2014 the stunting prevalence was about twice as high in children in the poorest wealth quintile compared to children in the richest quintile, with the prevalence of stunting decreasing from the poorest to the richest households.
Wasting was a public health problem over time: very high in both sexes in 2000, medium in both sexes in 2005, high in both sexes in 2010, and again medium in both sexes in 2014 (
Table 3), according to the WHO classification. Wasting was similarly prevalent in both sexes over time and not significantly different according to the education of mothers. Until the 2014 survey, wasting did not differ significantly between urban and rural area, whereas in 2014 rural prevalence was significantly higher than urban ones. The risk of being wasted was significantly higher in children in the poorest wealth quintile compared to children in the richest quintile in 2005 and 2014.
The prevalence of underweight (
Table 4) was not significantly different between the sexes over time, representing a very high public health problem in 2000 and a high public health problem thereafter. The prevalence of underweight decreased significantly over the study period. The risk of being underweight was significantly higher in children whose mother had no education than in those with mothers with secondary education (the prevalence of underweight was intermediate in women with primary education). The risk of being underweight was also significantly higher in children living in rural areas compared to urban areas in the 2000, 2010, and 2014 surveys. The prevalence of underweight was about twice as high in the poorest quintile compared to the richest quintile, with the prevalence of underweight decreasing form the poorest to the richest households.
The prevalence of overweight (OW) did not change significantly over the study period (it was always below 10%) and was significantly lower in females than in males in 2005 and 2014 (
Table 5). OW increased over time in urban areas and decreased in rural areas; the prevalence of OW in urban areas was almost twice that of rural areas in 2010 and 2014. In 2014, belonging to the richest category was a risk factor for OW compared to the poorest.
Anemia prevalence was a significant severe public health problem in all four surveys and the risk of being anemic was significantly higher in males compared to females in 2005 and 2010 (
Table 6). The prevalence of anemia decreased significantly over the years in both males and females, especially between 2000 and 2010, and remained stable thereafter. In the 2000 and 2005 surveys, the prevalence of anemia was significantly higher in children whose mothers had no education (compared to secondary education) and no more statistically different thereafter. In contrast, the differences between rural and urban areas were significant only in 2010 and 2014, with the children living in rural areas being more at risk than those in urban areas. The differences between the poorest and the richest quintiles were significant whatever the survey. The poorest were the most at risk, with the prevalence of anemia decreasing from the poorest to the richest households.
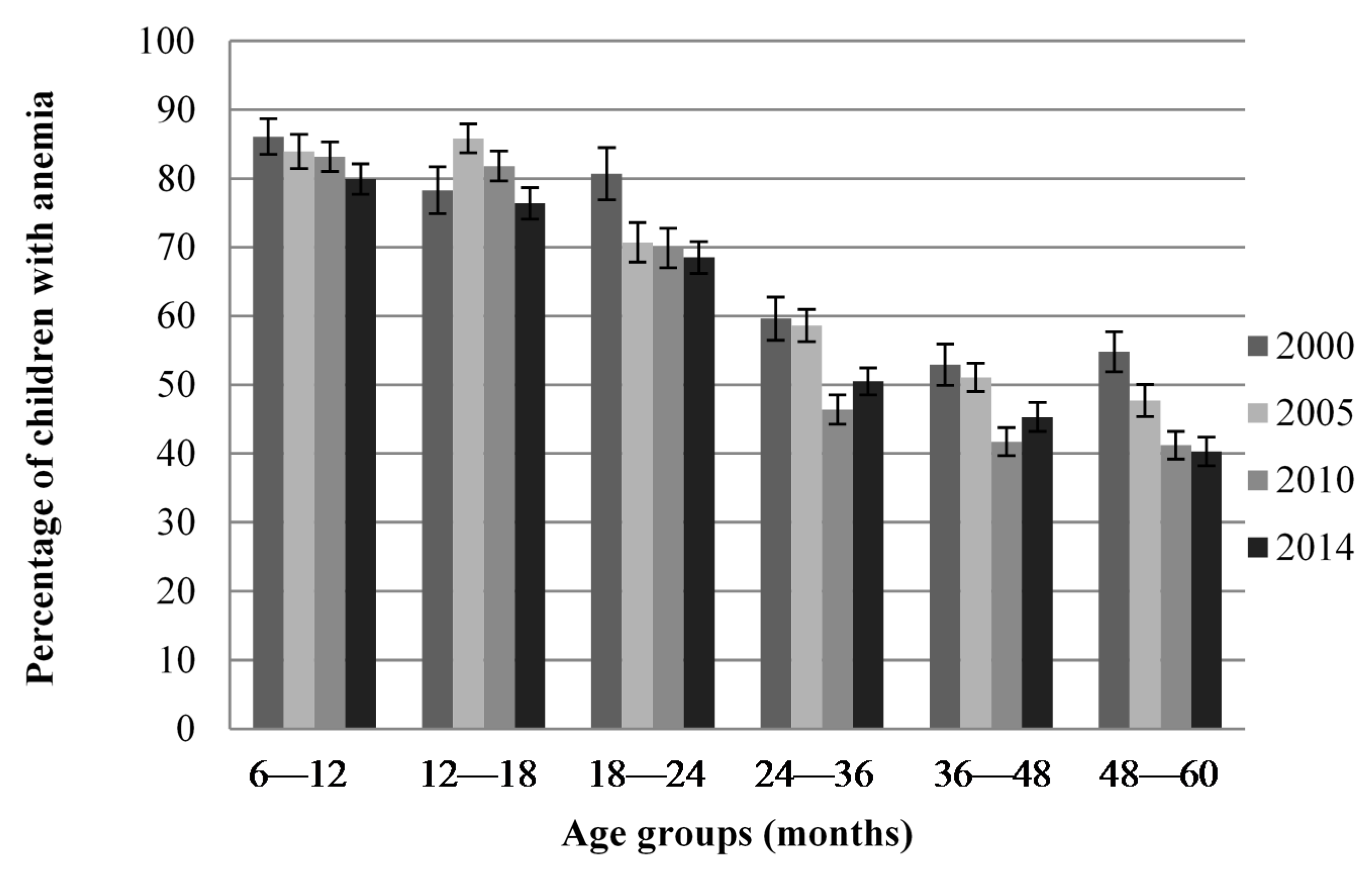
Figure 1 shows that anemia prevalence was higher in 6–18-month-old children compared to older children in all four surveys but was still a public health problem in all age groups and in all surveys. Data were also available for 0–6-month-old children in the 2000 survey and was 60.6% compared to 86.1% in 6–12-month-old children (data not shown).
The multivariate analysis indicated that, in the 2014 survey, the significant factors contributing to undernutrition,
i.e., stunting, wasting, and underweight, were birth weight, BMI of mothers, and wealth index, with the risk of being stunted, wasted, and underweight higher in children who had a low birth weight, a mother with low BMI, and the lowest category of wealth (
Table 7). Age was a contributing factor for stunting and underweight, with a higher risk in older children. Being younger, living in urban settings, and a higher BMI of mother were risk factors for overweight in children. Being younger, living in a rural area, having a mother with low BMI, and belonging to the poorest wealth quintile were associated with anemia. Wasting and stunting were risk factors for having anemia.
4. Discussion
Undernutrition was a public health problem in Cambodian children under five years of age in all four surveys conducted from 2000 to 2014. Stunting, underweight, and anemia were the most worrisome nutritional problems in both male and female children, affecting one third, one fourth, and more than half of children, respectively [
17]. The prevalence of these three nutritional problems decreased consistently over the 14-year period. Wasting also decreased from 2000 to 2010 but slightly increased again thereafter to affect one child in 10, thus still representing a mild health problem in 2014. Globally, undernutrition was higher in the poorest children, children living in rural areas and, except for wasting, in children with mothers with no education in all four surveys. Overweight prevalence was less than 10% and inequalities towards overweight between wealth quintiles and living areas appeared only recently, in 2010 and 2014, respectively.
Most of the low- and middle-income countries in Southeast Asia (SEA) suffer from stunting and its prevalence in Cambodia in the 2010 and 2014 surveys (39% and 33%, respectively) were in the range of other SEA countries—from 23.2% in Vietnam [
18] to 35% in Myanmar [
19] and 44% in Laos (44%) [
20]. In all surveys from 2000 to 2014, stunting prevalence was significantly higher in children living in rural areas and having a mother with no education, and had an inverse relationship with wealth quintiles.
The higher prevalence of stunting in the poorest wealth categories is in line with a recent analysis of the inequalities in child undernutrition in 80 countries [
8]. This underlines the inequality of access to economic development for Cambodian households, which probably results in inequality of access to an adequately nutritious diet for children during the critical window of the first two years of age. Indeed, access to a diversity of nutrient-rich foods is a key link in the relationship between higher income and lower prevalence of stunting [
21]. In addition, it is worth noting that, in the four surveys, the prevalence of stunting among the poor was approximately and consistently twice as high as among the richest. These findings indicate that the significant global decrease in stunting prevalence since 2000 was not accompanied by a narrowing of the wealth gap. However, in the 2014 survey stunting still affected about one fifth of the richest.
No decrease in inequality for living area toward stunting was observed since 2000 because stunting decreased in a similar trend in both rural and urban areas. Similar changes were observed in other SEA countries, where the height of children and stunting improved in both rural and urban areas from 1985 to 2011 [
6] but the poorest people living in rural areas remain the most at risk for undernutrition [
7,
21]. In the most recent Cambodian survey in 2014, the prevalence of stunting was 11% higher in rural areas but a multivariate model indicated that the living area was not significantly associated with stunting, suggesting that the differences in stunting prevalence between rural and urban areas were related to the difference in household wealth status in these two areas instead.
The oldest children were more at risk of being stunted, probably because growth retardation is a cumulative process that develops mainly during the two first years of age [
22]. Stunting prevalence was consistently lower in children with mothers having the highest level of education. This result is consistent with findings from cross-sectional studies carried out in different contexts [
23] and in Asia [
24]. In Cambodia, the inequalities toward stunting related to the mother’s education decreased significantly between 2005 and 2014, mainly due to a significant higher decrease of stunting prevalence in the category with no education, whereas no significant changes were observed in the category of higher education after 2005. These different trends of stunting according to mother’s education might explain why, in 2014, the multivariate model indicated that mothers’ education was not associated with stunting.
The prevalence of wasting decreased by half between 2000 and 2005 but remained a medium-high health problem thereafter, affecting approximately 10% of children in 2014, placing Cambodia in the scope of several SEA countries—where wasting prevalence ranges from 4.1% in Vietnam to 12.1% in Indonesia [
25]. In Cambodia, wasting was strongly related to the socioeconomic status of the household in 2005 and in 2014, with the poorest households exhibiting the highest prevalence. From 2000 to 2014 wasting prevalence was neither significantly different between boys and girls, nor between mothers with different education levels. Inequality between residence areas appeared only in 2014 and became significantly higher in rural areas. Multivariate analysis showed that, in 2014, the wealth index is the only socioeconomic factor significantly linked to wasting prevalence. The other contributing factors were linked to the nutritional status of mothers instead (low BMI of mothers and low birth weight). This high prevalence of wasting in Cambodia is of concern because wasting has a direct and immediate impact on the mortality risk in children and was recently shown to be a stronger predictor of mortality than stunting or underweight [
26]. The origin of wasting, often described as “acute malnutrition”, is reported to be a sudden and drastic lack of nutrients due to sickness and/or lack of food availability (including the hunger gap season, drought, flood, and displaced populations) [
27]. However, most of the risk factors for wasting are also associated with stunting, and wasting can also became a “chronic” problem deeply linked to stunting when these situations are frequent or accumulate [
28]. Thus these two forms of malnutrition share common causal factors, suggesting that some interventions could address both problems.
In Cambodia, the prevalence of underweight was approximately 10% less in each survey than the prevalence of stunting. This can be explained by the fact that some stunted children have a weight-for-height higher than the reference for their age. For instance, in the most recent survey in 2014, 7.7% of stunted children had weight-for-height
z-scores higher than 1 (3.5% higher than 2) so that 92% of these children were not underweight. A recent analysis noted that the positive and significant correlations between underweight and stunting observed in all regions of the world prove their close relationship even if these two indicators describe different physiological and biological processes [
26]. The authors indicate that even in regions with a high level of wasting, similar to the prevalence found in the four surveys in Cambodia, the correlation of underweight with stunting was consistent; and that in Asian sub-regions such as SEA, underweight accounted for more than 70% of stunting. It was thus not surprising that, in the four surveys, underweight mainly followed the same pattern of inequality as stunting, with the prevalence being significantly higher in the poorest children, those living in rural areas, and those with low education mothers.
The regression analysis carried out in the 2014 survey provided information on the causes of wasting, stunting, and underweight, showing similarities in their determinants. In addition to wealth status, low BMI of mothers was a contributing factor to undernutrition of children as well as low birth weight. Similar findings for wealth status and low BMI as explanatory factors for wasting and stunting have been showed in two studies in India [
27,
29], suggesting that intergenerational associations in wasting and stunting are not only driven by maternal intrauterine influences but also by undernutrition in the household. A previous study suggested that the reduction of child stunting in Cambodia was mainly linked to improvements in household wealth, sanitation, parental education, birth spacing, and reduction of maternal tobacco use [
30]. It has been demonstrated that direct interventions such as improving complementary foods and feeding practices of infants, micronutrient supplementation and fortification, and reduction of disease burden can decrease the prevalence of undernutrition [
31,
32] but that improvement of underlying determinants, such as poverty, poor education, disease burden, and lack of women’s empowerment also have to be improved to eliminate malnutrition in the long term [
33]. Regarding results for Cambodia, improving wealth status of households and of mothers, as well as nutritional status and empowerment of women, would be the keys to preventing stunting and wasting by diminishing the prevalence of small-for-gestational-age infants. Furthermore, dietary diversity and consumption of animal products were shown to be protective factors against stunting in Cambodia [
34]. Thus investing in agricultural programs to increase the availability and accessibility of nutrient-rich food in Cambodia for all population groups but especially for adolescent girls and women before and during pregnancy would have a positive impact on child nutritional status.
The prevalence of overweight was of concern in all surveys, and after a decrease between 2000 and 2005 remained stable around 7% thereafter. These findings are in line with data from the SEA region. In Vietnam, two recent surveys indicated a prevalence of about 7% in children under five [
18,
35], while a study in Indonesia reported a prevalence of 6.2% in urban settings and 3.2% in rural settings for children aged six months to two years [
36]. In Thailand in 2011, 4.2% of children aged six months to three years in urban areas and 7.1% in rural areas were overweight or obese [
37], and a study in Malaysia indicated 8% of overweight or obesity in children 0–13 years old [
38].
However, it is worth noting that, in Cambodia, the prevalence of overweight did not increase over the last 10 years, contrary to what is observed in many countries over the world, even in developing countries [
4,
39]. Recently, Black
et al. [
4] reported a 54% increase in global overweight prevalence in children from 1990 to 2011 and considered that this trend of increase is expected to continue in most parts of the word, even if their projections for 2025 suggest the plausibility of either an increase or a decrease in overweight prevalence in Asia.
It is interesting to note that whereas the global prevalence of overweight did not increase over the last 15 years, inequalities for overweight between socioeconomic subgroups appeared in 2014, with a higher prevalence in the richest wealth group compared to other wealth groups. Moreover, the prevalence of OW in 2014 was higher in boys than in girls. We also observed a widening of inequalities between rural and urban areas, with a constant increase of overweight in urban areas from 2005. Thus, in 2014, the urban prevalence of overweight was double that in 2005. Furthermore, in the 2014 survey, overweight was significantly related to low age, mother with higher BMI, and residence in urban settings. This link between maternal high BMI and children’s overweight was already reported by several authors and maternal obesity is identified as one of the strongest risk factors for child obesity, since the child’s eating habits resemble those of the family diet patterns [
40,
41]. The level of education of the mother was not related to child overweight whereas overweight in women was related to their low education level [
42].
These results suggest that effective strategies to prevent and control overweight and obesity in children should take into account the fact that prevalence increases more quickly in urban areas and in the richest populations and that programs improving household feeding practices in the most-at-risk populations should benefit both mothers and their offspring.
Anemia represented a severe public health problem in Cambodia without any significant improvement since 2000; in 2014 55% of children were still anemic. Anemia affected the youngest children more, from six months to two years old, during the critical period of immunity development and mutation from breastmilk feeding to family-like feeding. This strong link between age and anemia prevalence was confirmed in the multivariate model of 2014, which indicated that anemia most affected the poorest people and those living in rural areas. The gap between rural and urban areas widened after 2005, while anemia inequality for wealth did not change significantly. In contrast, the inequalities that existed in the different mother education subgroups disappeared from 2010. The failure to significantly decrease anemia prevalence over the years called into question the main possible causes of anemia in Cambodia. Micronutrient deficiencies and especially iron deficiency, as well as vitamin A and vitamin B12 deficiencies, are one of the most commonly encountered causes of anemia in the world. The Cambodian DHS reported that in 2014 only 3%, 11%, and 11% of children had iron deficiency, and vitamin A and B12 deficiencies, respectively. However, the prevalence of these micronutrient deficiencies followed the same picture as that for anemia, with children between six and 24 months of age being affected more than the older ones. Furthermore, anemia was associated with stunting and wasting. These findings suggest that anemia partly reflected the general undernutrition of children, especially in the first two years of age, and probably also the poor quality and quantity of diet including complementary feeding practices. Nevertheless, these results also suggest that other causes such as the high prevalence of hemoglobinopathies in this country, which have been indicated in several studies [
43,
44], might also contribute to anemia.