Effects of Arginine Supplementation on Amino Acid Profiles in Blood and Tissues in Fed and Overnight-Fasted Rats
Abstract
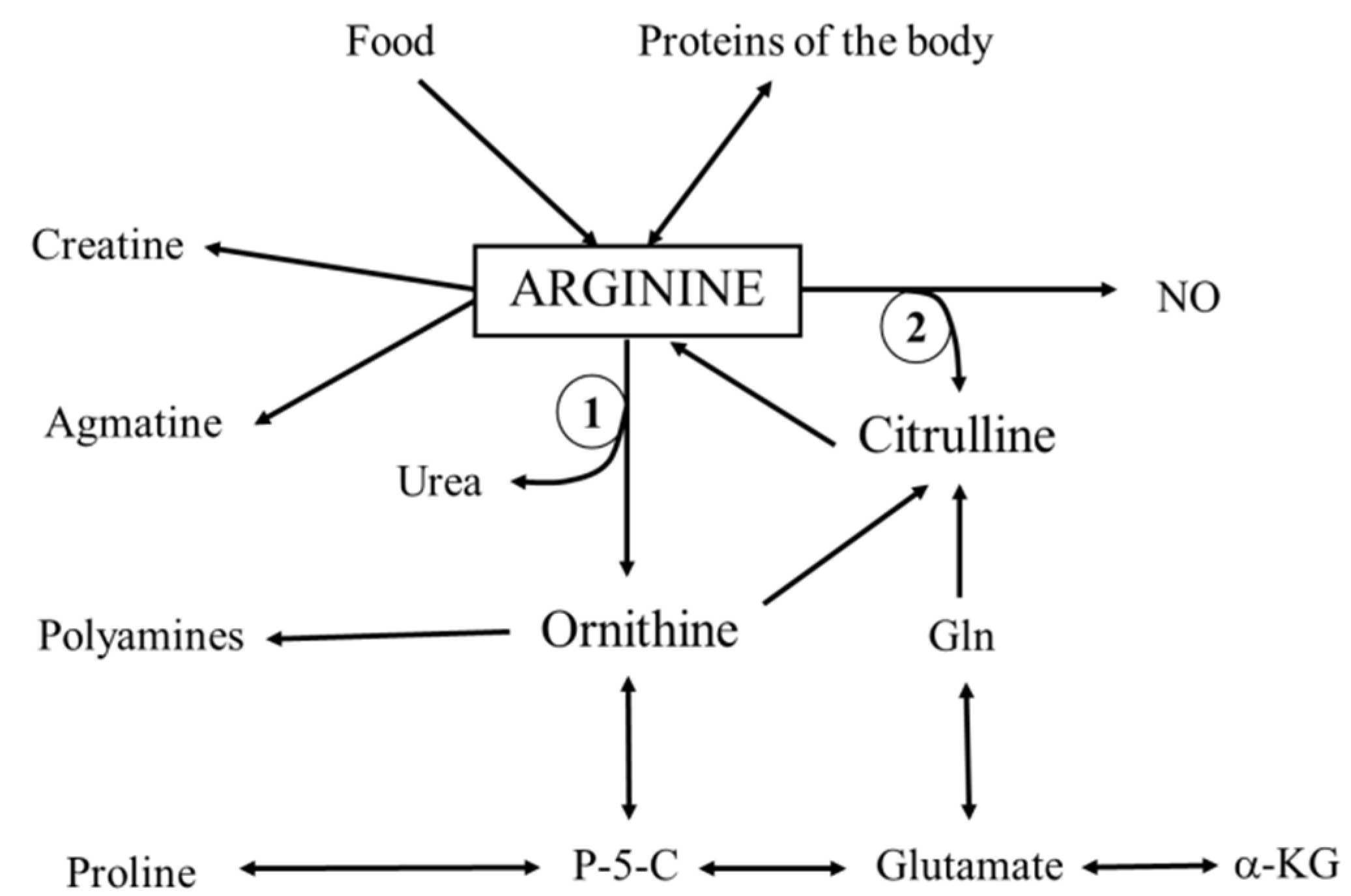
:1. Introduction
2. Materials and Methods
2.1. Animals and Materials
2.2. Experimental Design
2.3. Amino Acid Concentrations in Blood Plasma and Tissues
2.4. Other Techniques
2.5. Statistical Analyses
3. Results
3.1. Alterations in Food Intake, Body Weight Gain, and Weight and Protein Content of Tissues
3.2. Alterations in Blood Plasma
3.3. Alterations in Tissues
4. Discussion
4.1. Alterations in Arginine Levels
4.2. Alterations in Arginine Metabolites
4.3. Alterations in Other Amino Acids
4.4. Other Alterations Induced by HAD
4.5. Effect of Overnight Starvation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SLD | standard laboratory diet |
| HAD | high-arginine diet |
| EDL | extensor digitorum longus muscle |
| SOL | soleus muscle |
| EAA | essential amino acids |
| NEAA | non-essential amino acids |
References
- Morris, S.M. Arginine: Beyond protein. Am. J. Clin. Nutr. 2006, 83, 508S–512S. [Google Scholar]
- Das, U.N.; Repossi, G.; Dain, A.; Eynard, A.R. l-arginine, NO and asymmetrical dimethylarginine in hypertension and type 2 diabetes. Front. Biosci. 2011, 16, 13–20. [Google Scholar] [CrossRef]
- Zhou, M.; Martindale, R.G. Arginine in the critical care setting. J. Nutr. 2007, 137, 1687S–1692S. [Google Scholar] [PubMed]
- Barbul, A.; Sisto, D.A.; Wasserkrug, H.L.; Efron, G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery 1981, 90, 244–251. [Google Scholar] [PubMed]
- Evans, R.W.; Fernstrom, J.D.; Thompson, J.; Morris, S.M.; Kuller, L.H. Biochemical responses of healthy subjects during dietary supplementation with l-arginine. J. Nutr. Biochem. 2004, 15, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Fürst, P.; Norée, L.O.; Vinnars, E. Intracellular free amino acid concentration in human muscle tissue. J. Appl. Physiol. 1974, 36, 693–697. [Google Scholar] [PubMed]
- Graham, J.A.; Lamb, J.F.; Linton, A.L. Measurement of body water and intracellular electrolytes by means of muscle biopsy. Lancet 1976, 2, 1172–1176. [Google Scholar] [CrossRef]
- Xiong, Y.; Fru, M.F.; Yu, Y.; Montani, J.P.; Ming, X.F.; Yang, Z. Long term exposure to l-arginine accelerates endothelial cell senescence through arginase-II and S6K1 signaling. Aging 2014, 6, 369–379. [Google Scholar] [CrossRef]
- Rabier, D.; Kamoun, P. Metabolism of citrulline in man. Amino Acids 1995, 9, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meininger, C.J.; Hawker, J.R.; Haynes, T.E.; Kepka-Lenhart, D.; Mistry, S.K.; Morris, S.M.; Wu, G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E75–E82. [Google Scholar] [PubMed]
- Holecek, M.; Kovarik, M. Alterations in protein metabolism and amino acid concentrations in rats fed by a high-protein (casein-enriched) diet—Effect of starvation. Food Chem. Toxicol. 2011, 49, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 1990, 70, 43–77. [Google Scholar] [PubMed]
- Kovarik, M.; Muthny, T.; Sispera, L.; Holecek, M. The dose-dependent effects of endotoxin on protein metabolism in two types of rat skeletal muscle. J. Physiol. Biochem. 2012, 68, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Sispera, L. Glutamine deficiency in extracellular fluid exerts adverse effects on protein and amino acid metabolism in skeletal muscle of healthy, laparotomized, and septic rats. Amino Acids 2014, 46, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Kandar, R.; Sispera, L.; Kovarik, M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: Different sensitivity of red and white muscle. Amino Acids 2011, 40, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Sispera, L.; Skalska, H. Enhanced glutamine availability exerts different effects on protein and amino acid metabolism in skeletal muscle from healthy and septic rats. J. Parenter. Enter. Nutr. 2015, 39, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Flodin, N.W. The metabolic roles, pharmacology, and toxicology of lysine. J. Am. Coll. Nutr. 1997, 16, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Fürst, P.; Vinnars, E. Effect of a test meal, without and with protein, on muscle and plasma free amino acids. Clin. Sci. 1990, 79, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A.; Gennari, F.J. Life-threatening hyperkalemia induced by arginine. Ann. Intern. Med. 1978, 89, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.L.; Klaff, L.J.; Levitt, N.S.; Ling, N.; Millar, R.P. Arginine hydrochloride stimulation of serum potassium and aldosterone is enhanced by somatostatin-28. Acta Endocrinol. 1984, 105, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Whang, R.; Papper, S.; Llach, F. Arginine-induced hypermagnesemia and hyperkalemia in nephrectomized rats. Magnesium 1988, 7, 23–26. [Google Scholar] [PubMed]
- Schulze, F.; Glos, S.; Petruschka, D.; Altenburg, C.; Maas, R.; Benndorf, R.; Schwedhelm, E.; Beil, U.; Böger, R.H. l-Arginine enhances the triglyceride-lowering effect of simvastatin in patients with elevated plasma triglycerides. Nutr. Res. 2009, 29, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Z.; Jia, S.; Dahanayaka, S.; Feng, S.; Meininger, C.J.; McNeal, C.J.; Wu, G. Safety of long-term dietary supplementation with l-arginine in rats. Amino Acids 2015, 47, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
| SLD (n = 20) | HAD (n = 20) | |
|---|---|---|
| Body weight (g) | ||
| initial | 198 ± 4 | 200 ± 5 |
| 1 week | 257 ± 6 | 247 ± 6 |
| 6 weeks | 450 ± 10 | 438 ± 10 |
| 70 days | 525 ± 12 | 498 ± 12 |
| Food intake (g/kg b.w./day) | ||
| 1st week | 116 ± 4 | 122 ± 4 |
| 6th week | 74 ± 1 | 73 ± 1 |
| last week | 64 ± 2 | 66 ± 1 |
| Fed Animals | Overnight-Starved Animals | |||
|---|---|---|---|---|
| SLD (n = 10) | HAD (n = 10) | SLD + S (n = 10) | HAD + S (n = 10) | |
| Liver | ||||
| weight (g/kg b.w.) | 32.42 ± 0.63 | 33.34 ± 1.16 | 23.13 ± 0.42 | 23.66 ± 0.44 |
| protein (mg/g wet t.w.) | 160 ± 9 | 147 ± 4 | 168 ± 4 | 156 ± 3 |
| protein (g/kg b.w.) | 5.17 ± 0.29 | 4.88 ± 0.18 | 3.88 ± 0.11 | 3.70 ± 0.10 |
| Kidney | ||||
| weight (g/kg b.w.) | 2.91 ± 0.06 | 3.45 ± 0.07 * | 2.85 ± 0.09 | 3.22 ± 0.08 * |
| protein (mg/g wet t.w.) | 128 ± 2 | 125 ± 4 | 126 ± 3 | 115 ± 3 |
| protein (g/kg b.w.) | 0.37 ± 0.01 | 0.43 ± 0.01 * | 0.36 ± 0.01 | 0.37 ± 0.01 # |
| EDL | ||||
| weight (g/kg b.w.) | 0.43 ± 0.01 | 0.41 ± 0.01 | 0.43 ± 0.01 | 0.45 ± 0.01 |
| protein (mg/g wet t.w.) | 150 ± 3 | 146 ± 3 | 150 ± 3 | 145 ± 4 |
| protein (g/kg b.w.) | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 |
| SOL | ||||
| weight (g/kg b.w.) | 0.46 ± 0.01 | 0.46 ± 0.01 | 0.48 ± 0.01 | 0.50 ± 0.01 |
| protein (mg/g wet t.w.) | 127 ± 4 | 128 ± 4 | 129 ± 3 | 118 ± 2 |
| protein (g/kg b.w.) | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 |
| Fed Animals | Overnight-Starved Animals | |||
|---|---|---|---|---|
| SLD (n = 10) | HAD (n = 10) | SLD + S (n = 10) | HAD + S (n = 10) | |
| Glucose (mmol/L) | 10.7 ± 0.2 | 10.2 ± 0.2 | 9.1 ± 0.3 # | 9.9 ± 0.1 * |
| Urea (mmol/L) | 7.1 ± 0.2 | 10.4 ± 0.4 * | 6.6 ± 0.2 | 7.1 ± 0.2 # |
| Creatinine (µmol/L) | 27.6 ± 0.8 | 33.1 ± 0.9 * | 31.8 ± 1.5 # | 30.2 ± 1.1 |
| Sodium (mmol/L) | 142.3 ± 0.5 | 141.6 ± 0.4 | 143.1 ± 0.3 | 142.5 ± 0.2 |
| Potassium (mmol/L) | 4.4 ± 0.1 | 3.8 ± 0.1 * | 3.8 ± 0.1 # | 3.8 ± 0.1 |
| Chloride (mmol/L) | 100.9 ± 0.6 | 102.1 ± 0.5 | 103.2 ± 0.3 # | 102.3 ± 0.5 |
| ALT (µkat/L) | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.6 ± 0.1 # | 0.7 ± 0.0 |
| AST (µkat/L) | 1.3 ± 0.1 | 1.5 ± 0.1 | 1.2 ± 0.0 | 1.2 ± 0.0 # |
| Cholesterol (mmol/L) | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| HDL cholesterol (mmol/L) | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.0 |
| LDL cholesterol (mmol/L) | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 # |
| Atherogenicity index | 0.7 ± 0.0 | 0.5 ± 0.0 * | 0.3 ± 0.0 # | 0.3 ± 0.0 # |
| Triglycerides (mmol/L) | 1.6 ± 0.2 | 1.2 ± 0.1 * | 1.0 ± 0.2 # | 0.8 ± 0.1 # |
| Total protein (g/L) | 63.7 ± 0.8 | 62.6 ± 0.5 | 61.1 ± 0.5 # | 62.5 ± 0.6 |
| Albumin (g/L) | 38.6 ± 0.9 | 40.1 ± 0.7 | 39.5 ± 0.5 | 40.4 ± 0.3 |
| Fed Animals | Overnight-Starved Animals | |||
|---|---|---|---|---|
| Plasma | SLD (n = 10) | HAD (n = 10) | SLD + S (n = 10) | HAD + S (n = 10) |
| Essential amino acids | ||||
| Histidine | 63 ± 1 | 54 ± 2 * | 54 ± 2 # | 55 ± 1 |
| Isoleucine | 84 ± 2 | 81 ± 3 | 93 ± 2 # | 89 ± 3 |
| Leucine | 148 ± 5 | 141 ± 5 | 149 ± 6 | 152 ± 4 |
| Lysine | 293 ± 8 | 257 ± 7 * | 308 ± 8 | 289 ± 10 # |
| Methionine | 53 ± 1 | 41 ± 1 * | 48 ± 1 # | 48 ± 2 # |
| Phenylalanine | 66 ± 2 | 55 ± 2 * | 65 ± 2 | 65 ± 1 # |
| Threonine | 248 ± 7 | 123 ± 5 * | 234 ± 8 | 216 ± 7 # |
| Valine | 189 ± 5 | 166 ± 5 * | 179 ± 5 | 182 ± 5 |
| ∑ EAA | 1143 ± 21 | 917 ± 26 * | 1130 ± 24 | 1097 ± 27 |
| Non-essential amino acids | ||||
| Alanine | 507 ± 13 | 495 ± 23 | 387 ± 16 # | 377 ± 16 # |
| Asparagine | 59 ± 2 | 45 ± 3 * | 58 ± 1 | 55 ± 2 # |
| Aspartate | 28 ± 21 | 17 ± 1 * | 15 ± 1 # | 12 ± 1 # |
| Glutamine | 671 ± 16 | 624 ± 16 | 620 ± 15 | 544 ± 13 * |
| Glycine | 297 ± 7 | 161 ± 7 * | 364 ± 13 # | 244 ± 7 *,# |
| Serine | 252 ± 9 | 155 ± 5 * | 230 ± 6 | 198 ± 5 *,# |
| Taurine | 570 ± 40 | 181 ± 15 * | 241 ± 15 # | 192 ± 8 |
| Tyrosine | 85 ± 2 | 57 ± 3 * | 81 ± 3 | 64 ± 3 |
| Arginine and its metabolites | ||||
| Arginine | 173 ± 5 | 387 ± 27 * | 151 ± 7 | 132 ± 5 # |
| Citrulline | 71 ± 3 | 73 ± 2 | 70 ± 2 | 54 ± 2 *,# |
| Glutamate | 123 ± 8 | 112 ± 7 | 104 ± 5 | 96 ± 5 |
| Ornithine | 53 ± 3 | 132 ± 25 * | 41 ± 1 | 30 ± 1 # |
| Proline | 229 ± 15 | 180 ± 15 * | 133 ± 3 # | 117 ± 4 # |
| ∑ NEAA-Arg | 2883 ± 78 | 2223 ± 62 * | 2345 ± 39 # | 1984 ± 46 *,# |
| ∑ AA-Arg | 4026 ± 95 | 3150 ± 78 * | 3475 ± 58 # | 3081 ± 69 * |
| Fed Animals | Overnight-Starved Animals | |||
|---|---|---|---|---|
| Liver | SLD (n = 10) | HAD (n = 10) | SLD + S (n = 10) | HAD + S (n = 10) |
| Essential amino acids | ||||
| Histidine | 1688 ± 41 | 1486 ± 29 * | 1424 ± 43 # | 1390 ± 20 |
| Isoleucine | 379 ± 14 | 342 ± 19 | 386 ± 12 | 359 ± 16 |
| Leucine | 652 ± 42 | 754 ± 109 | 619 ± 21 | 646 ± 28 |
| Lysine | 853 ± 45 | 885 ± 50 | 1107 ± 40 # | 1132 ± 53 # |
| Methionine | 103 ± 5 | 92 ± 6 | 97 ± 8 | 83 ± 3 |
| Phenylalanine | 237 ± 9 | 214 ± 15 | 238 ± 10 | 227 ± 11 |
| Threonine | 894 ± 61 | 472 ± 23 * | 970 ± 73 | 775 ± 47 *,# |
| Valine | 614 ± 38 | 500 ± 27 * | 561 ± 19 | 561 ± 26 |
| ∑ EAA | 5420 ± 178 | 4746 ± 238 * | 5401 ± 136 | 5173 ± 157 |
| Non-essential amino acids | ||||
| Alanine | 7860 ± 326 | 7786 ± 373 | 3871 ± 351 # | 5073 ± 329 # |
| Asparagine | 215 ± 8 | 180 ± 9 * | 199 ± 11 | 174 ± 9 |
| Aspartate | 1740 ± 84 | 1477 ± 71 | 2014 ± 85 | 1827 ± 80 # |
| Glutamine | 13,485 ± 487 | 12,790 ± 474 | 13,693 ± 476 | 12,027 ± 365 * |
| Glycine | 6602 ± 249 | 4270 ± 174 * | 7977 ± 327 # | 6840 ± 158 *,# |
| Serine | 1436 ± 126 | 656 ± 36 * | 1170 ± 107 | 747 ± 59 * |
| Taurine | 9388 ± 787 | 11,127 ± 865 | 10,420 ± 508 | 12,095 ± 896 |
| Tyrosine | 216 ± 12 | 185 ± 15 | 221 ± 13 | 186 ± 10 |
| Arginine and its metabolites | ||||
| Arginine | 48 ± 4 | 42 ± 6 | 20 ± 4 # | 46 ± 4 * |
| Citrulline | 69 ± 4 | 78 ± 5 | 83 ± 6 | 71 ± 7 |
| Glutamate | 4234 ± 18 | 4893 ± 239 * | 3879 ± 119 | 3599 ± 83 # |
| Ornithine | 810 ± 58 | 1010 ± 130 * | 804 ± 33 | 638 ± 47 # |
| Proline | 387 ± 25 | 312 ± 14 * | 325 ± 10 | 292 ± 21 |
| ∑ NEAA-Arg | 46,441 ± 791 | 44,807 ± 1022 | 44,656 ± 818 | 43,568 ± 812 |
| ∑ AA-Arg | 51,862 ± 909 | 49,511 ± 1184 | 50,058 ± 840 | 48,741 ± 859 |
| Fed Animals | Overnight-Starved Animals | |||
|---|---|---|---|---|
| Kidney | SLD (n = 10) | HAD (n = 10) | SLD + S (n = 10) | HAD + S (n = 10) |
| Essential amino acids | ||||
| Histidine | 462 ± 22 | 349 ± 12 * | 391 ± 15 # | 403 ± 20 |
| Isoleucine | 280 ± 14 | 254 ± 10 | 270 ± 13 | 261 ± 11 |
| Leucine | 529 ± 32 | 454 ± 20 | 493 ± 26 | 451 ± 17 |
| Lysine | 765 ± 31 | 636 ± 25 * | 737 ± 37 | 730 ± 21 |
| Methionine | 110 ± 5 | 86 ± 5 * | 102 ± 6 | 102 ± 6 |
| Phenylalanine | 232 ± 13 | 181 ± 8 * | 209 ± 10 | 188 ± 7 |
| Threonine | 1395 ± 83 | 799 ± 30 * | 1222 ± 56 | 1232 ± 37 # |
| Valine | 554 ± 28 | 453 ± 22 * | 474 ± 25 | 481 ± 19 |
| ∑ EAA | 4326 ± 200 | 3214 ± 114 * | 3898 ± 157 | 3849 ± 119 # |
| Non-essential amino acids | ||||
| Alanine | 2198 ± 107 | 2019 ± 78 | 1653 ± 59 # | 1848 ± 46 |
| Asparagine | 381 ± 16 | 278 ± 14 * | 331 ± 12 # | 313 ± 8 |
| Aspartate | 3819 ± 184 | 2644 ± 119 * | 3184 ± 151 # | 3333 ± 128 # |
| Glutamine | 3609 ± 210 | 3370 ± 123 | 2932 ± 179 # | 2674 ± 102 # |
| Glycine | 7270 ± 217 | 4587 ± 170 * | 7179 ± 384 | 6086 ± 193 *,# |
| Serine | 2057 ± 83 | 1374 ± 38 * | 1961 ± 83 | 2073 ± 92 # |
| Taurine | 33,328 ± 599 | 30,228 ± 1426 | 31,425 ± 658 | 32,065 ± 752 |
| Tyrosine | 367 ± 17 | 263 ± 14 * | 314 ± 16 | 295 ± 21 |
| Arginine and its metabolites | ||||
| Arginine | 455 ± 15 | 663 ± 21 * | 386 ± 19 | 430 ± 37 # |
| Citrulline | 61 ± 8 | 117 ± 7 * | 55 ± 6 | 128 ± 8 * |
| Glutamate | 16,320 ± 622 | 14,244 ± 712 * | 13,971 ± 411 # | 14,388 ± 286 |
| Ornithine | 126 ± 19 | 214 ± 16 * | 89 ± 6 | 107 ± 11 # |
| Proline | 546 ± 56 | 395 ± 20 * | 350 ± 15 # | 346 ± 17 |
| ∑ NEAA-Arg | 70,082 ± 1413 | 59,732 ± 1517 * | 63,444 ± 1028 # | 63,656 ± 1226 |
| ∑ AA-Arg | 74,408 ± 1588 | 62,946 ± 1578 * | 67,342 ± 1144 # | 67,505 ± 1331 |
| Fed Animals | Overnight-Starved Animals | |||
|---|---|---|---|---|
| EDL | SLD (n = 10) | HAD (n = 10) | SLD + S (n = 10) | HAD + S (n = 10) |
| Essential amino acids | ||||
| Histidine | 373 ± 17 | 391 ± 11 | 288 ± 11 # | 315 ± 9 # |
| Isoleucine | 134 ± 5 | 153 ± 4 * | 162 ± 7 # | 174 ± 5 # |
| Leucine | 223 ± 6 | 223 ± 8 | 256 ± 13 # | 281 ± 7 # |
| Lysine | 782 ± 61 | 1034 ± 58 * | 635 ± 35 | 657 ± 29 # |
| Methionine | 87 ± 3 | 63 ± 2 * | 83 ± 4 | 86 ± 3 # |
| Phenylalanine | 123 ± 3 | 108 ± 4 * | 122 ± 4 | 136 ± 3 *,# |
| Threonine | 993 ± 31 | 602 ± 19 * | 882 ± 34 # | 914 ± 15 # |
| Valine | 293 ± 10 | 290 ± 7 | 293 ± 13 | 335 ± 8 *,# |
| ∑ EAA | 3008 ± 109 | 2865 ± 84 | 2721 ± 102 | 2898 ± 50 |
| Non-essential amino acids | ||||
| Alanine | 3727 ± 143 | 4580 ± 118 * | 3556 ± 158 | 3915 ± 112 # |
| Asparagine | 337 ± 18 | 279 ± 22 * | 367 ± 13 | 402 ± 6 # |
| Aspartate | 524 ± 22 | 680 ± 20 * | 754 ± 30 # | 960 ± 62 *,# |
| Glutamine | 7538 ± 247 | 7362 ± 303 | 6424 ± 290 # | 5824 ± 221 # |
| Glycine | 4240 ± 300 | 2928 ± 99 * | 4362 ± 346 | 4052 ± 246 # |
| Serine | 1497 ± 76 | 943 ± 31 * | 1217 ± 37 # | 1168 ± 17 # |
| Taurine | 27,014 ± 467 | 21,951 ± 645 * | 26,070 ± 597 | 24,280 ± 531 # |
| Tyrosine | 201 ± 6 | 150 ± 8 * | 189 ± 7 | 175 ± 6 # |
| Arginine and its metabolites | ||||
| Arginine | 534 ± 34 | 2018 ± 195 * | 393 ± 26 | 405 ± 17 # |
| Citrulline | 362 ± 12 | 416 ± 16 * | 351 ± 14 | 297 ± 7 *,# |
| Glutamate | 2536 ± 62 | 3066 ± 63 * | 2493 ± 205 | 2757 ± 176 |
| Ornithine | 78 ± 6 | 314 ± 75 * | 49 ± 3 | 44 ± 2 # |
| Proline | 569 ± 40 | 584 ± 43 | 343 ± 12 # | 381 ± 7 # |
| ∑ NEAA-Arg | 48,623 ± 783 | 43,253 ± 840 * | 46,175 ± 917 | 44,253 ± 654 |
| ∑ AA-Arg | 51,631 ± 856 | 46,117 ± 904 * | 48,896 ± 990 | 47,151 ± 687 |
| Fed Animals | Overnight-Starved Animals | |||
|---|---|---|---|---|
| SOL | SLD (n = 10) | HAD (n = 10) | SLD + S (n = 10) | HAD + S (n = 10) |
| Essential amino acids | ||||
| Histidine | 759 ± 40 | 748 ± 28 | 655 ± 30 | 779 ± 43 |
| Isoleucine | 106 ± 5 | 120 ± 5 | 122 ± 4 # | 130 ± 4 |
| Leucine | 175 ± 8 | 172 ± 7 | 192 ± 6 | 209 ± 6 # |
| Lysine | 1327 ± 73 | 1704 ± 115 * | 1562 ± 99 | 2012 ± 130 * |
| Methionine | 74 ± 3 | 56 ± 2 * | 68 ± 2 | 68 ± 2 # |
| Phenylalanine | 107 ± 4 | 92 ± 3 * | 104 ± 2 | 113 ± 4 # |
| Threonine | 926 ± 19 | 607 ± 17 * | 918 ± 36 | 1047 ± 34 *,# |
| Valine | 228 ± 9 | 214 ± 8 | 222 ± 9 | 250 ± 5 # |
| ∑ EAA | 3701 ± 108 | 3713 ± 136 | 3843 ± 143 | 4608 ± 179 *,# |
| Non-essential amino acids | ||||
| Alanine | 3298 ± 129 | 3964 ± 167 * | 3517 ± 215 | 3887 ± 214 |
| Asparagine | 636 ± 30 | 517 ± 36 * | 743 ± 23 | 879 ± 42 *,# |
| Aspartate | 2114 ± 213 | 2764 ± 285 | 3797 ± 193 # | 3351 ± 224 |
| Glutamine | 11,552 ± 464 | 11,408 ± 378 | 10,861 ± 403 | 11,224 ± 416 |
| Glycine | 2764 ± 88 | 2252 ± 50 * | 3400 ± 92 # | 3293 ± 90 # |
| Serine | 2904 ± 127 | 1673 ± 56 * | 2631 ± 104 | 2571 ± 108 # |
| Taurine | 33,561 ± 633 | 31,283 ± 467 | 32,960 ± 844 | 33,547 ± 659 |
| Tyrosine | 157 ± 5 | 111 ± 5 * | 148 ± 6 | 132 ± 6 # |
| Arginine and its metabolites | ||||
| Arginine | 940 ± 53 | 3739 ± 186 * | 871 ± 56 | 1006 ± 62 # |
| Citrulline | 586 ± 36 | 657 ± 22 | 646 ± 18 | 619 ± 34 |
| Glutamate | 5550 ± 121 | 6095 ± 137 | 6248 ± 221 | 6182 ± 286 |
| Ornithine | 128 ± 7 | 405 ± 69 * | 95 ± 5 | 88 ± 4 # |
| Proline | 676 ± 49 | 724 ± 54 | 378 ± 9 # | 389 ± 12 # |
| ∑ NEAA-Arg | 63,926 ± 1385 | 61,853 ± 1046 | 65,423 ± 1435 | 66,108 ± 1619 |
| ∑ AA-Arg | 67,628 ± 1455 | 65,566 ± 1102 | 69,266 ± 1549 | 70,716 ± 1736 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holecek, M.; Sispera, L. Effects of Arginine Supplementation on Amino Acid Profiles in Blood and Tissues in Fed and Overnight-Fasted Rats. Nutrients 2016, 8, 206. https://doi.org/10.3390/nu8040206
Holecek M, Sispera L. Effects of Arginine Supplementation on Amino Acid Profiles in Blood and Tissues in Fed and Overnight-Fasted Rats. Nutrients. 2016; 8(4):206. https://doi.org/10.3390/nu8040206
Chicago/Turabian StyleHolecek, Milan, and Ludek Sispera. 2016. "Effects of Arginine Supplementation on Amino Acid Profiles in Blood and Tissues in Fed and Overnight-Fasted Rats" Nutrients 8, no. 4: 206. https://doi.org/10.3390/nu8040206
APA StyleHolecek, M., & Sispera, L. (2016). Effects of Arginine Supplementation on Amino Acid Profiles in Blood and Tissues in Fed and Overnight-Fasted Rats. Nutrients, 8(4), 206. https://doi.org/10.3390/nu8040206





