Effects of Trigonelline, an Alkaloid Present in Coffee, on Diabetes-Induced Disorders in the Rat Skeletal System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
- Control rats,
- Streptozotocin-treated control rats,
- Streptozotocin-treated rats receiving trigonelline (50 mg/kg p.o. daily),
- Nicotinamide/streptozotocin-treated control rats, and
- Nicotinamide/streptozotocin-treated rats receiving trigonelline (50 mg/kg p.o. daily).
2.2. Biochemical Studies
2.3. Bone Mechanical Properties Studies
2.4. Bone Mineralization Studies
2.5. Statistical Analysis
3. Results
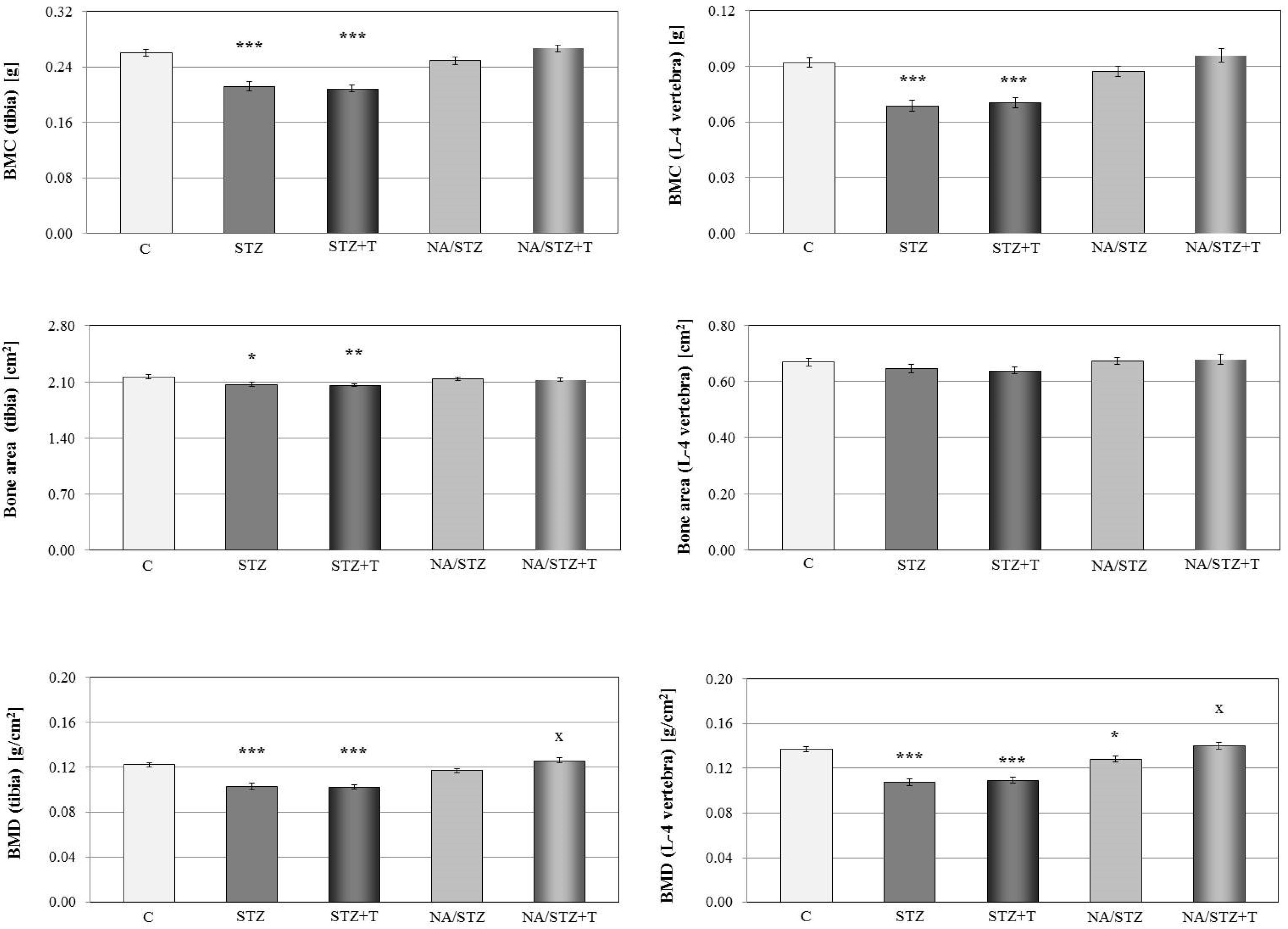
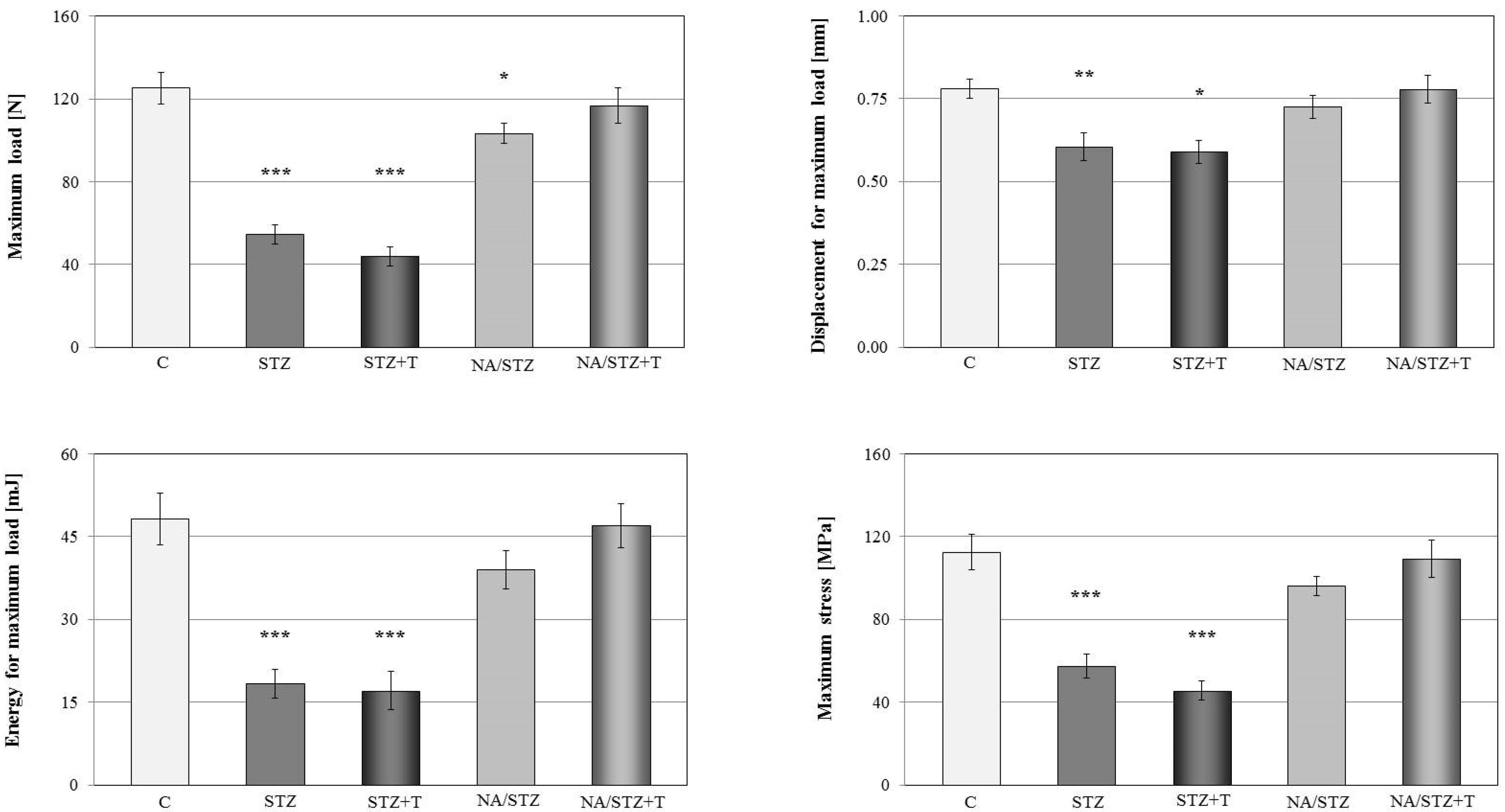
3.1. Skeletal Changes in Streptozotocin-Treated Rats
3.2. Effect of Trigonelline on the Skeletal System of Rats with Streptozotocin-Induced Diabetes
3.3. Skeletal Changes in Nicotinamide/Streptozotocin-Treated Rats
3.4. Effect of Trigonelline on the Skeletal System of Rats Treated with Nicotinamide and Streptozotocin
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar]
- Yan, W.; Li, X. Impact of diabetes and its treatments on skeletal diseases. Front. Med. 2013, 7, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Starup-Linde, J.; Vestergaard, P. Management of endocrine disease: Diabetes and osteoporosis: Cause for concern? Eur. J. Endocrinol. 2015, 173, R93–R99. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.S.; Graham, T.E. Methylxanthines and human health: Epidemiological and experimental evidence. Handb. Exp. Pharmacol. 2011, 200, 509–548. [Google Scholar] [PubMed]
- Jiang, X.; Zhang, D.; Jiang, W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Lee, J.; Rota, M.; Lee, J.; Ahn, H.S.; Park, S.M.; Shin, D. Coffee consumption and risk of fractures: A systematic review and dose-response meta-analysis. Bone 2014, 63, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dai, Z.; Wu, Q. Effect of coffee intake on hip fracture: A meta-analysis of prospective cohort studies. Nutr. J. 2015, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Donangelo, C.M.; Farah, A. Fast simultaneous analysis of caffeine, trigonelline, nicotinic acid and sucrose in coffee by liquid chromatography-mass spectrometry. Food Chem. 2008, 110, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo Carvalho, D.; Brigagão, M.R.; dos Santos, M.H.; de Paula, F.B.; Giusti-Paiva, A.; Azevedo, L. Organic and conventional Coffea arabica L.: A comparative study of the chemical composition and physiological, biochemical and toxicological effects in Wistar rats. Plant Foods Hum. Nutr. 2011, 66, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.P.; Salva, T.J.G.; Bragagnolo, N. Influence of coffee genotype on bioactive compounds and the in vitro capacity to scavenge reactive oxygen and nitrogen species. J. Agric. Food Chem. 2015, 63, 4815–4826. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.; Baquer, N.Z. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Zych, M.; Nowińska, B.; Pytlik, M.; Janas, A. Unfavorable effect of trigonelline, an alkaloid present in coffee and fenugreek, on bone mechanical properties in estrogen-deficient rats. Mol. Nutr. Food Res. 2014, 58, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Pytlik, M.; Zych, M.; Cegieła, U.; Kaczmarczyk-Sedlak, I.; Nowińska, B.; Śliwiński, L. Favorable effect of moderate dose caffeine on the skeletal system in ovariectomized rats. Mol. Nutr. Food Res. 2013, 57, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Folwarczna, J.; Zych, M.; Trzeciak, H.I. Effects of curcumin on the skeletal system in rats. Pharmacol. Rep. 2010, 62, 900–909. [Google Scholar] [CrossRef]
- Turner, C.H.; Burr, D.B. Basic biomechanical measurements of bone: A tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef]
- Stürmer, E.K.; Seidlová-Wuttke, D.; Sehmisch, S.; Rack, T.; Wille, J.; Frosch, K.H.; Wuttke, W.; Stürmer, K.M. Standardized bending and breaking test for the normal and osteoporotic metaphyseal tibias of the rat: Effect of estradiol, testosterone, and raloxifene. J. Bone Miner. Res. 2006, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.S.; Fraser, L.A. Type 1 diabetes and osteoporosis: From molecular pathways to bone phenotype. J. Osteoporos. 2015, 2015, 174186. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, V.V.; Mitchell, D.M.; Rosen, C.J.; Bouxsein, M.L. Type 2 diabetes and the skeleton: New insights into sweet bones. Lancet Diabetes Endocrinol. 2016, 4, 159–173. [Google Scholar] [CrossRef]
- Oei, L.; Rivadeneira, F.; Zillikens, M.C.; Oei, E.H. Diabetes, diabetic complications, and fracture risk. Curr. Osteoporos. Rep. 2015, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. New insights for oxidative stress and diabetes mellitus. Oxid. Med. Cell Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Schwartz, A.V.; Egger, A.; Lecka-Czernik, B. Effects of diabetes drugs on the skeleton. Bone 2016, 82, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Brodt, M.D.; Lynch, M.A.; McKenzie, J.A.; Tanouye, K.M.; Nyman, J.S.; Wang, X. Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J. Bone Miner. Res. 2009, 24, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.; Gürgül, S.; Demirel, C.; Yildiz, A. The effect of insulin therapy on biomechanical deterioration of bone in streptozotocin (STZ)-induced type 1 diabetes mellitus in rats. Diabetes Res. Clin. Pract. 2012, 97, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Coe, L.M.; Zhang, J.; McCabe, L.R. Both spontaneous Ins2(+/-) and streptozotocin-induced type I diabetes cause bone loss in young mice. J. Cell. Physiol. 2013, 228, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Thrailkill, K.M.; Clay Bunn, R.; Nyman, J.S.; Rettiganti, M.R.; Cockrell, G.E.; Wahl, E.C.; Uppuganti, S.; Lumpkin, C.K., Jr.; Fowlkes, J.L. SGLT2 inhibitor therapy improves blood glucose but does not prevent diabetic bone disease in diabetic DBA/2J male mice. Bone 2016, 82, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P. Animal models of type 2 diabetes with reduced pancreatic beta-cell mass. Int. J. Biochem. Cell Biol. 2006, 38, 873–893. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Zywert, A.; Szkudelska, K. Metabolic disturbances and defects in insulin secretion in rats with streptozotocin-nicotinamide-induced diabetes. Physiol. Res. 2013, 62, 663–670. [Google Scholar] [PubMed]
- Wei, J.; Ferron, M.; Clarke, C.J.; Hannun, Y.A.; Jiang, H.; Blaner, W.S.; Karsenty, G. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J. Clin. Investig. 2014, 124, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Lacombe, J. Regulation of energy metabolism by the skeleton: Osteocalcin and beyond. Arch. Biochem. Biophys. 2014, 561, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Bengara, A.; Amri, Z.; Elfeki, A. Experimental diabetes treated with trigonelline: Effect on key enzymes related to diabetes and hypertension, β-cell and liver function. Mol. Cell. Biochem. 2013, 381, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, S.; Zeng, S. Experimental diabetes treated with trigonelline: Effect on β cell and pancreatic oxidative parameters. Fundam. Clin. Pharmacol. 2013, 27, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kamble, H.V.; Bodhankar, S.L. Cardioprotective effect of concomitant administration of trigonelline and sitagliptin on cardiac biomarkers, lipid levels, electrocardiographic and heamodynamic modulation on cardiomyopathy in diabetic Wistar rats. Biomed. Aging Pathol. 2014, 4, 335–342. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Wu, J.J.; Liu, Z.Q.; Lin, N. Development of a hydrophilic interaction chromatography-UPLC assay to determine trigonelline in rat plasma and its application in a pharmacokinetic study. Chin. J. Nat. Med. 2013, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kida, Y.; Kato, S.; Marumo, K. Diabetes, collagen, and bone quality. Curr. Osteoporos. Rep. 2014, 12, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, O.; Takenake, A.; Igarashi, K. Trigonelline ameliorates oxidative stress in type 2 diabetic Goto-Kakizaki rats. J. Med. Food. 2013, 16, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ghule, A.E.; Jadhav, S.S.; Bodhankar, S.L. Trigonelline ameliorates diabetic hypertensive nephropathy by suppression of oxidative stress in kidney and reduction in renal cell apoptosis and fibrosis in streptozotocin induced neonatal diabetic (nSTZ) rats. Int. Immunopharmacol. 2012, 14, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Tharaheswari, M.; Jayachandra Reddy, N.; Kumar, R.; Varshney, K.C.; Kannan, M.; Sudha Rani, S. Trigonelline and diosgenin attenuate ER stress, oxidative stress-mediated damage in pancreas and enhance adipose tissue PPARγ activity in type 2 diabetic rats. Mol. Cell. Biochem. 2014, 396, 161–714. [Google Scholar] [CrossRef] [PubMed]
- Boettler, U.; Sommerfeld, K.; Volz, N.; Pahlke, G.; Teller, N.; Somoza, V.; Lang, R.; Hofmann, T.; Marko, D. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J. Nutr. Biochem. 2011, 22, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.; Shi, Y.; Otoukesh, A.; Ghosh, A.; Lo, C.S.; Chenier, I.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S. Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice. Diabetes 2014, 63, 3483–3496. [Google Scholar] [PubMed]
- Barroso, E.; Rodríguez-Rodríguez, R.; Chacón, M.R.; Maymó-Masip, E.; Ferrer, L.; Salvadó, L.; Salmerón, E.; Wabistch, M.; Palomer, X.; Vendrell, J.; et al. PPARβ/δ ameliorates fructose-induced insulin resistance in adipocytes by preventing Nrf2 activation. Biochim. Biophys. Acta 2015, 1852, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Choi, D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B. Role of the Nrf2 signaling system in health and disease. Clin. Genet. 2014, 86, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Arasu, M.V.; Lee, J.C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, G.J.; Mayakrishnan, V.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Allred, K.F.; Yackley, K.M.; Vanamala, J.; Allred, C.D. Trigonelline is a novel phytoestrogen in coffee beans. J. Nutr. 2009, 139, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Dieminger, N.; Beusch, A.; Lee, Y.M.; Dunkel, A.; Suess, B.; Skurk, T.; Wahl, A.; Hauner, H.; Hofmann, T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal. Bioanal. Chem. 2013, 405, 8487–8503. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Wahl, A.; Stark, T.; Hofmann, T. Urinary N-methylpyridinium and trigonelline as candidate dietary biomarkers of coffee consumption. Mol. Nutr. Food Res. 2011, 55, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
| Parameter/Group | Control | STZ | STZ + Trigonelline | NA/STZ | NA/STZ + Trigonelline | |
|---|---|---|---|---|---|---|
| Body mass (g) | Initial | 193.8 ± 4.4 | 194.4 ± 5.6 | 195.6 ± 3.1 | 193.4 ± 4.2 | 191.5 ± 3.1 |
| 2 weeks after STZ administration | 202.8 ± 5.0 | 175.4 ± 7.6 * | 182.0 ± 4.6 ** | 202.1 ± 4.1 | 199.9 ± 3.3 | |
| 6 weeks after STZ administration # | 217.2 ± 4.6 | 177.4 ± 5.5 *** | 173.2 ± 7.1 ** | 216.5 ± 4.2 | 213.8 ± 3.7 | |
| Non-fasting glucose level (mg/100 mL) | Initial | 117.1 ± 3.8 | 119.8 ± 3.1 | 118.4 ± 5.1 | 119.7 ± 2.5 | 115.7 ± 4.1 |
| 2 weeks after STZ administration | 103.7 ± 3.6 | 470.7 ± 22.9 *** | 528.7 ± 15.8 *** | 121.7 ± 7.4 | 112.3 ± 4.2 | |
| 6 weeks after STZ administration # | 111.2 ± 3.6 | 574.8 ± 16.3 *** | 576.7 ± 11.7 *** | 109.3 ± 3.0 | 107.2 ± 2.5 | |
| Total cholesterol (mg/100 mL) | 36.32 ± 2.28 | 40.80 ± 3.61 | 33.44 ± 5.75 | 34.05 ± 2.43 | 33.52 ± 3.52 | |
| Total calcium (mg/100 mL) | 9.88 ± 0.09 | 8.95 ± 0.40 | 9.57 ± 0.49 | 10.03 ± 0.17 | 10.17 ± 0.29 | |
| Osteocalcin (ng/mL) | 182.4 ± 16.9 | 129.1 ± 24.3 | 179.4 ± 5.6 | 235.7 ± 25.0 * | 243.3 ± 14.1 ** | |
| C-terminal type I collagen fragments (RatLaps) (ng/mL) | 14.51 ± 1.04 | 59.25 ± 9.36 *** | 58.36 ± 5.13 *** | 16.47 ± 0.98 | 15.60 ± 1.14 | |
| Parameter/Group | Control | STZ | STZ + Trigonelline | NA/STZ | NA/STZ +Trigonelline | |
|---|---|---|---|---|---|---|
| Bone mineral mass/bone mass ratio | femur | 0.466 ± 0.006 | 0.440 ± 0.007 ** | 0.421 ± 0.004 **,O | 0.458 ± 0.004 | 0.462 ± 0.007 |
| tibia | 0.466 ± 0.005 | 0.441 ± 0.006 ** | 0.419 ± 0.005 ***,O | 0.451 ± 0.006 | 0.467 ± 0.004 | |
| L-4 vertebra | 0.429 ± 0.012 | 0.408 ± 0.006 * | 0.394 ± 0.006 ** | 0.424 ± 0.010 | 0.436 ± 0.006 | |
| Mass of bone water/bone mass ratio | femur | 0.289 ± 0.006 | 0.319 ± 0.008 ** | 0.337 ± 0.006 ***,O | 0.301 ± 0.005 | 0.299 ± 0.008 |
| tibia | 0.275 ± 0.005 | 0.303 ± 0.007 ** | 0.332 ± 0.005 ***,O | 0.295 ± 0.008 * | 0.280 ± 0.006 | |
| L-4 vertebra | 0.316 ± 0.017 | 0.337 ± 0.008 * | 0.352 ± 0.007 ** | 0.319 ± 0.015 | 0.307 ± 0.010 | |
| Mass of bone organic substances/bo-ne mass ratio | femur | 0.244 ± 0.002 | 0.241 ± 0.002 | 0.241 ± 0.002 | 0.240 ± 0.001 | 0.240 ± 0.002 |
| tibia | 0.258 ± 0.002 | 0.255 ± 0.004 | 0.249 ± 0.002 | 0.254 ± 0.004 | 0.252 ± 0.002 | |
| L-4 vertebra | 0.255 ± 0.006 | 0.255 ± 0.003 | 0.254 ± 0.002 | 0.257 ± 0.005 | 0.258 ± 0.005 | |
| Parameter/Group | Control | STZ | STZ + Trigonelline | NA/STZ | NA/STZ + Trigonelline |
|---|---|---|---|---|---|
| Young’s modulus (MPa) | 3299 ± 287 | 2971 ± 340 | 3037 ± 341 | 3366 ± 252 | 3436 ± 242 |
| Yield point load (N) | 70.1 ± 11.4 | 31.6 ± 4.7 ** | 21.8 ± 3.1 ** | 70.0 ± 8.6 | 62.0 ± 7.7 |
| Displacement for yield point load (mm) | 0.426 ± 0.065 | 0.278 ± 0.031 | 0.165 ± 0.028 **,O | 0.463 ± 0.067 | 0.375 ± 0.059 |
| Energy for yield point load (mJ) | 16.62 ± 4.49 | 4.15 ± 0.91 * | 1.98 ± 0.50 ** | 17.77 ± 4.69 | 12.36 ± 3.64 |
| Stress for yield point load (MPa) | 62.6 ± 9.8 | 33.1 ± 4.9 * | 23.2 ± 3.9 ** | 64.7 ± 7.4 | 58.1 ± 8.1 |
| Fracture load (N) | 93.5 ± 6.0 | 41.2 ± 2.9 *** | 33.1 ± 4.1 *** | 78.0 ± 4.1 | 90.9 ± 7.2 |
| Displacement for fracture load (mm) | 1.092 ± 0.042 | 0.859 ± 0.049 ** | 1.121 ± 0.093 O | 1.124 ± 0.041 | 1.021 ± 0.032 |
| Energy for fracture load (mJ) | 79.76 ± 4.81 | 30.90 ± 3.69 *** | 37.10 ± 4.66 *** | 72.88 ± 3.75 | 69.71 ± 2.65 |
| Stress for fracture load (MPa) | 83.5 ± 5.6 | 43.2 ± 3.6 *** | 33.9 ± 3.6 *** | 73.0 ± 4.6 | 85.3 ± 7.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folwarczna, J.; Janas, A.; Pytlik, M.; Cegieła, U.; Śliwiński, L.; Krivošíková, Z.; Štefíková, K.; Gajdoš, M. Effects of Trigonelline, an Alkaloid Present in Coffee, on Diabetes-Induced Disorders in the Rat Skeletal System. Nutrients 2016, 8, 133. https://doi.org/10.3390/nu8030133
Folwarczna J, Janas A, Pytlik M, Cegieła U, Śliwiński L, Krivošíková Z, Štefíková K, Gajdoš M. Effects of Trigonelline, an Alkaloid Present in Coffee, on Diabetes-Induced Disorders in the Rat Skeletal System. Nutrients. 2016; 8(3):133. https://doi.org/10.3390/nu8030133
Chicago/Turabian StyleFolwarczna, Joanna, Aleksandra Janas, Maria Pytlik, Urszula Cegieła, Leszek Śliwiński, Zora Krivošíková, Kornélia Štefíková, and Martin Gajdoš. 2016. "Effects of Trigonelline, an Alkaloid Present in Coffee, on Diabetes-Induced Disorders in the Rat Skeletal System" Nutrients 8, no. 3: 133. https://doi.org/10.3390/nu8030133
APA StyleFolwarczna, J., Janas, A., Pytlik, M., Cegieła, U., Śliwiński, L., Krivošíková, Z., Štefíková, K., & Gajdoš, M. (2016). Effects of Trigonelline, an Alkaloid Present in Coffee, on Diabetes-Induced Disorders in the Rat Skeletal System. Nutrients, 8(3), 133. https://doi.org/10.3390/nu8030133




