A Prospective Study on Serum Methylmalonic Acid and Homocysteine in Pregnant Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Pregnancy and Neonatal Outcomes
2.4. Laboratory Analyses
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
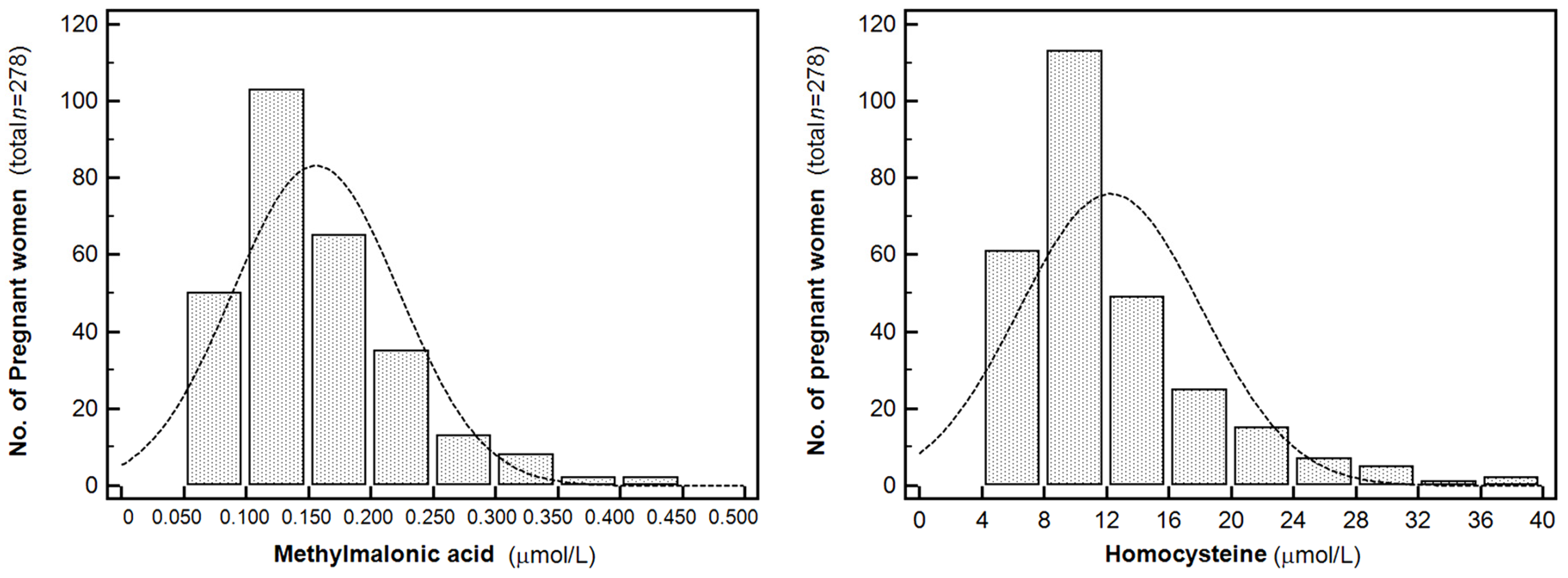
3.2. Serum MMA and Homocysteine Levels in Pregnant Korean Women
3.3. Association between MMA and Homocysteine Levels and Maternal and Neonatal Outcomes
4. Discussion
4.1. MMA and Homocysteine Levels in Pregnant Women
4.2. MMA and Homocysteine Levels in Maternal and Neonatal Outcomes
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| GC-MS | gas chromatography-mass spectrometry |
| GDM | gestational diabetes; HPLC, high performance liquid chromatography |
| LC-MS | liquid chromatography-mass spectrometry |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| MMA | methylmalonic acid |
| SGA | small for gestational age |
References
- Bae, S.; West, A.A.; Yan, J.; Jiang, X.; Perry, C.A.; Malysheva, O.; Stabler, S.P.; Allen, R.H.; Caudill, M.A. Vitamin B-12 status differs among pregnant, lactating, and control women with equivalent nutrient intakes. J. Nutr. 2015, 145, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; Gonzalez-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2015, 53, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Clinical practice. Vitamin b12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.K.; McGowan, C.A.; Gibney, E.R.; Donnelly, J.M.; McAuliffe, F.M. The association between maternal dietary micronutrient intake and neonatal anthropometry—Secondary analysis from the rolo study. Nutr. J. 2015, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Nasri, K.; Ben Fradj, M.K.; Touati, A.; Aloui, M.; Ben Jemaa, N.; Masmoudi, A.; Elmay, M.V.; Omar, S.; Feki, M.; Kaabechi, N.; et al. Association of maternal homocysteine and vitamins status with the risk of neural tube defects in tunisia: A case-control study. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 103, 1011–1020. [Google Scholar] [PubMed]
- Muthayya, S.; Kurpad, A.V.; Duggan, C.P.; Bosch, R.J.; Dwarkanath, P.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Vaz, M.; Bhat, S.; et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban south Indians. Eur. J. Clin. Nutr. 2006, 60, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Bondevik, G.T.; Schneede, J.; Refsum, H.; Lie, R.T.; Ulstein, M.; Kvale, G. Homocysteine and methylmalonic acid levels in pregnant Nepali women. Should cobalamin supplementation be considered? Eur. J. Clin. Nutr. 2001, 55, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Molloy, A.M.; Ueland, P.M.; Fernandez-Ballart, J.D.; Schneede, J.; Arija, V.; Scott, J.M. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J. Nutr. 2007, 137, 1863–1867. [Google Scholar] [PubMed]
- Hogeveen, M.; Blom, H.J.; van der Heijden, E.H.; Semmekrot, B.A.; Sporken, J.M.; Ueland, P.M.; den Heijer, M. Maternal homocysteine and related B vitamins as risk factors for low birthweight. Am. J. Obstet. Gynecol. 2010, 202, 572.e1–572.e6. [Google Scholar] [CrossRef] [PubMed]
- Hay, G.; Clausen, T.; Whitelaw, A.; Trygg, K.; Johnston, C.; Henriksen, T.; Refsum, H. Maternal folate and cobalamin status predicts vitamin status in newborns and 6-month-old infants. J. Nutr. 2010, 140, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Hong, S.C.; Choi, J.S.; Han, J.Y.; Oh, M.J.; Kim, H.J.; Nava-Ocampo, A.; Koren, G. Homocysteine, folate and pregnancy outcomes. J. Obstet. Gynaecol. 2012, 32, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, Y.J.; Ha, E.H.; Kim, K.N.; Chang, N. The risk of folate and vitamin B(12) deficiencies associated with hyperhomocysteinemia among pregnant women. Am. J. Perinatol. 2004, 21, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, H.; Mito, N.; Umegaki, K.; Ishiwaki, A.; Kusama, K.; Abe, S.; Yamawaki, M.; Fukuoka, H.; Ohta, C.; Yoshiike, N. Relationship between dietary folate intakes, maternal plasma total homocysteine and B-vitamins during pregnancy and fetal growth in Japan. Eur. J. Nutr. 2007, 46, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Coustan, D.R.; Carpenter, M.W. The diagnosis of gestational diabetes. Diabetes Care 1998, 21 (Suppl. 2), B5–B8. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.R.; Cho, L.S. Hypertension in pregnancy. Curr. Atheroscler. Rep. 2014, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Analysis for the Behavioral Sciences; Lawrance Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Milman, N.; Byg, K.E.; Bergholt, T.; Eriksen, L.; Hvas, A.M. Cobalamin status during normal pregnancy and postpartum: A longitudinal study comprising 406 Danish women. Eur. J. Haematol. 2006, 76, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Bergholt, T.; Byg, K.E.; Eriksen, L.; Hvas, A.M. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. Eur. J. Haematol. 2007, 79, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Greibe, E.; Andreasen, B.H.; Lildballe, D.L.; Morkbak, A.L.; Hvas, A.M.; Nexo, E. Uptake of cobalamin and markers of cobalamin status: A longitudinal study of healthy pregnant women. Clin. Chem. Lab. Med. 2011, 49, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Cikot, R.J.L.M.; Steegers-Theunissen, R.P.M.; Thomas, C.M.G.; de Boo, T.M.; Merkus, H.M.W.M.; Steegers, E.A.P. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br. J. Nutr. 2001, 85, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Scott, J.M.; McPartlin, J.M.; Fernandez-Ballart, J.D. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am. J. Clin. Nutr. 2002, 76, 614–619. [Google Scholar] [PubMed]
- Hong, S.-C.; Choi, J.S.; Han, J.Y.; Nava-Ocampo, A.A.; Koren, G. Essence of preconception counseling and care. J. Korean Med. Assoc. 2011, 54, 799–807. [Google Scholar] [CrossRef]
- Azadbakht, L.; Mirmiran, P.; Azizi, F. Variety scores of food groups contribute to the specific nutrient adequacy in Tehranian men. Eur. J. Clin. Nutr. 2005, 59, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Duggan, C.; Thomas, T.; Bosch, R.; Rajendran, R.; Virtanen, S.M.; Srinivasan, K.; Kurpad, A.V. Vitamin B(12) intake and status in early pregnancy among urban south Indian women. Ann. Nutr. Metab. 2013, 62, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, M.; Blom, H.J.; den Heijer, M. Maternal homocysteine and small-for-gestational-age offspring: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Shinohara, E.M.; Pereira, P.M.; Kubota, A.M.; Silva, T.A.; Reis, J.L.; Miyashita, G.S.; D’Almeida, V.; Allen, R.H.; Stabler, S.P. Increased mma concentration and body mass index are associated with spontaneous abortion in Brazilian women: A pilot study. Clin. Chim. Acta 2010, 411, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Garrod, M.G.; Rockwood, A.L.; Kushnir, M.M.; Allen, L.H.; Haan, M.N.; Green, R. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin. Chem. 2006, 52, 278–285. [Google Scholar] [CrossRef] [PubMed]

| Variables | Participants | |
|---|---|---|
| n | % | |
| Trimester at sampling (total) | 278 | |
| First | 65 | (23.4) |
| Second | 113 | (40.6) |
| Third | 100 | (36.0) |
| Job (total) | 269 | |
| Home maker | 90 | (33.5) |
| Any employment | 179 | (66.5) |
| Education period (total) | 270 | |
| <12 years | 18 | 6.67 |
| ≥12 years | 252 | 93.33 |
| Alcohol ingestion (total) | 276 | |
| No | 273 | (98.9) |
| Yes | 3 | (1.1) |
| Smoking (total) | 278 | |
| No | 277 | (99.6) |
| Yes | 1 | (0.4) |
| Concurrent medical history (total) | 278 | |
| No | 237 | (85.3) |
| Yes | 41 | (14.7) |
| Parity (total) | 278 | |
| 0 (nullipara) | 168 | (60.4) |
| ≥1 | 110 | (39.6) |
| Type of pregnancy (total) a | 278 | |
| Spontaneous pregnancy | 271 | (97.5) |
| Artificial pregnancy | 7 | (2.5) |
| Multivitamin or folate supplementation (total) | 278 | |
| No | 7 | (2.5) |
| Yes | 271 | (97.5) |
| Variables | Participants | Methylmalonic Acid Level (µmol/L) | Homocysteine Level (µmol/L) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | Median | IQR | p | Median | IQR | p | |
| Trimester at sampling (total) | 278 | 0.03 | 0.11 | |||||
| First | 65 | (23.4) | 0.138 | 0.104–0.176 | 10.4 | 8.9–15.7 | ||
| Second | 113 | (40.6) | 0.133 | 0.103–0.181 | 10.7 | 8.2–13.9 | ||
| Third | 100 | (36.0) | 0.151 | 0.114–0.210 | 10.3 | 7.9–14.0 | ||
| Job (total) | 269 | 0.47 | 0.26 | |||||
| Home maker | 90 | (33.5) | 0.144 | 0.106–0.201 | 11.3 | 8.6–15.3 | ||
| Any employment | 179 | (66.5) | 0.140 | 0.107–0.185 | 10.1 | 7.9–13.1 | ||
| Education period (total) | 270 | 0.93 | 0.23 | |||||
| <12 years | 18 | 6.67 | 0.145 | 0.112–0.195 | 9.2 | 7.8–11.3 | ||
| ≥12 years | 252 | 93.33 | 0.143 | 0.107–0.187 | 10.6 | 8.2–14.3 | ||
| Alcohol ingestion (total) | 276 | 0.34 | 0.58 | |||||
| No | 273 | (98.9) | 0.142 | 0.107–0.186 | 10.6 | 8.2–14.4 | ||
| Yes | 3 | (1.1) | 0.220 | –b, c | 9.0 | –b, c | ||
| Smoking (total) | 278 | 0.06 | 0.96 | |||||
| No | 277 | (99.6) | 0.142 | 0.107–0.186 | 10.5 | 8.3–14.4 | ||
| Yes | 1 | (0.4) | 0.195 | 0.155–0.307 | 11.3 | 9.0–16.2 | ||
| Concurrent medical history (total) | 278 | 0.93 | 0.37 | |||||
| No | 237 | (85.3) | 0.143 | 0.108–0.184 | 10.4 | 8.2–14.5 | ||
| Yes | 41 | (14.7) | 0.134 | 0.104–0.201 | 10.6 | 8.3–14.3 | ||
| Parity (total) | 278 | 0.50 | 0.68 | |||||
| 0 (nullipara) | 168 | (60.4) | 0.140 | 0.107–0.186 | 10.7 | 8.5–13.9 | ||
| ≥1 | 110 | (39.6) | 0.144 | 0.108–0.187 | 10.3 | 7.9–14.6 | ||
| Type of pregnancy (total) a | 278 | 0.27 | 0.27 | |||||
| Spontaneous pregnancy | 271 | (97.5) | 0.142 | 0.107–0.185 | 10.4 | 8.2–13.9 | ||
| Artificial pregnancy | 7 | (2.5) | 0.189 | 0.124–0.217 | 15.1 | 9.6–18.1 | ||
| Multivitamin or folate supplementation (total) | 278 | 0.57 | 0.41 | |||||
| No | 7 | (2.5) | 0.157 | 0.096–0.188 | 14.2 | 11.1–15.7 | ||
| Yes | 271 | (97.5) | 0.142 | 0.107–0.187 | 10.4 | 8.2–13.9 | ||
| Participants | Methylmalonic Acid Level (µmol/L) | Homocysteine Level (µmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal and Neonatal Outcomes | n | % | Median | IQR | p a | p b | Median | IQR | p a | p b |
| Gestational diabetes (total) | 278 | 0.40 | 0.59 | 0.40 | 0.47 | |||||
| No | 255 | (91.7) | 0.143 | 0.109–0.187 | 10.5 | 8.3–14.3 | ||||
| Yes | 23 | (8.3) | 0.134 | 0.091–0.168 | 10.6 | 7.5–14.7 | ||||
| Preeclampsia (total) | 257 | 0.29 | 0.30 | 0.49 | 0.40 | |||||
| No | 252 | (98.0) | 0.142 | 0.108–0.186 | 10.4 | 8.2–14.1 | ||||
| Yes | 5 | (2.0) | 0.195 | 0.151–0.235 | 11.7 | 9.9–18.7 | ||||
| Preterm delivery (total) | 263 | 0.41 | 0.75 | 0.27 | 0.19 | |||||
| No | 246 | (93.5) | 0.142 | 0.107–0.187 | 10.4 | 8.2–13.9 | ||||
| Yes | 17 | (6.5) | 0.145 | 0.109–0.184 | 9.4 | 7.3–12.6 | ||||
| Small for gestational age (total) | 256 | 0.12 | 0.30 | 0.62 | 0.64 | |||||
| No | 217 | (84.8) | 0.141 | 0.108–0.185 | 10.6 | 8.2–14.8 | ||||
| Yes | 39 | (15.2) | 0.161 | 0.109–0.216 | 10.2 | 8.6–13.0 | ||||
| Congenital abnormality (total) | 256 | 0.69 | 0.81 | 0.12 | 0.15 | |||||
| No | 240 | (93.8) | 0.143 | 0.107–0.185 | 10.3 | 8.2–13.5 | ||||
| Yes | 16 | (6.3) | 0.150 | 0.111–0.219 | 12.7 | 9.4–18.5 | ||||
| Ref | Study Region | Study Design | Participants | Specimen | Measurement | Method | Sampling Time | Values | Levels (µmol/L) | Range | Range def. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] | Spain and Ireland | Longitudinal | n = 92 healthy preg | Plasma | MMA a | GC-MS | Preconception | G- Mean | 0.12 | (0.09–0.17) | 10‰–90‰ |
| 8 wk | 0.11 | (0.09–0.17) | |||||||||
| 20 wk | 0.11 | (0.08–0.15) | |||||||||
| 32 wk | 0.14 | (0.09–0.20) | |||||||||
| at labor | 0.14 | (0.09–0.21) | |||||||||
| Cord blood | 0.24 | (0.13–0.40) | |||||||||
| [18] | Denmark | Longitudinal | n = 406 healthy preg | Plasma | MMA a | GC-MS | 18 wk | Median | 0.11 | (0.06–0.25) | 5‰–95‰ |
| 32 wk | 0.13 | (0.06–0.31) | |||||||||
| 39 wk | 0.14 | (0.07–0.36) | |||||||||
| 8 wk postpartum | 0.16 | (0.09–0.30) | |||||||||
| Plasma | HCY a | GC-MS | 18 wk | Median | 6.4 | (3.6–9.4) | 5‰–95‰ | ||||
| 32 wk | 7.0 | (4.0–9.7) | |||||||||
| 39 wk | 7.7 | (5.2–12.0) | |||||||||
| 8 wk postpartum | 10.8 | (6.8–19.3) | |||||||||
| [19] | Denmark | Longitudinal | n = 434 healthy preg | Plasma | MMA a | GC-MS | 18 wk | Mean | 0.11 | (0.04–0.29) | Mean ± 1.96 × SD |
| 32 wk | 0.13 | (0.05–0.34) | |||||||||
| 39 wk | 0.15 | (0.06–0.36) | |||||||||
| 8 wk postpartum | 0.16 | (0.08–0.35) | |||||||||
| Plasma | HCY a | GC-MS | 18 wk | Mean | 6.06 | (3.34–11.00) | Mean ± 1.96 × SD | ||||
| 32 wk | 6.61 | (3.93–11.10) | |||||||||
| 39 wk | 7.78 | (4.72–12.81) | |||||||||
| 8 wk postpartum | 10.99 | (5.85–20.64) | |||||||||
| [7] | Nepal | Cross-sectional | n = 382 preg | Serum | MMA a | GC-MS | 1st trimester | Mean | 0.37 | (0.32–0.41) | 95% CI |
| 2nd trimester | 0.41 | (0.36–0.47) | |||||||||
| 3rd trimester | 0.39 | (0.31–0.48) | |||||||||
| Serum | HCY | GC-MS | 1st trimester | Mean | 9.9 | (9.1–10.6) | 95% CI | ||||
| 2nd trimester | 9.3 | (8.6–10.1) | |||||||||
| 3rd trimester | 9.4 | (8.5–10.3) | |||||||||
| [25] | South India | Cross-sectional | n = 360 preg | Plasma | MMA | GC-MS | <14 wk | Median | 0.47 | (0.28–0.67) | IQR |
| Plasma | HCY | GC-MS | <14 wk | Median | 9.22 | (5.74–15.08) | IQR | ||||
| [10] | Nordic descent | Longitudinal | n = 364 healthy preg | Serum | MMA | GC-MS | 17–19 wk | G-Mean | 0.10 | (0.10–0.11) | 95% CI |
| Serum | HCY | GC-MS | 17–19 wk | G-Mean | 4.7 | (4.5–4.9) | 95% CI | ||||
| [9] | Netherlands | prospective | n = 366 preg (not high-risk preg) | Plasma | MMA | LC-MS/MS | 30–34 wk | Median | 0.16 | (0.13–0.22) | IQR |
| Plasma | HCY | LC-MS/MS | 30–34 wk | Median | 5.5 | (4.5–6.7) | IQR | ||||
| This study | South Korea | Prospective cohort | n = 278 preg | Serum | MMA a | LC-MS/MS | 5–13 wk | Median | 0.13 | (0.10–0.18) | IQR |
| 14–26 wk | 0.13 | (0.10–0.18) | |||||||||
| 27–40 wk | 0.15 | (0.11–0.21) | |||||||||
| Serum | HCY | LC-MS/MS | 5–13 wk | Median | 10.6 | (8.9–15.7) | IQR | ||||
| 14–26 wk | 10.6 | (8.2–13.9) | |||||||||
| 27–40 wk | 10.2 | (7.9–14.0) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, R.; Choi, S.; Lim, Y.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.-y.; Lee, S.-Y. A Prospective Study on Serum Methylmalonic Acid and Homocysteine in Pregnant Women. Nutrients 2016, 8, 797. https://doi.org/10.3390/nu8120797
Choi R, Choi S, Lim Y, Cho YY, Kim HJ, Kim SW, Chung JH, Oh S-y, Lee S-Y. A Prospective Study on Serum Methylmalonic Acid and Homocysteine in Pregnant Women. Nutrients. 2016; 8(12):797. https://doi.org/10.3390/nu8120797
Chicago/Turabian StyleChoi, Rihwa, Sunkyu Choi, Yaeji Lim, Yoon Young Cho, Hye Jeong Kim, Sun Wook Kim, Jae Hoon Chung, Soo-young Oh, and Soo-Youn Lee. 2016. "A Prospective Study on Serum Methylmalonic Acid and Homocysteine in Pregnant Women" Nutrients 8, no. 12: 797. https://doi.org/10.3390/nu8120797
APA StyleChoi, R., Choi, S., Lim, Y., Cho, Y. Y., Kim, H. J., Kim, S. W., Chung, J. H., Oh, S.-y., & Lee, S.-Y. (2016). A Prospective Study on Serum Methylmalonic Acid and Homocysteine in Pregnant Women. Nutrients, 8(12), 797. https://doi.org/10.3390/nu8120797





