Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes
Abstract
:1. Introduction
2. Methods
2.1. Definitions
2.2. Statistical Analysis
3. Results
3.1. Vitamin B12, Folate Status, Maternal BMI, and GDM
3.2. Vitamin B12, Folate, and Birth Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heslehurst, N.; Rankin, J.; Wilkinson, J.R.; Summerbell, C.D. A nationally representative study of maternal obesity in England, UK: Trends in incidence and demographic inequalities in 619,323 births, 1989–2007. Int. J. Obes. (Lond.) 2010, 34, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. The Confidential Enquiry into Maternal and Child Health (CEMACH). In Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer—2003–2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom; CEMACH: London, UK, 2007. [Google Scholar]
- Buckley, B.S.; Harreiter, J.; Damm, P.; Corcoy, R.; Chico, A.; Simmons, D.; Vellinga, A.; Dunne, F.; DALI Core Investigator Group. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet. Med. 2012, 29, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Cundy, T.; Ackermann, E.; Ryan, E.A. Gestational diabetes: New criteria may triple the prevalence but effect on outcomes is unclear. BMJ 2014, 348, g1567. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Yajnik, C.S. Role of maternal vitamin B12 on the metabolic health of the offspring: A contributor to the diabetes epidemic? Br. J. Diabetes Vasc. Dis. 2010, 10, 109–114. [Google Scholar] [CrossRef]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The role of the one-carbon cycle in the developmental origins of type 2 diabetes and obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Adaikalakoteswari, A.; Jayashri, R.; Sukumar, N.; Venkataraman, H.; Pradeepa, R.; Gokulakrishnan, K.; Anjana, R.M.; McTernan, P.G.; Tripathi, G.; Patel, V.; et al. Vitamin B12 deficiency is associated with adverse lipid profile in Europeans and Indians with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Brindle, N.P.; Zammit, V.A.; Pogson, C.I. Regulation of carnitine palmitoyltransferase activity by malonyl-CoA in mitochondria from sheep liver, a tissue with a low capacity for fatty acid synthesis. Biochem. J. 1985, 232, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Lalitha, A.; Pavithra, D.; Padmavathi, I.J.; Ganeshan, M.; Rao, K.R.; Venu, L.; Balakrishna, N.; Shanker, N.H.; Reddy, S.U.; et al. Maternal dietary folate and/or vitamin B12 restrictions alter body composition (adiposity) and lipid metabolism in wistar rat offspring. J. Nutr. Biochem. 2013, 24, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Adaikalakoteswari, A.; Finer, S.; Voyias, P.D.; McCarthy, C.M.; Vatish, M.; Moore, J.; Smart-Halajko, M.; Bawazeer, N.; Al-Daghri, N.M.; McTernan, P.G.; et al. Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin. Epigenet. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, N.; Rafnsson, S.B.; Kandala, N.B.; Bhopal, R.; Yajnik, C.S.; Saravanan, P. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1232–1251. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Hill, J.C.; Veena, S.R.; Bhat, D.S.; Wills, A.K.; Karat, C.L.; Yajnik, C.S.; Fall, C.H. Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009, 52, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Christian, P.; Schulze, K.J.; Arguello, M.; LeClerq, S.C.; Khatry, S.K.; West, K.P., Jr. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J. Nutr. 2011, 141, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The pune maternal nutrition study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Adaikalakoteswari, A.; Vatish, M.; Lawson, A.; Wood, C.; Sivakumar, K.; McTernan, P.G.; Webster, C.; Anderson, N.; Yajnik, C.S.; Tripathi, G.; et al. Low maternal vitamin B12 status is associated with lower cord blood HDL cholesterol in white caucasians living in the UK. Nutrients 2015, 7, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.A.; Shields, B.M.; Brook, A.; Hill, A.; Bhat, D.S.; Hattersley, A.T.; Yajnik, C.S. Lower circulating B12 is associated with higher obesity and insulin resistance during pregnancy in a non-diabetic white British population. PLoS ONE 2015, 10, e0135268. [Google Scholar] [CrossRef] [PubMed]
- Koukoura, O.; Sifakis, S.; Spandidos, D.A. DNA methylation in the human placenta and fetal growth (review). Mol. Med. Rep. 2012, 5, 883–889. [Google Scholar] [PubMed]
- Hogeveen, M.; Blom, H.J.; van der Heijden, E.H.; Semmekrot, B.A.; Sporken, J.M.; Ueland, P.M.; den Heijer, M. Maternal homocysteine and related B vitamins as risk factors for low birthweight. Am. J. Obstet. Gynecol. 2010, 202, 572. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Kurpad, A.V.; Duggan, C.P.; Bosch, R.J.; Dwarkanath, P.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Vaz, M.; Bhat, S.; et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban south Indians. Eur. J. Clin. Nutr. 2006, 60, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, N.; Adaikalakoteswari, A.; Venkataraman, H.; Maheswaran, H.; Saravanan, P. Vitamin B12 status in women of childbearing age in the UK and its relationship with national nutrient intake guidelines: Results from two national diet and nutrition surveys. BMJ Open 2016, 6, e011247. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, L.; Ferraro, Z.M.; Wen, S.W.; Walker, M. Maternal obesity and occurrence of fetal macrosomia: A systematic review and meta-analysis. BioMed Res. Int. 2014, 2014, 640291. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Qin, F.Y.; Hu, C.L.; Zhu, M.; Tian, C.Q.; Li, L. Is gestational diabetes mellitus an independent risk factor for macrosomia: A meta-analysis? Arch. Gynecol. Obstet. 2015, 291, 729–735. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Diabetes in pregnancy: Management of diabetes and its complications from pre-conception to the postnatal period. In NICE Clinical Guideline 63; National Institute for Health and Care Excellence: Manchester, UK, 2008; pp. 1–42. [Google Scholar]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The newborn cross-sectional study of the intergrowth-21st project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- IBM Corp. IBM Spss Statistics for Windows, version 22.0; IBM Corp: Armonk, NY, USA, 2013. [Google Scholar]
- Ho, M.; Halim, J.H.; Gow, M.L.; El-Haddad, N.; Marzulli, T.; Baur, L.A.; Cowell, C.T.; Garnett, S.P. Vitamin B12 in obese adolescents with clinical features of insulin resistance. Nutrients 2014, 6, 5611–5618. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, D.; Kutlucan, A.; Turker, Y.; Yilmaz, A.; Karacam, S.; Deler, H.; Ucgun, T.; Kara, I.H. Association of vitamin B12 with obesity, overweight, insulin resistance and metabolic syndrome, and body fat composition; primary care-based study. Med. Glas. (Zenica) 2013, 10, 203–210. [Google Scholar] [PubMed]
- Kaya, C.; Cengiz, S.D.; Satiroglu, H. Obesity and insulin resistance associated with lower plasma vitamin B12 in PCOS. Reprod. Biomed. Online 2009, 19, 721–726. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. USDA Table of Nutrient Retention Factors, Release 6. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/retn/retn06.pdf (accessed on 5 November 2016).
- Karatela, R.A.; Sainani, G.S. Plasma homocysteine in obese, overweight and normal weight hypertensives and normotensives. Indian Heart J. 2009, 61, 156–159. [Google Scholar] [PubMed]
- Guven, A.; Inanc, F.; Kilinc, M.; Ekerbicer, H. Plasma homocysteine and lipoprotein (a) levels in Turkish patients with metabolic syndrome. Heart Vessels 2005, 20, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Setola, E.; Monti, L.D.; Galluccio, E.; Palloshi, A.; Fragasso, G.; Paroni, R.; Magni, F.; Sandoli, E.P.; Lucotti, P.; Costa, S.; et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: Relationship between homocysteine levels and hyperinsulinemia. Eur. J. Endocrinol. 2004, 151, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Koebnick, C.; Heins, U.A.; Dagnelie, P.C.; Wickramasinghe, S.N.; Ratnayaka, I.D.; Hothorn, T.; Pfahlberg, A.B.; Hoffmann, I.; Lindemans, J.; Leitzmann, C. Longitudinal concentrations of vitamin B(12) and vitamin B(12)-binding proteins during uncomplicated pregnancy. Clin. Chem. 2002, 48, 928–933. [Google Scholar] [PubMed]
- Greibe, E.; Andreasen, B.H.; Lildballe, D.L.; Morkbak, A.L.; Hvas, A.M.; Nexo, E. Uptake of cobalamin and markers of cobalamin status: A longitudinal study of healthy pregnant women. Clin. Chem. Lab. Med. 2011, 49, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Molloy, A.M.; Ueland, P.M.; Fernandez-Ballart, J.D.; Schneede, J.; Arija, V.; Scott, J.M. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J. Nutr. 2007, 137, 1863–1867. [Google Scholar] [PubMed]
- Guerra-Shinohara, E.M.; Morita, O.E.; Peres, S.; Pagliusi, R.A.; Neto, L.F.S.; D’Almeida, V.; Irazusta, S.P.; Allen, R.H.; Stabler, S.P. Low ratio of S-adenosylmethionine to S-adenosylhomocysteine is associated with vitamin deficiency in Brazilian pregnant women and newborns. Am. J. Clin. Nutr. 2004, 80, 1312–1322. [Google Scholar] [PubMed]
- Gadgil, M.; Joshi, K.; Pandit, A.; Otiv, S.; Joshi, R.; Brenna, J.T.; Patwardhan, B. Imbalance of folic acid and vitamin B12 is associated with birth outcome: An Indian pregnant women study. Eur. J. Clin. Nutr. 2014, 68, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Langman, L.J.; Boss, S.C.; Cole, D.E. Persistence of vitamin B12 insufficiency among elderly women after folic acid food fortification. Clin. Biochem. 2003, 36, 387–391. [Google Scholar] [CrossRef]
- Wyckoff, K.F.; Ganji, V. Proportion of individuals with low serum vitamin B-12 concentrations without macrocytosis is higher in the post folic acid fortification period than in the pre folic acid fortification period. Am. J. Clin. Nutr. 2007, 86, 1187–1192. [Google Scholar] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [PubMed]
| Variables | Total | GDM | No GDM |
|---|---|---|---|
| Number (%) | 344 (100) | 143 (41.6) | 201 (58.4) |
| Age (years) | 30.3 ± 5.88 | 31.4 + 5.8 | 29.6 ± 5.9 **,a |
| BMI (kg/m2) § | 28.8 ± 7.46 | 31.7 ± 7.0 | 26.7 ± 7.1 *** |
| Obesity (BMI > 30 kg/m2) (%) | 38.0 | 60.6 | 22.0 *** |
| Current smokers (%) | 18.7 | 15.2 | 19.9 |
| Parity | 1.1 ± 1.18 | 1.2 ± 1.18 | 1.0 ± 1.18 |
| Ethnicity (%) | |||
| European | 86.9 | 86.0 | 87.6 |
| South Asian | 9.3 | 11.2 | 8.0 |
| Afro-Caribbean | 1.2 | 0.7 | 1.5 |
| Other | 1.2 | 1.4 | 1.0 |
| Gestation of GTT (weeks) b | 26.6 ± 3.95 | 26.4 ± 4.40 | 26.8 ± 3.10 |
| Mean fasting glucose (mmol/L) § | 4.9 ± 1.01 | 5.2 ± 1.15 | 4.4 ± 0.39 *** |
| Mean 2 h glucose (mmol/L) § | 7.5 ± 1.94 | 8.7 ± 1.26 | 5.6 ± 1.13 *** |
| Gestation of B12 bloods (weeks) | 26.9 ± 5.3 | 28.0 ± 4.3 | 26.2 + 5.7 ** |
| Vitamin B12 (pmol/L) § | 187.5 (146.9, 235.4) | 169.0 (140.2, 217.7) | 195.6 (157.9, 244.6) ** |
| Vitamin B12 deficiency (<150 pmol/L), n (%) | 90 (26.2) | 46 (32.2) | 44 (21.9) * |
| Serum folate (nmol/L) § | 21.3 (14.0, 34.4) | 21.5 (13.5, 34.5) | 20.8 (14.5, 34.4) |
| Serum folate deficiency (<7 nmol/L), n (%) | 5 (1.5) | 3 (2.1) | 2 (1.0) |
| Folic acid supplements taken (%) | 91.4 | 90.9 | 91.5 |
| Variables | Serum B12 § | Serum Folate § | ||
|---|---|---|---|---|
| β-Coefficient | p-Value | β-Coefficient | p-Value | |
| Age | - | NS | 0.32 | <0.001 |
| Parity | - | NS | −0.24 | <0.001 |
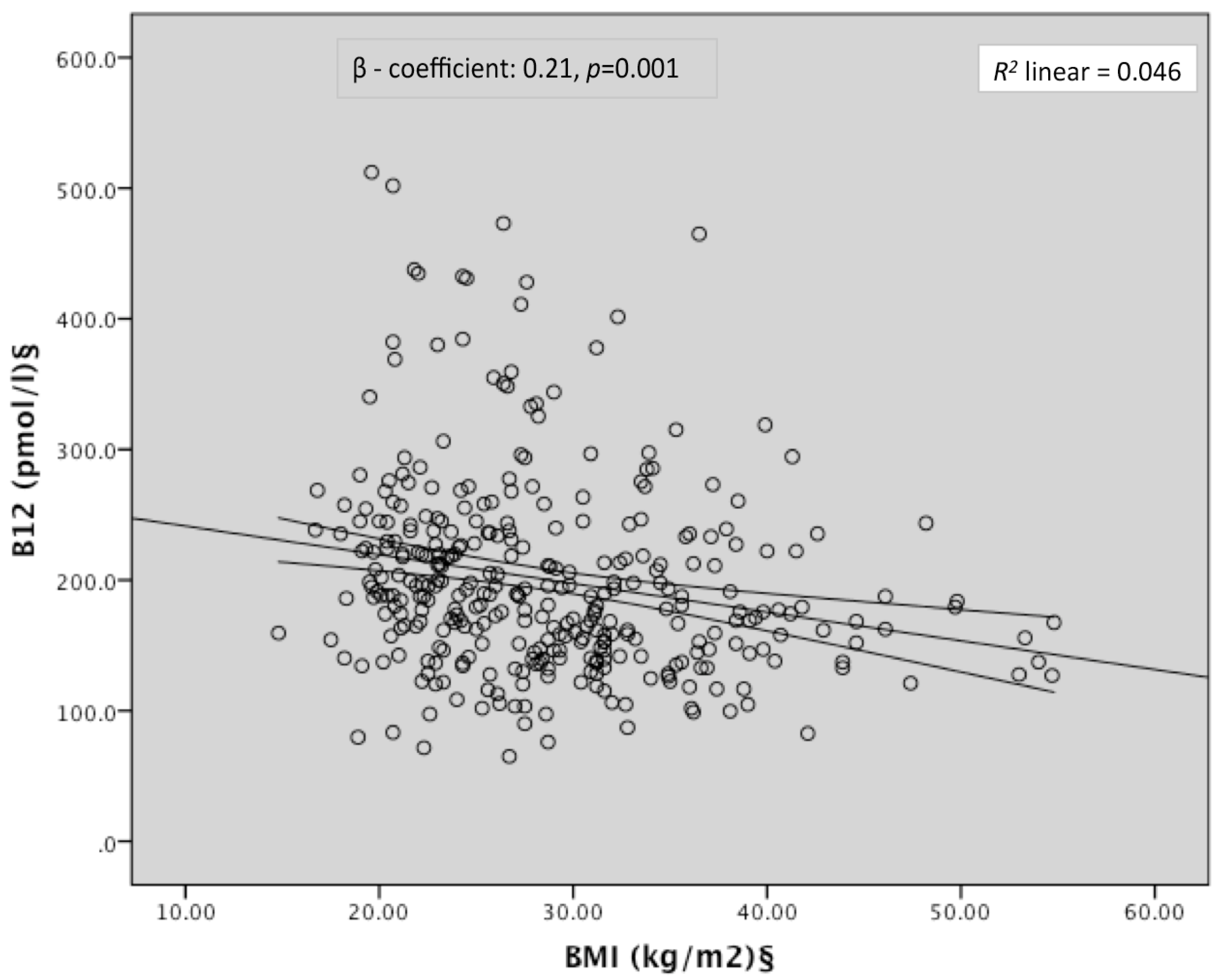
| BMI § | −0.21 | 0.001 | −0.12 | 0.05 |
| Ethnicity | - | NS | - | NS |
| Smoking | - | NS | - | NS |
| Gestation of B12/folate bloods | - | NS | −0.28 | <0.001 |
| Serum B12 § | 0.12 | 0.05 | ||
| Serum folate § | 0.23 | <0.001 | ||
| Folic acid supplements | - | NS | - | NS |
| n (%) | Obesity, n (%) | GDM, n (%) | |
|---|---|---|---|
| Vitamin B12 deficiency | |||
| Yes | 90 | 44 (49.4) | 46 (51.1) |
| No | 254 | 86 (34.0) | 97 (38.2) |
| Model 1 OR (95% CI) a | 2.40 (1.31, 4.40) | 2.59 (1.35, 4.98) | |
| adjusted p | 0.004 | 0.004 | |
| Model 2 OR (95% CI) b | N/A | 2.05 (1.03, 4.10) | |
| adjusted p | N/A | 0.042 | |
| Folate deficiency | |||
| Yes | 5 | 4 (80.0) | 3 (60.0) |
| No | 332 | 125 (37.9) | 139 (41.9) |
| Model 1 OR (95% CI) a | 6.29 (0.48, 82.79) | 1.93 (0.17, 22.23) | |
| adjusted p | NS | NS | |
| Model 2 OR (95% CI) b | N/A | 0.89 (0.07, 11.38) | |
| adjusted p | N/A | NS |
| n | Range of Values (pmol/L) | Macrosomia, n (%) | LGA, n (%) | LBW, n (%) | SGA, n (%) | |
|---|---|---|---|---|---|---|
| Vitamin B12 (quartiles) | ||||||
| 1 | 48 | 71.6, 157.2 | 11 (22.9) | 12 (25.0) | 1 (2.1) | 4 (8.3) |
| 2 | 48 | 158.7, 195.6 | 10 (20.8) | 12 (25.0) | 2 (4.2) | 2 (4.2) |
| 3 | 47 | 196.3, 244.3 | 9 (19.1) | 10 (21.3) | 3 (6.4) | 3 (6.4) |
| 4 | 50 | 245.0, 512.2 | 4 (8.0) | 5 (10.0) | 3 (6.0) | 5 (10.0) |
| Relative risk (95% CI) a | 5.26 (1.26, 21.91) | 3.18 (0.96, 10.56) | 0.10 (0.002, 5.75) | 1.35 (0.28, 6.47) | ||
| p b | 0.02 | 0.06 | 0.27 | 0.71 | ||
| p c | 0.05 | 0.13 | 0.37 | 0.52 | ||
| Folate (quartiles) | ||||||
| 1 | 44 | 4.5, 14.3 | 5 (11.4) | 7 (15.9) | 4 (9.1) | 4 (9.1) |
| 2 | 47 | 14.5, 20.6 | 7 (14.9) | 9 (19.1) | 1 (2.1) | 2 (4.3) |
| 3 | 48 | 20.8, 34.2 | 11 (22.9) | 10 (20.8) | 1 (2.1) | 3 (6.3) |
| 4 | 48 | 34.4, 45.3 | 10 (20.8) | 12 (25.0) | 3 (6.3) | 5 (10.4) |
| Relative risk (95% CI) a | 4.99 (1.15, 21.62) | 2.32 (0.74, 7.34) | 0.21 (0.01, 9.64) | 1.52 (0.26, 8.93) | ||
| p b | 0.03 | 0.15 | 0.42 | 0.64 | ||
| p c | 0.02 | 0.06 | 0.41 | 0.90 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients 2016, 8, 768. https://doi.org/10.3390/nu8120768
Sukumar N, Venkataraman H, Wilson S, Goljan I, Selvamoni S, Patel V, Saravanan P. Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients. 2016; 8(12):768. https://doi.org/10.3390/nu8120768
Chicago/Turabian StyleSukumar, Nithya, Hema Venkataraman, Sean Wilson, Ilona Goljan, Selvin Selvamoni, Vinod Patel, and Ponnusamy Saravanan. 2016. "Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes" Nutrients 8, no. 12: 768. https://doi.org/10.3390/nu8120768
APA StyleSukumar, N., Venkataraman, H., Wilson, S., Goljan, I., Selvamoni, S., Patel, V., & Saravanan, P. (2016). Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients, 8(12), 768. https://doi.org/10.3390/nu8120768






