Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Inclusion Criteria
2.3. Quality Assessment and Data Extraction
2.4. Statistical Analysis
3. Results
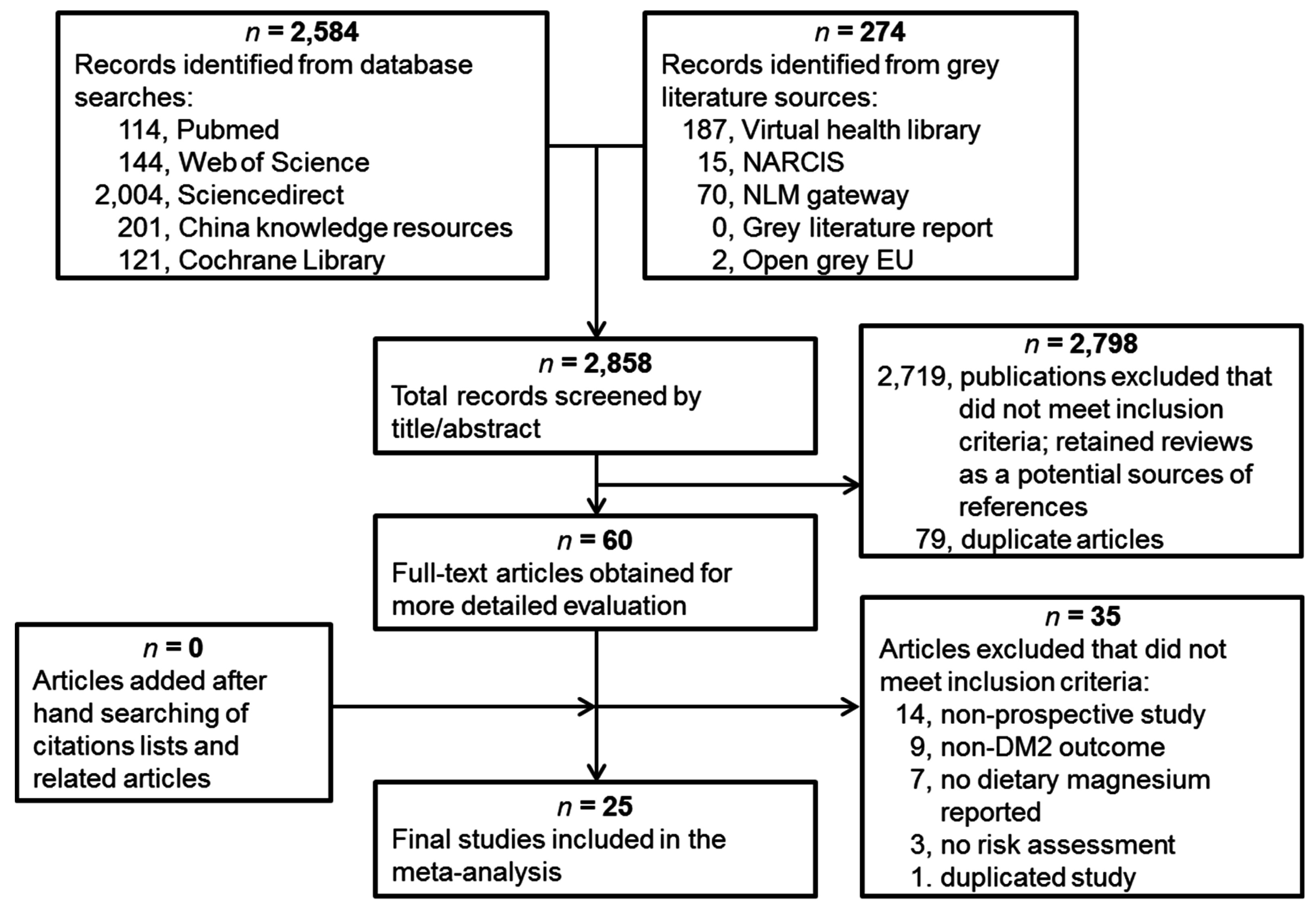
3.1. Eligible Studies and Characteristics
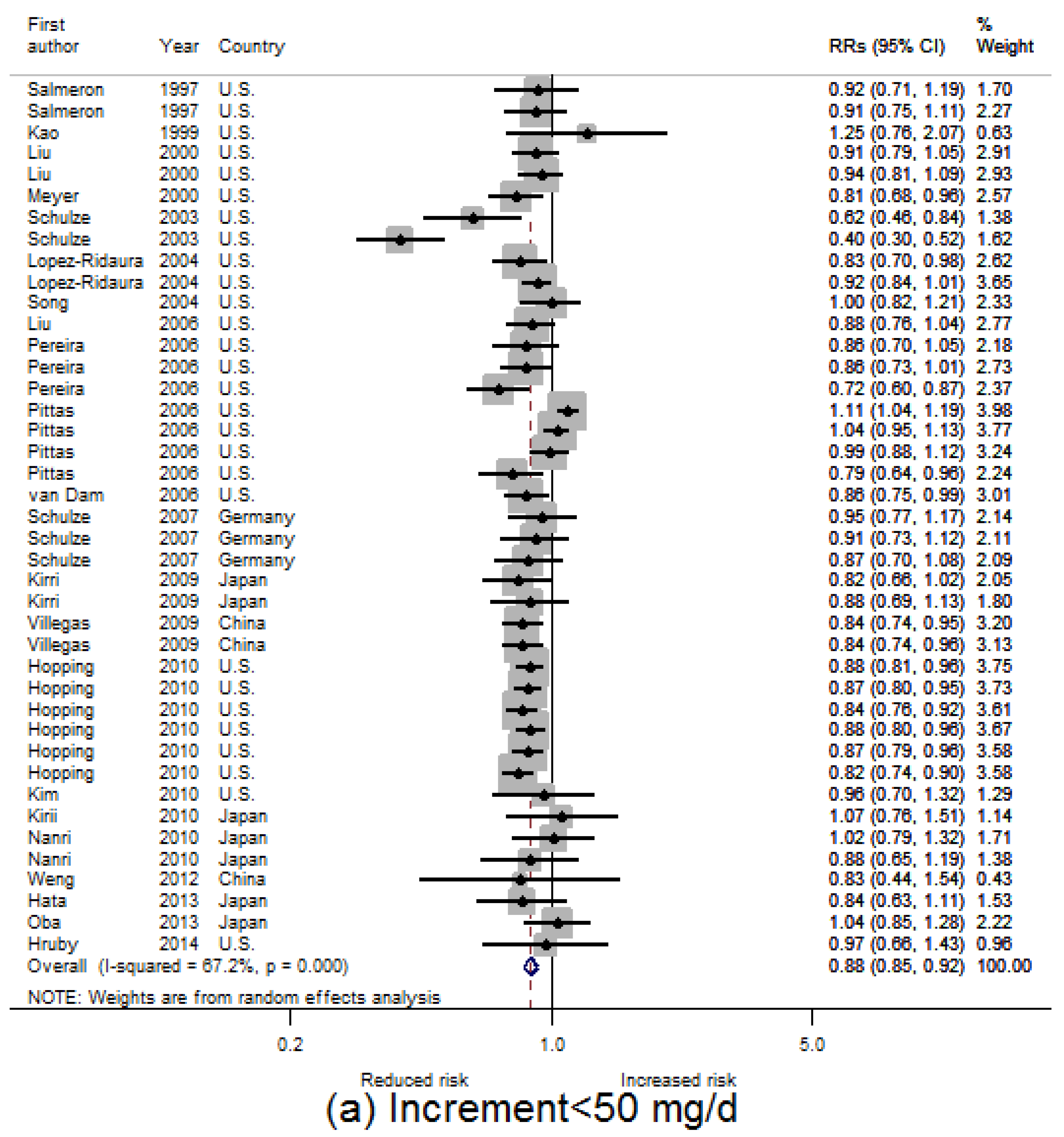
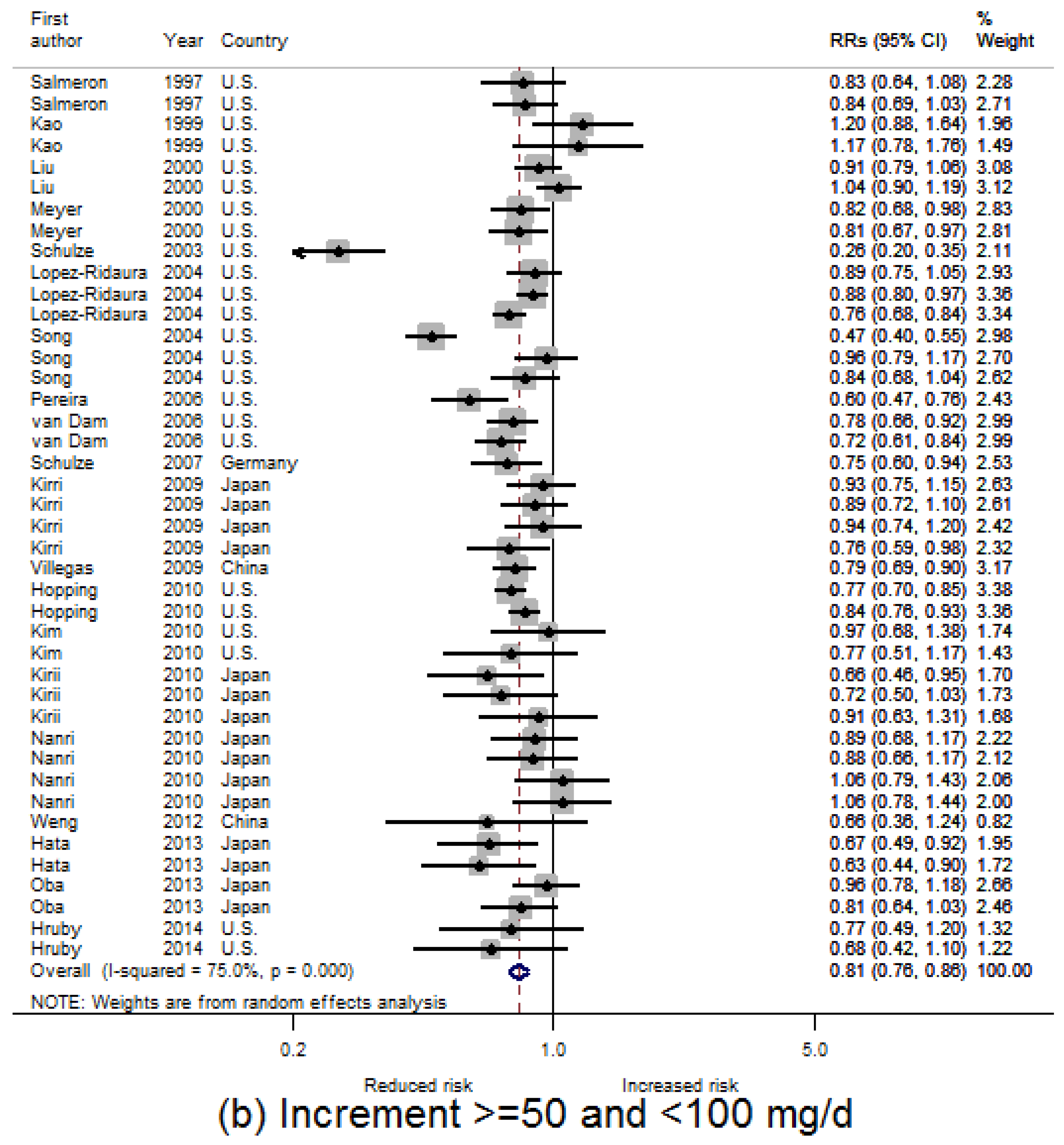
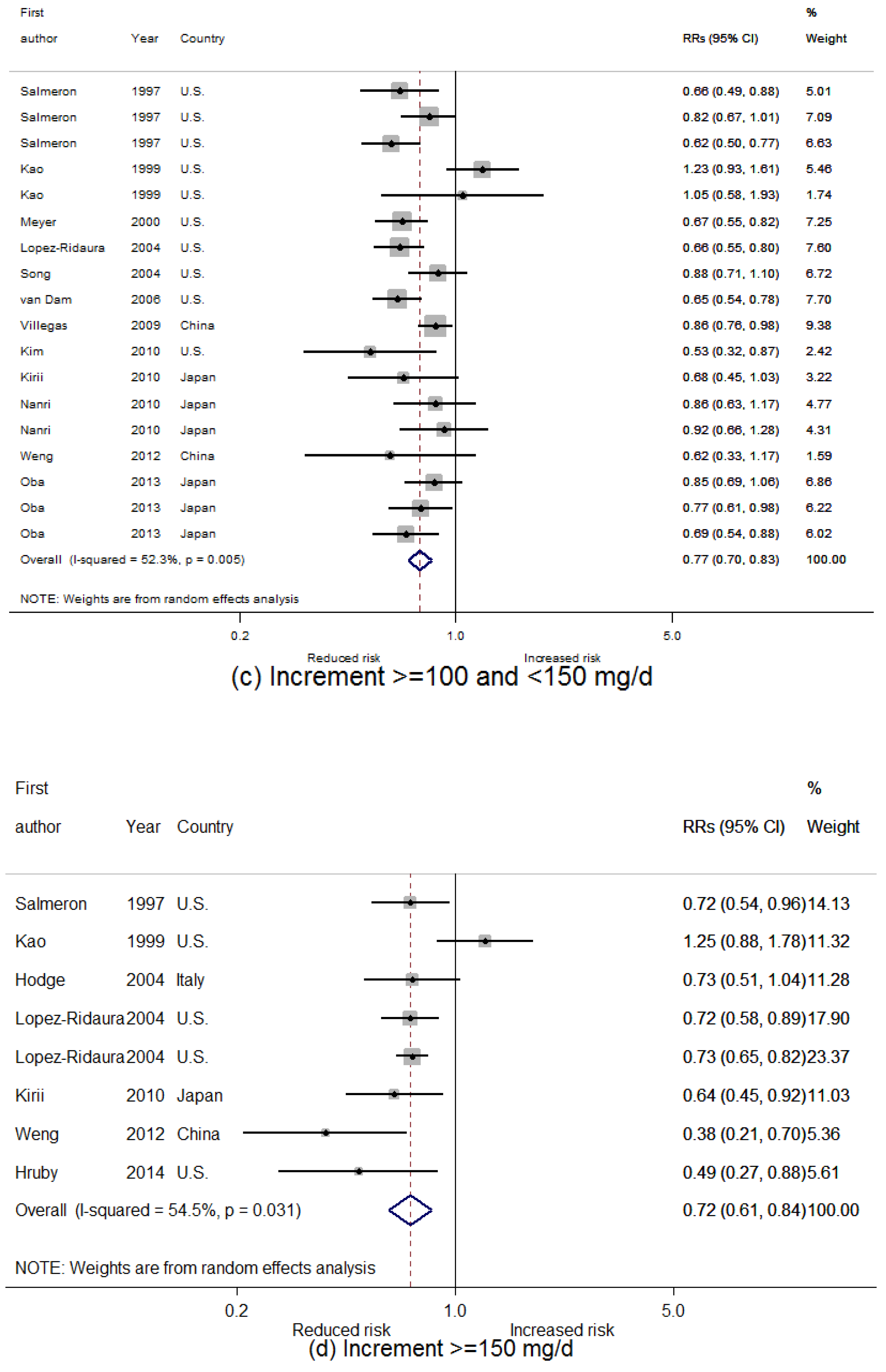
3.2. Dietary Magnesium Intake and Type 2 Diabetes Mellitus (T2D) Incidence
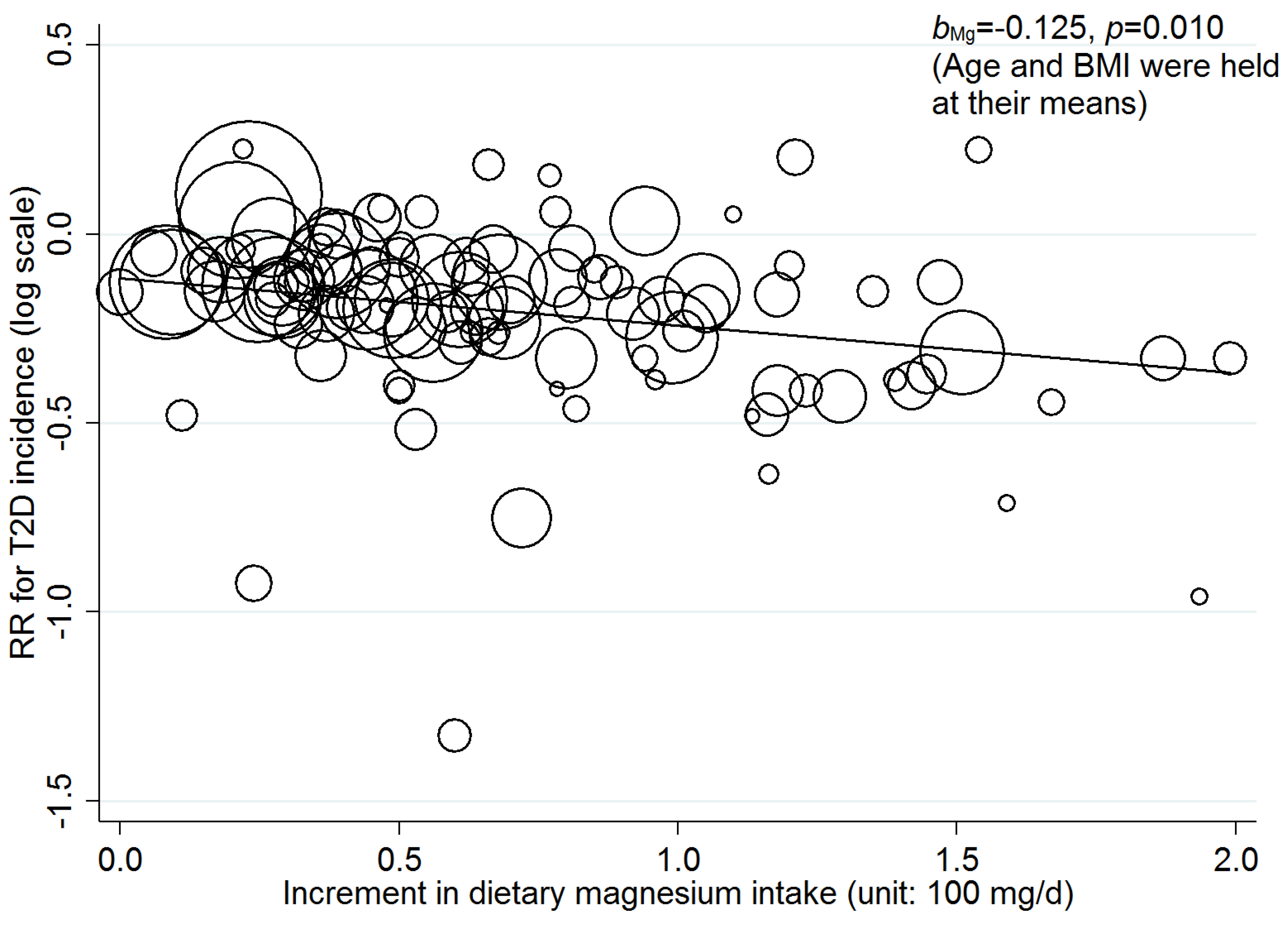
3.3. Linear Dose-Response Relationship
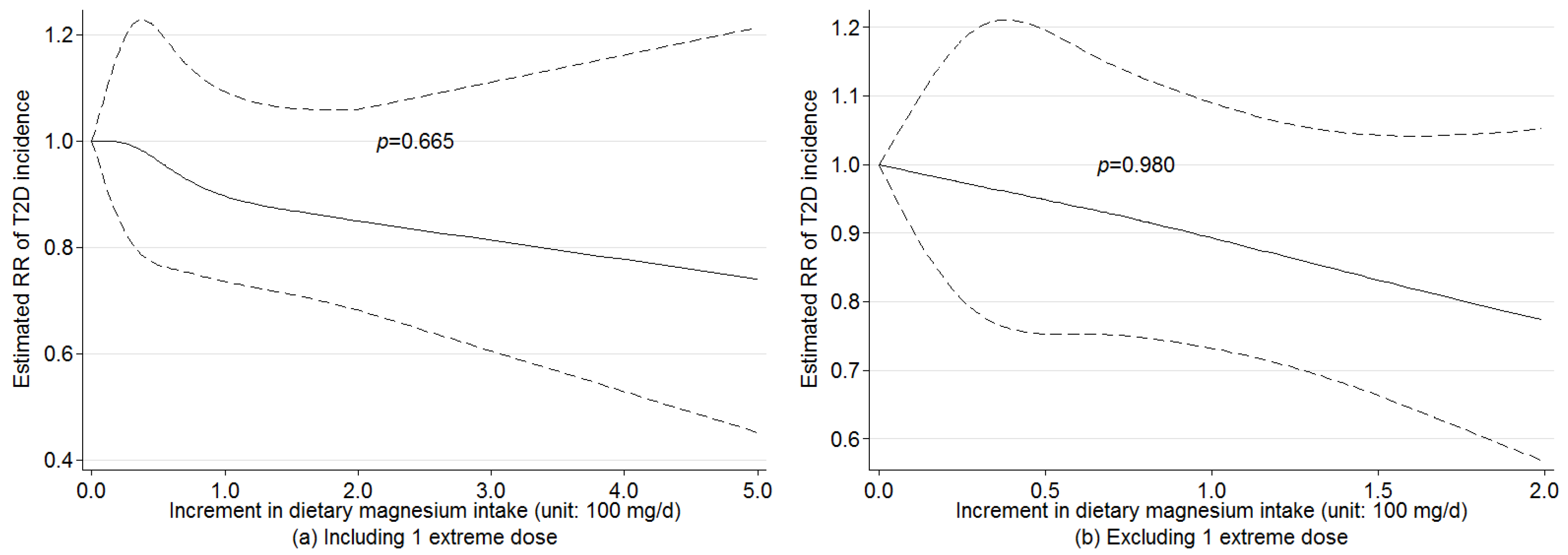
3.4. Nonlinear Dose-Response Relationship
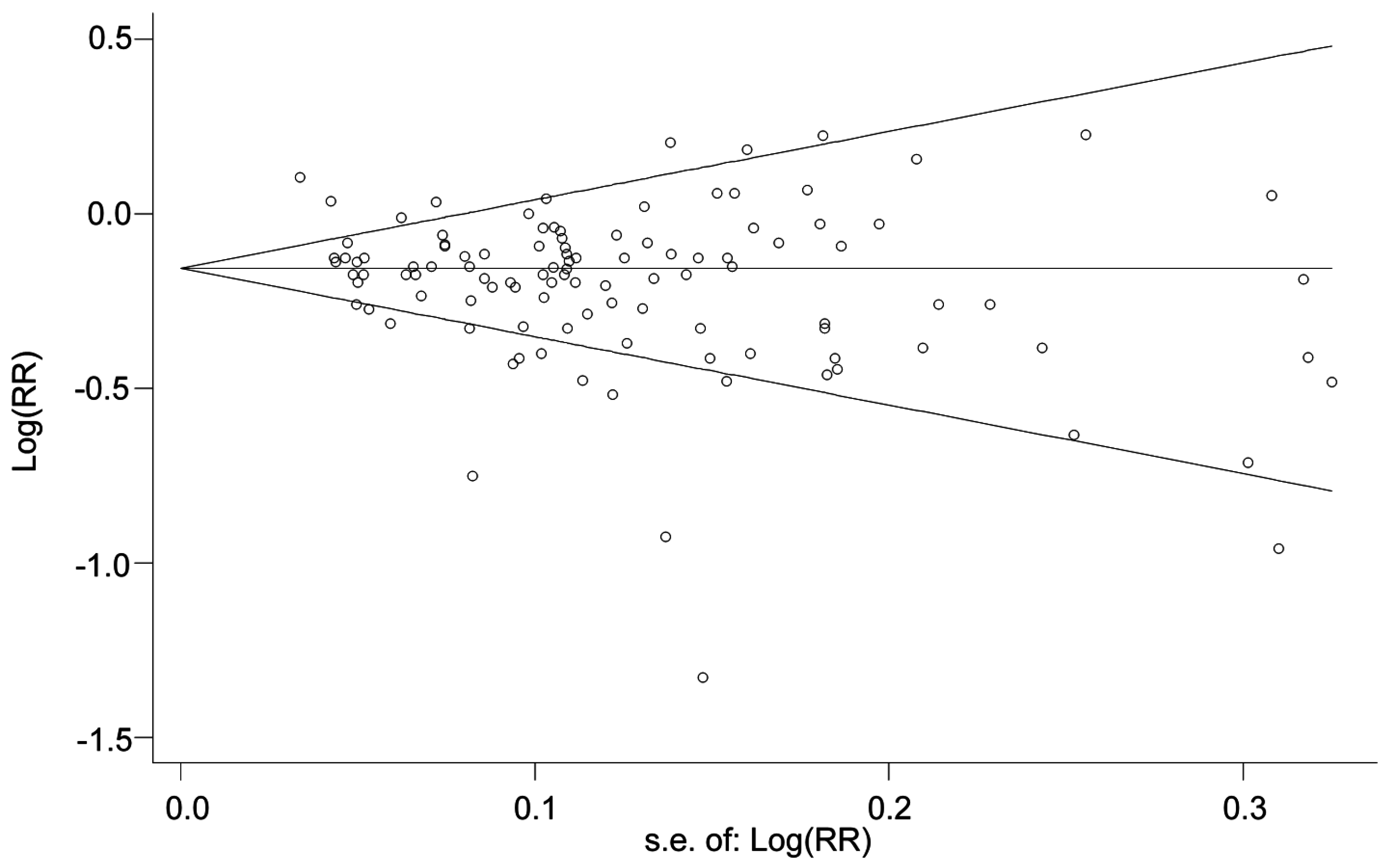
3.5. Sensitivity Analysis
4. Discussion
4.1. Dose-Response Association of Dietary Magnesium Intake with T2D Incidence
4.2. Implication for Practice
4.3. Strengths and Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| FFQ | Food frequency questionnaires |
| HR | Hazard ratio |
| OR | Odds ratio |
| RCT | Randomized controlled trial |
| REML | Restricted maximum likelihood |
| RR | Relative risk |
| T2D | Type 2 diabetes |
| US | United States |
References
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, F.B. The global implications of diabetes and cancer. Lancet 2014, 383, 1947–1948. [Google Scholar] [CrossRef]
- Colditz, G.A.; Manson, J.E.; Stampfer, M.J.; Rosner, B.; Willett, W.C.; Speizer, F.E. Diet and risk of clinical diabetes in women. Am. J. Clin. Nutr. 1992, 55, 1018–1023. [Google Scholar] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Balon, T.W.; Jasman, A.; Scott, S.; Meehan, W.P.; Rude, R.K.; Nadler, J.L. Dietary magnesium prevents fructose-induced insulin insensitivity in rats. Hypertension 1994, 23, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Meigs, J.B.; O’Donnell, C.J.; Jacques, P.F.; McKeown, N.M. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care 2014, 37, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Pisu, E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 2008, 19, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardo, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Ramadass, S.; Basu, S.; Srinivasan, A.R. Serum magnesium levels as an indicator of status of diabetes mellitus type 2. Diabetes Metab. Syndr. 2015, 9, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Folsom, A.R.; Melnick, S.L.; Eckfeldt, J.H.; Sharrett, A.R.; Nabulsi, A.A.; Hutchinson, R.G.; Metcalf, P.A. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: The aric study. Atherosclerosis risk in communities study. J. Clin. Epidemiol. 1995, 48, 927–940. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Song, Y.; Poirier, P.; Dewailly, E.; Elin, R.J.; Egeland, G.M. Low serum magnesium concentrations are associated with a high prevalence of premature ventricular complexes in obese adults with type 2 diabetes. Cardiovasc. Diabetol. 2012, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.H.; Folsom, A.R.; Nieto, F.J.; Mo, J.P.; Watson, R.L.; Brancati, F.L. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The atherosclerosis risk in communities study. Arch. Intern. Med. 1999, 159, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R., Jr.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [PubMed]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004, 27, 2701–2706. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 2004, 27, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Hu, F.B.; Rosenberg, L.; Krishnan, S.; Palmer, J.R. Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 2006, 29, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Gao, Y.T.; Dai, Q.; Yang, G.; Cai, H.; Li, H.; Zheng, W.; Shu, X.O. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: The Shanghai women’s health study. Am. J. Clin. Nutr. 2009, 89, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Hopping, B.N.; Erber, E.; Grandinetti, A.; Verheus, M.; Kolonel, L.N.; Maskarinec, G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J. Nutr. 2010, 140, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Xun, P.; Liu, K.; Loria, C.; Yokota, K.; Jacobs, D.R., Jr.; He, K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care 2010, 33, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Kirii, K.; Iso, H.; Date, C.; Fukui, M.; Tamakoshi, A. Magnesium intake and risk of self-reported type 2 diabetes among Japanese. J. Am. Coll. Nutr. 2010, 29, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Kirii, K.; Inoue, M.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Magnesium intake and type ii diabetes in japanese men and women: The Japan public health center-based prospective study. Eur. J. Clin. Nutr. 2010, 64, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Xun, P.; He, K.; Qin, L.Q. Magnesium intake and risk of type 2 diabetes: Meta-analysis of prospective cohort studies. Diabetes Care 2011, 34, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Magnesium intake and risk of type 2 diabetes: A meta-analysis. J. Intern. Med. 2007, 262, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, G.C.; Zhai, L.; Ke, K.F. Nonlinear reduction in risk for type 2 diabetes by magnesium intake: An updated meta-analysis of prospective cohort studies. Biomed. Environ. Sci. 2015, 28, 527–534. [Google Scholar] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Gargon, E.; Williamson, P.R.; Clarke, M. Collating the knowledge base for core outcome set development: Developing and appraising the search strategy for a systematic review. BMC Med. Res. Methodol. 2015, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, S.; Tatt, I.D.; Higgins, J.P. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. Int. J. Epidemiol. 2007, 36, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Carletta, J. Assessing agreement on classification tasks: The kappa statistic. Comput. Linguist. 1996, 22, 249–254. [Google Scholar]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Harbord, R.M.; Higgins, J.P.T. Meta-regression in Stata. Stata J. 2008, 8, 493–519. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey, S.G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Pollock, B.G. Regression models in clinical studies: Determining relationships between predictors and response. J. Natl. Cancer Inst. 1988, 80, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; White, I.R.; Thompson, S.G. Extending dersimonian and laird’s methodology to perform multivariate random effects meta-analyses. Stat. Med. 2010, 29, 1282–1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Choi, H.K.; Ford, E.; Song, Y.; Klevak, A.; Buring, J.E.; Manson, J.E. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006, 29, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Kirii, K.; Mizoue, T.; Iso, H.; Takahashi, Y.; Kato, M.; Inoue, M.; Noda, M.; Tsugane, S. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia 2009, 52, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Dawson-Hughes, B.; Li, T.; Van Dam, R.M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006, 29, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The women’s health study. Diabetes Care 2004, 27, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia 2003, 46, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Colditz, G.A.; Hennekens, C.H.; Willett, W.C. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am. J. Public Health 2000, 90, 1409–1415. [Google Scholar] [PubMed]
- Salmeron, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Parker, E.D.; Folsom, A.R. Coffee consumption and risk of type 2 diabetes mellitus: An 11-year prospective study of 28,812 postmenopausal women. Arch. Intern. Med. 2006, 166, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Nanri, A.; Kurotani, K.; Goto, A.; Kato, M.; Mizoue, T.; Noda, M.; Inoue, M.; Tsugane, S. Dietary glycemic index, glycemic load and incidence of type 2 diabetes in japanese men and women: The Japan public health center-based prospective study. Nutr. J. 2013, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Song, Y.; Belin, R.J.; Chen, Y. Magnesium intake and the metabolic syndrome: Epidemiologic evidence to date. J. Cardiometab. Syndr. 2006, 1, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Doi, Y.; Ninomiya, T.; Mukai, N.; Hirakawa, Y.; Hata, J.; Ozawa, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Magnesium intake decreases type 2 diabetes risk through the improvement of insulin resistance and inflammation: The Hisayama study. Diabet. Med. 2013, 30, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.C.; Lee, N.J.; Yeh, W.T.; Ho, L.T.; Pan, W.H. Lower intake of magnesium and dietary fiber increases the incidence of type 2 diabetes in Taiwanese. J. Formos. Med. Assoc. 2012, 111, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Lawson, A.B.; Liese, A.D.; Bell, R.A.; Mayer-Davis, E.J. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: Approaches to modeling a dose-dependent association. Am. J. Epidemiol. 2006, 164, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Moran, M.; Simental, M.L.; Zambrano, G.G.; Guerrero-Romero, F. The role of magnesium in type 2 diabetes: A brief based-clinical review. Magnes. Res. 2011, 24, 156–162. [Google Scholar] [PubMed]
- Meisinger, C.; Thorand, B.; Schneider, A.; Stieber, J.; Doring, A.; Lowel, H. Sex differences in risk factors for incident type 2 diabetes mellitus: The MONICA Augsburg cohort study. Arch. Intern. Med. 2002, 162, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Franco, M.; Dobs, A.S.; Ouyang, P.; Vaidya, D.; Bertoni, A.; Gapstur, S.M.; Golden, S.H. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J. Clin. Endocrinol. Metab. 2009, 94, 4127–4135. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.; Goldman, J.; Cleveland, L. What We Eat in America, NHANES 2001–2002: Usual Nutrient Intakes from Food Compared to Dietary References Intake; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 2005; pp. 1–56.
| First Author, Year, Study, Country | No. of Case (Cohort Size) | Years of Follow-Up | Dietary Assessment Method | Case Ascertainment | Sex, Mean Age at Baseline (Years) | Magnesium Intake (Magnesium/Day) for Highest vs. the Lowest Quantile [RR (95% CI)] | Confounders Adjusted for |
|---|---|---|---|---|---|---|---|
| Hruby, 2014, FHSO, U.S. [7] | 179 (2582) | 7 | Validated FFQ | Validated self-report | M and F, 53.9 | 395 vs. 236 (0.49 (0.27, 0.88)) | Age, sex, energy intake, family history of diabetes, BMI, physical activity, smoking, alcohol, hypertension, dietary fiber |
| Oba, 2013, JPHCPS, Japan [50] | Men: 690 (27,769) | 5 | Validated FFQ | Validated self-report | M: 56.5 | 349 vs. 232 (0.85 (0.69, 1.06)) | Crude * |
| Women: 500 (36,864) | F: 56.9 | 356 vs. 211 (0.69 (0.54, 0.88)) | |||||
| Hata, 2013, Hisayama, Japan [52] | 417 (1999) | 15.6 | FFQ | Self-report | M and F, 57.0 | 215 vs. 133 (0.63 (0.44, 0.90)) | Age, sex, family history of diabetes, BMI. HDL cholesterol, triglycerides, hypertension, smoking, alcohol, physical activity, total energy intake, carbohydrate, crude fiber, saturated fatty acid, polyunsaturated fatty acid and vitamin C |
| Weng, 2012, CVDFACTS, Taiwan [53] | 141 (1604) | 4.6 | Validated FFQ | Self-report | M and F, 50.0 | 406 vs. 212 (0.38 (0.21, 0.70)) | Age, sex, caloric intake, residential area, family history of diabetes, BMI, education, smoking, alcohol, physical activity, hypertension, hypercholesterolemia, hypertriglyceridemia, low HDL-cholesterol |
| Hopping, 2010, MEC, Hawaii [21] | Men: 4555 (36,255) | 14 | FFQ | Validated self-report | M: 57.4 | 185 vs. 129 (0.77 (0.70, 0.85)) | Ethnicity, BMI, physical activity, education, calories |
| Women: 4032 (39,255) | F: 57.2 | 200 vs. 139 (0.84 (0.76, 0.93)) | |||||
| Kirii, 2010, JACC, Japan [23] | Men: 237 (6480) | 5 | Validated FFQ | Validated self-report | M: 53.3 | 323 vs. 156 (0.64 (0.44, 0.91)) | Age, BMI, family history of diabetes, smoking, alcohol, physical activity, green tea, coffee, total energy intake |
| Women: 222 (11,112) | F: 53.1 | 298 vs. 159 (0.68 (0.33, 0.75)) | |||||
| Nanri, 2010, JPHCPS, Japan [24] | Men: 634 (25,872) | 5 | FFQ | Validated self-report | M: 56.5 | 348 vs. 213 (0.86 (0.63, 1.16)) | Age, study area, BMI, smoking, alcohol, family history of diabetes, physical activity, hypertension, coffee, calcium intake, total energy intake |
| Women: 480 (33,919) | F: 57.3 | 333 vs. 213 (0.92 (0.66, 1.28)) | |||||
| Kim, 2010, CARDIA, U.S. [22] | 330 (4497) | 20 | Validated FFQ | Validated self-report | M and F, 24.9 | 478 vs. 362 (0.53 (0.32, 0.86)) | Age, sex, ethnicity, study center, education, smoking, alcohol, physical activity, family history of diabetes, BMI, blood pressure, total energy intake, saturated fat, crude fiber |
| Kirri, 2009, JPHCPS, Japan [43] | Men: 634 (25,877) | 5 | FFQ | Validated self-report | M: 56.5 | 331 vs. 245 (0.89 (0.72, 1.10)) | Crude |
| Women: 480 (33,919) | F: 57.3 | 314 vs. 248 (0.76 (0.59, 0.98)) | |||||
| Villegas, 2009, SWHS, China [20] | 2270 (64,190) | 7 | Validated FFQ | Self-report | F: 51.0 | 318 vs. 214 (0.86 (0.75, 0.97)) | Age, energy intake, WHR, smoking, alcohol, physical activity, income, education level, occupation, hypertension |
| Schulze, 2007, EPIC, Germany [19] | 844 (27,550) | 7 | Validated FFQ | Validated self-report | M and F, 49.6 | 359 vs. 298 (0.75 (0.60, 0.94)) | Crude |
| Liu, 2006, WHS, U.S. [42] | 651 (14,874) | 10 | Validated FFQ | Validated self-report | F: 54.5 | 340 vs. 307 (0.88 (0.76, 1.04)) | Crude |
| Pereira, 2006, IWHS, U.S. [49] | 1415 (28,812) | 11 | FFQ | Validated self-report | F: 61.3 | 334 vs. 281 (0.60 (0.47, 0.76)) | Crude |
| van Dam, 2006, BWHS, U.S. [18] | 1964 (41,186) | 8 | Validated FFQ | Validated self-report | F: 38.7 | 244 vs. 115 (0.65 (0.54, 0.78)) | Age, energy intake, BMI, smoking, alcohol, physical activity, family diabetes history, education level, calcium, coffee, sugar, soft drink, processed meat, red meat |
| Pittas, 2006, NHS, U.S. [44] | 4843 (83,779) | 20 | FFQ | Validated self-report | F: 46.1 | 313 vs. 281 (0.79 (0.64, 0.96)) | Crude |
| Song, 2004, WHS, U.S. [45] | 708 (14,924) | 8.8 | Validated FFQ | Validated self-report | F: 53.9 | 377 vs. 305 (0.47 (0.41, 0.55)) | Crude |
| Song 2004, WHS, U.S. [17] | 918 (38,025) | 6 | Validated FFQ | Validated self-report | F: 53.9 | 399 vs. 252 (0.88 (0.71, 1.1)) | Age, smoking, BMI, exercise, alcohol, family history of diabetes and total calories |
| Lopez-Ridaura, 2004, NHS, U.S. [16] | 4085 (85,060) | 18 | FFQ | Validated self-report | F: 46.1 | 373 vs. 222 ([0.73 (0.65, 0.82)) | Age, energy, family history of diabetes, BMI, physical activity, smoking, alcohol, hypertension, hypercholesterolemia, other dietary variables |
| Lopez-Ridaura, 2004, HPFS, U.S. [16] | 1333 (42,872) | 12 | FFQ | Validated self-report | M: 53.3 | 457 vs. 270 (0.72 (0.58, 0.89)) | Age, energy, family history of diabetes, BMI, physical activity, smoking, alcohol, hypertension, hypercholesterolemia, other dietary variables |
| Hodge, 2004, MCCS, Italy [15] | 365 (31,641) | 4 | FFQ | Validated self-report | M and F, 54.5 | Per 500 magnesium increment (0.73 (0.51, 1.04)) | Age, sex, country of birth, physical activity, family history of diabetes, alcohol, education, weight change, energy intake, BMI and WHR |
| Schulze, 2003, NHS II, U.S. [46] | 741 (92,146) | 8 | Validated FFQ | Validated self-report | F: 36.0 | 341 vs. 281 [0.26 (0.20, 0.36)) | Crude |
| Liu, 2000, NHS, U.S. [47] | 1879 (75,521) | 10 | Validated FFQ | Validated self-report | F: 56.5 | 342 vs. 248 (1.04 (0.90, 1.19)) | Crude |
| Meyer, 2000, IWHS, U.S. [14] | 1141 (35,988) | 6 | FFQ | Validated self-report | F: 61.5 | 362 vs. 220 (0.67 (0.55, 0.82)) | Age, total energy intake, BMI, WTH ratio, education, smoking, alcohol intake,
physical activity |
| Kao, 1999, ARIC, U.S. [13] | White people: 739 (9506) | 6 | FFQ | Validated self-report | M and F, 54.2 | 418 vs. 308 (1.25 (0.88, 0.1.78)) | Age, sex education, family history of diabetes, BMI, WHR, physical activity, alcohol, diuretic use, dietary calcium, potassium, fasting insulin and glucose |
| Black people: 367 (2622) | M and F, 53.0 | 374 vs. 264 (1.05 (0.58, 1.93)) | |||||
| Salmeron, 1997, HPFS, U.S. [48] | 523 (42,759) | 6 | FFQ | Validated self-report | M: 53.3 | 461 vs. 262 (0.72 (0.54, 0.96)) | Age, BMI, alcohol, smoking, physical activity, family history of diabetes |
| Salmeron, 1997, NHS, U.S. [54] | 915 (65,173) | 6 | FFQ | Validated self-report | F: 46.1 | 338 vs. 222 (0.62 (0.50, 0.78)) | Age, BMI, alcohol, smoking, physical activity, family history of diabetes |
| Subgroup | No. of Studies (Dose Quantiles) | RR (95% CI) | I2 (%) | Heterogeneity-p |
|---|---|---|---|---|
| Sex | ||||
| Women | 17 (58) | 0.814 (0.774, 0.856) | 82.4 | <0.001 |
| Men | 7 (25) | 0.838 (0.803, 0.876) | 25.7 | 0.120 |
| Both | 7 (26) | 0.854 (0.775, 0.941) | 46.7 | 0.005 |
| Area | ||||
| U.S. | 16 (67) | 0.817 (0.780, 0.857) | 81.7 | <0.001 |
| Europe | 2 (5) | 0.858 (0.774, 0.951) | 0 | 0.498 |
| Asia | 7 (37) | 0.846 (0.811, 0.883) | 10.2 | 0.294 |
| Adjustment | ||||
| Adjusted † | 16 (76) | 0.830 (0.806, 0.855) | 39.6 | <0.001 |
| Crude ‡ | 9 (33) | 0.808 (0.741, 0.881) | 87.8 | <0.001 |
| Black people | 2 (7) | 0.815 (0.711, 0.935) | 59.3 | 0.022 |
| No. of Studies (Doses) | I2 (%) | RR (95% CI) | p-Value | |
|---|---|---|---|---|
| All studies | 25 (105) | 69.72 | 0.916 (0.852, 0.985) | 0.018 |
| All studies * | 24 (104) | 69.13 | 0.882 (0.803, 0.969) | 0.010 |
| Sex | ||||
| Women | 17 (56) | 78.87 | 0.879 (0.756, 1.023) | 0.094 |
| Men | 7 (23) | 0 | 0.865 (0.767, 0.975) | 0.020 |
| Both | 7 (26) | 26.51 | 0.935 (0.853, 1.026) | 0.148 |
| Both * | 6 (25) | 29.00 | 0.857 (0.695, 1.057) | 0.141 |
| Area | ||||
| U.S. | 16 (63) | 79.09 | 0.910 (0.796, 1.042) | 0.169 |
| Europe | 2 (5) | 0 | 1.071 (0.264, 4.351) | 0.644 |
| Europe * | 1 (4) | - | - | - |
| Asia | 7 (37) | 0 | 0.867 (0.768, 0.978) | 0.022 |
| Adjustment | ||||
| Adjusted | 16 (72) | 25.73 | 0.911 (0.864, 0.961) | 0.001 |
| Adjusted * | 15 (71) | 24.09 | 0.885 (0.830, 0.944) | <0.001 |
| Crude | 9 (33) | 85.14 | 0.653 (0.462, 0.924) | 0.018 |
| Black people | 2 (7) | 0 | 0.747 (0.232, 2.409) | 0.486 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Han, H.; Li, M.; Liang, C.; Fan, Z.; Aaseth, J.; He, J.; Montgomery, S.; Cao, Y. Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients 2016, 8, 739. https://doi.org/10.3390/nu8110739
Fang X, Han H, Li M, Liang C, Fan Z, Aaseth J, He J, Montgomery S, Cao Y. Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients. 2016; 8(11):739. https://doi.org/10.3390/nu8110739
Chicago/Turabian StyleFang, Xin, Hedong Han, Mei Li, Chun Liang, Zhongjie Fan, Jan Aaseth, Jia He, Scott Montgomery, and Yang Cao. 2016. "Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies" Nutrients 8, no. 11: 739. https://doi.org/10.3390/nu8110739
APA StyleFang, X., Han, H., Li, M., Liang, C., Fan, Z., Aaseth, J., He, J., Montgomery, S., & Cao, Y. (2016). Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients, 8(11), 739. https://doi.org/10.3390/nu8110739






