“English Disease”: Historical Notes on Rickets, the Bone–Lung Link and Child Neglect Issues
Abstract
:1. Introduction
↓ Nutritional intake of Vitamin D
- Exclusive breastfeeding > 6 months
- ↓ Maternal Vitamin D stores
- Malabsorption
- ○
- Gluten-sensitive enteropathy (GSE)
- ○
- Non-GSE malabsorption syndromes
- ○
- Cystic fibrosis (CF)
- ○
- Non-CF-related pancreatic insufficiency
- ○
- Biliary atresia (BA)
- ○
- Non-BA extrahepatic obstructive cholangiopathies
↓ Synthesis or ↑ degradation of 25(OH)D
- Liver cirrhosis
- Non-cirrhotic chronic liver disease
- ↑ Vitamin D metabolism (e.g., isoniazid, rifampicin, and anticonvulsants therapy)
- ↑ Skin pigmentation
- ↓ Sun exposure:
- ○
- Sunscreen with protection factor > 8, type of clothing, shades
- ○
- Latitude > 40 degrees (North or South), “long winters”, air polluted geographical areas, and “perennial clouding” geographical areas
2. Historical Notes
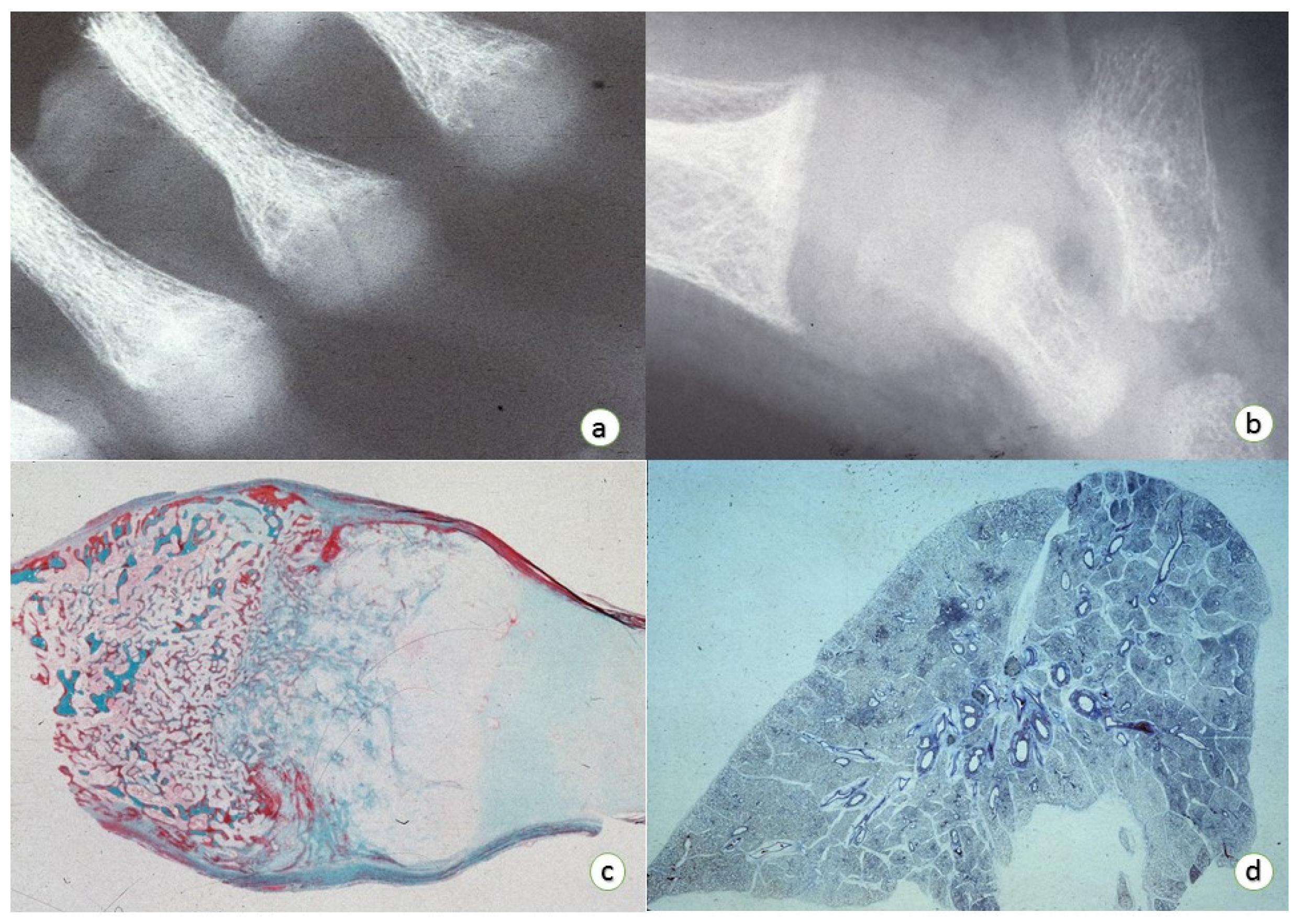
3. Osteopathology
Epiphyseal Physis:
- (1)
- Enlargement of the epiphyseal plate in height and width.
- (2)
- Enlargement and distortion of the hypertrophic cell zone with poorly definition of the zone of provisional calcification associated with loss of the normal architecture of the cellular columns with small amount of intervening matrix.
- (3)
- Failure of adequate mineralization and vascular invasion.
- (4)
- Normal resting cartilage with prolongations (so-called “tongues”) of viable cartilage without histologic evidence of active endochondral replacement.
Medullary Bone:
- (1)
- ↓ Medullary bone with thin and irregular bony trabeculae with an extended layer of un-mineralized bone (osteoid seams) surrounding mineralized bony segments.
- (2)
- ↓ Bony component in both trabecular and cortical bones.
4. Respiratory Tract Pathology
5. Public Health and Socio-Ideological Debate
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morris, H.A.; Turner, A.G.; Anderson, P.H. Vitamin-D regulation of bone mineralization and remodelling during growth. Front. Biosci. 2012, 4, 677–689. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Boot, A.M.; Czech-Kowalska, J.; Weber, G.; Camargo, C.A., Jr.; Mallet, E.; Fanos, M.; Shaw, N.J.; Holick, M.F. Vitamin D in childhood and adolescence: An expert position statement. Eur. J. Pediatr. 2015, 174, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Vojinovic, J.; Cimaz, R. Vitamin D-update for the pediatric rheumatologists. Pediatr. Rheumatol. Online J. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Aggarwal, S. Vitamin D deficiency: Diagnosis and patient centred management. J. Pak. Med. Assoc. 2015, 65, 569–573. [Google Scholar] [PubMed]
- Kitanaka, S. Vitamin D dependency and its treatment. Clin. Calcium 2016, 26, 277–283. [Google Scholar] [PubMed]
- Kepron, C.; Pollanen, M.S. Rickets or abuse? A histologic comparison of rickets and child abuse-related fractures. Forensic. Sci. Med. Pathol. 2015, 11, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Linderkamp, O. Pathological case of the month: Classic rickets in a setting of significant psychosocial deprivation. Arch. Pediatr. Adolesc. Med. 2001, 155, 967–968. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W.; Mehls, O.; Anast, C.S.; Brown, E.; Hammerman, M.R.; Portale, A.; Fallon, M.D.; Mahan, J., Jr.; Alfrey, A.C. Renal osteodystrophy in children: The role of Vitamin D, phosphorus, and parathyroid hormone. Am. J. Kidney Dis. 1986, 7, 275–284. [Google Scholar] [CrossRef]
- Pettifor, J.M.; Prentice, A. The role of Vitamin D in paediatric bone health. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A. Nutritional rickets around the world. J. Steroid Biochem. Mol. Biol. 2013, 136, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A. Vitamin D deficiency: A global perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Schoenmakers, I.; Laskey, M.A.; de Bono, S.; Ginty, F.; Goldberg, G.R. Nutrition and bone growth and development. Proc. Nutr. Soc. 2006, 65, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W. Vitamin D and the magic mountain: The anti-infectious role of the vitamin. J. Pediatr. 2010, 156, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Beck-Nielsen, S.S. Rickets in Denmark. Dan. Med. J. 2012, 59, B4384. [Google Scholar] [PubMed]
- Alles, M.S.; Eussen, S.R.; van der Beek, E.M. Nutritional challenges and opportunities during the weaning period and in young childhood. Ann. Nutr. Metab. 2014, 64, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ameen, S.; Staub, L.; Ulrich, S.; Vock, P.; Ballmer, F.; Anderson, S.E. Harris lines of the tibia across centuries: A comparison of two populations, medieval and contemporary in central Europe. Skelet. Radiol. 2005, 34, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.; Saeed, A.; AlSarkhy, A.; El Mouzan, M.I.; El Matary, W. Celiac disease presenting as rickets in Saudi children. Ann. Saudi Med. 2013, 33, 49–51. [Google Scholar] [PubMed]
- Holick, M.F. High prevalence of Vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Kaganov, B.; Caroli, M.; Mazur, A.; Singhal, A.; Vania, A. Suboptimal micronutrient intake among children in Europe. Nutrients 2015, 7, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Pettifor, J.M. Vitamin D &/or calcium deficiency rickets in infants & children: A global perspective. Indian J. Med. Res. 2008, 127, 245–249. [Google Scholar] [PubMed]
- Vahlquist, B. Two-century perspective of some major nutritional deficiency diseases in childhood. Acta Paediatr. Scand. 1975, 64, 161–171. [Google Scholar] [PubMed]
- Wandel, M. Nutrition-related diseases and dietary change among third world immigrants in northern Europe. Nutr. Health 1993, 9, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P.T.; Shaikh, U. The effects of Vitamin D deficiency and insufficiency on the endocrine and paracrine systems. Biol. Res. Nurs. 2007, 9, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Amorim Cruz, J.A. Nutrition and osteoporosis: Facts and uncertainties about calcium and Vitamin D recommendations. Forum. Nutr. 2003, 56, 178–181. [Google Scholar] [PubMed]
- Calvo, M.S.; Uribarri, J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am. J. Clin. Nutr. 2013, 98, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Vitamin D in childhood and adolescence. Postgrad. Med. J. 2007, 83, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.E.; Coleman, L.A. Vitamin D and influenza. Adv. Nutr. 2012, 3, 517–525. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Vitamin D: Historical overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar] [PubMed]
- Ogunkolade, B.W.; Boucher, B.J.; Fairclough, P.D.; Hitman, G.A.; Dorudi, S.; Jenkins, P.J.; Bustin, S.A. Expression of 25-hydroxyVitamin D-1-alpha-hydroxylase mRNA in individuals with colorectal cancer. Lancet 2002, 359, 1831–1832. [Google Scholar] [CrossRef]
- Bah, S.Y.; Dickinson, P.; Forster, T.; Kampmann, B.; Ghazal, P. Immune oxysterols: Role in mycobacterial infection and inflammation. J. Steroid Biochem. Mol. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Pilz, S.; Hoffmann, H.; Marz, W. Vitamin D and airway infections: A European perspective. Eur. J. Med. Res. 2016, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Immunological aspects of sport nutrition. Immunol. Cell Biol. 2016, 94, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Neme, A.; Nurminen, V.; Seuter, S.; Carlberg, C. The Vitamin D-dependent transcriptome of human monocytes. J. Steroid Biochem. Mol. Biol. 2016, 164, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyVitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Provvedini, D.M.; Tsoukas, C.D. Interactions of 1,25-dihydroxyVitamin D3 and the immune system. Mol. Cell. Endocrinol. 1985, 43, 113–122. [Google Scholar] [CrossRef]
- Kyle, R.A.; Shampo, M.A. Jeanne Mance: Founder of Hotel Dieu of Montreal. Mayo Clin. Proc. 1988, 63, 212. [Google Scholar] [CrossRef]
- Whistler, D. “De morbo puerile anglorum, quem patrio idiomate indigenae vocant the Rickets” (Concerning the disease of English children, which in English it is called “Rickets”). M.D. Thesis, Universiteit Leiden, Academia Lugduno-Batava, Leiden, The Netherland, 1645. [Google Scholar]
- Nova et Vetera. Paedosplanchnosteocaces. BMJ 1925, 2, 1080. [Google Scholar]
- Cone, T.E., Jr. A rachitic infant painted by Burgkmair 136 years before Dr. Whistler described rickets. Clin. Pediatr. 1980, 19, 194. [Google Scholar] [CrossRef]
- Vitiello, A.; Fornaciari, A.; Giusiani, S.; Fornaciari, G.; Giuffra, V. The Medici children (Florence, XVI–XVII centuries): Anthropological study and proposal of identification. Med. Secoli. 2015, 27, 29–49. [Google Scholar] [PubMed]
- Castagna, M.; Giuffra, V.; Fattori, S.; Vitiello, A.; Caramella, D.; Giustini, D.; Fornaciari, G. Rickets at the medici court of florence: The case of Don Filippino (1577–1582). Med. Secoli 2014, 26, 779–792. [Google Scholar] [PubMed]
- Fornaciari, G.; Vitiello, A.; Giusiani, S.; Giuffra, V.; Fornaciari, A.; Villari, N. The Medici project first anthropological and paleopathological results of the exploration of the Medici tombs in Florence. Med. Secoli 2007, 19, 521–543. [Google Scholar] [PubMed]
- Giuffra, V.; Vitiello, A.; Caramella, D.; Fornaciari, A.; Giustini, D.; Fornaciari, G. Rickets in a high social class of renaissance Italy: The Medici children. Int. J. Osteoarchaeol. 2015, 25, 608–624. [Google Scholar] [CrossRef]
- Frenk, S.; Faure-Fontenla, M.A. Rachitis, not arthritis, in Caravaggio’s sleeping child. Lancet 1995, 345, 801. [Google Scholar] [CrossRef]
- Frenk, S. Rickets. Int. Child Health 1993, 4, 69–75. [Google Scholar]
- Foote, J. Evidence of rickets prior to 1650. Am. J. Dis. Child. 1927, 34, 443–452. [Google Scholar] [CrossRef]
- Ihde, A.J. Studies on the history of rickets. II. The roles of cod liver oil and light. Pharm. Hist. 1975, 17, 13–20. [Google Scholar] [PubMed]
- Ihde, A.J. Studies on the history of rickets. I. Recognition of rickets as a deficiency disease. Pharm. Hist. 1974, 16, 83–88. [Google Scholar] [PubMed]
- Ebstein, W. Uber das Vorkommen rachitischer Skelettveränderungen im Altertum und im Mittelalter. Virchow’s Arch. 1908, 193, 519–545. [Google Scholar]
- Soranus, E. «On Gynecology» (Sorani Gynaeciorium, Libri IV); The Johns Hopkins University Press: Baltimore, MD, USA; London, UK, 1991. [Google Scholar]
- Galenus, C. «On Hygiene» (de Sanitate Tuenda); Charles Thomas Books: Springfield, MD, USA, 1951. [Google Scholar]
- Gwei-Djen, L.; Needham, J. Records of diseases in ancient China. Am. J. Chin. Med. 1976, 4, 3–16. [Google Scholar] [PubMed]
- Ernst, H. Die Alt Niederlandische Malerei; Eugen Diederichs Verlag: Jena, Germany, 1924. [Google Scholar]
- Ernst, H. Die Alt Deutsche Malerei; Eugen Diederichs Verlag: Jena, Germany, 1909. [Google Scholar]
- Glisson, F. De Rachitide Sive Morbo Puerili Qui Vulgo the Rickets Dicitur; Dugard: London, UK, 1650. [Google Scholar]
- Locke, J. The Correspondence of John Locke to John Locke Senior 20 December 1660; Clarendon Press: Oxford, UK, 1989; Volume 1, p. 162. [Google Scholar]
- Dewhurst, K. John Locke (1632–1704), Physician and Philosopher: A Medical Biography; Wellcome Historical Medical Library: London, UK, 1963; p. 6. [Google Scholar]
- Fox Bourne, H.R. The life of John Locke. In Bibliographica Brittanica; Kessinger Press: Whitefish, MT, USA, 1876; pp. 3009–4298. [Google Scholar]
- Conrad LI, N.M.; Nutton, V.; Porter, R.; Wear, A. The Western Medical Tradition; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Williams, A.N. John Locke, “Rhickets” and the cardiopulmonary circulation. Pediatr. Cardiol. 2012, 33, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.N.; Wilson, N.; Sunderland, R. Philosopher, pediatrician, pathologist? John Locke’s thoughts on Rickets and a missed case of Ebstein’s anomaly. Pediatr. Cardiol. 2009, 30, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, K. Post-mortem examination on case of rickets performed by John Locke. Br. Med. J. 1962, 2, 1466. [Google Scholar] [PubMed]
- Macewan, W. Osteotomy with an Enquiry into the Aetiology and Pathology of Knock-Knee, Bow-Leg and other Osseous Deformities of the Lower Limbs; Churchill: London, UK, 1880. [Google Scholar]
- Fenwick, S. Outlines of Medical Treatment, 3rd ed.; Churchill: London, UK, 1891. [Google Scholar]
- Gibbs, D. Rickets and the crippled child: An historical perspective. J. R. Soc. Med. 1994, 87, 729–732. [Google Scholar] [PubMed]
- Nightingale, F. Nursing the sick. In A Dictionary of Medicine; Quain, R., Ed.; Longmans Green: London, UK, 1882. [Google Scholar]
- Sergi, C.; Graf, M.; Jung, C.; Sohn, C.; Adam, S.; Krempien, B.; Otto, H.F. Resting cartilage and the growth plate in dystrophic dysplasia: Case report and clinicopathologic characteristics as compared to pseudodystrophic dysplasia and type II atelosteogenesis. Pathologe 1998, 19, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.J. Hugh Owen Thomas the cripple’s champion. BMJ 1991, 303, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- British Medical Association (BMA). Report of the collective investigation committee of the BMA. BMJ 1888, 1, 1309–1322. [Google Scholar]
- Palm, T.A. The geographical distribution and aetiology of rickets. Practitioner 1890, 45, 270–279. [Google Scholar]
- Chesney, R.W. Theobald Palm and his remarkable observation: How the sunshine vitamin came to be recognized. Nutrients 2012, 4, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Huldschinsky, K. Heilung von Rachitis durch kunssliche Hohensonne. Dtsch. Med. Wochenschr. 1919, 45, 712. [Google Scholar] [CrossRef]
- György, P. Die Behandlung und Verhütung der Rachitis und Tetanie: Nebst Bemerkungen zu ihrer Pathogenese und Aetiologie; Verlag von Julius Springer: Berlin, Germany, 1929. [Google Scholar]
- Huldschinsky, K. Die Behandlung der Rachitis durch Ultraviolettbestrahlung. Z. Orthop. Chir. 1920, 39, 426. [Google Scholar]
- Riedel, G. Die Erfolge der Quartzlichtbestrahlung bei Rachitis. Munchener Med. Wochenschr. 1920, 67, 838. [Google Scholar]
- Rosenfeld, L. Vitamine—Vitamin. The early years of discovery. Clin. Chem. 1997, 43, 680–685. [Google Scholar] [PubMed]
- Hawgood, B.J. Sir Edward Mellanby (1884–1955) GBE KCB FRCP FRS: Nutrition scientist and medical research mandarin. J. Med. Biogr. 2010, 18, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, E. An experimental investigation on rickets. 1919. Nutrition 1989, 5, 81–86. [Google Scholar] [PubMed]
- Parascandola, J.; Ihde, A.J. Edward Mellanby and the antirachitic factor. Bull. Hist. Med. 1977, 51, 507–515. [Google Scholar] [PubMed]
- Medical Research Council. Studies of Rickets in Vienna 1919–22 (Special Report Series No. 77); HMSO: London, UK, 1923. [Google Scholar]
- Baumgartner-Sigl, S.; Haberlandt, E.; Mumm, S.; Scholl-Burgi, S.; Sergi, C.; Ryan, L.; Ericson, K.L.; Whyte, M.P.; Hogler, W. Pyridoxine-responsive seizures as the first symptom of infantile hypophosphatasia caused by two novel missense mutations (c.677t>c, p.M226t; c.1112c>t, p.T371i) of the tissue-nonspecific alkaline phosphatase gene. Bone 2007, 40, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Mornet, E.; Troeger, J.; Voigtlaender, T. Perinatal hypophosphatasia: Radiology, pathology and molecular biology studies in a family harboring a splicing mutation (648+1a) and a novel missense mutation (n400s) in the tissue-nonspecific alkaline phosphatase (TNSALP) gene. Am. J. Med. Genet. 2001, 103, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Rosella, D.; Papi, P.; Giardino, R.; Cicalini, E.; Piccoli, L.; Pompa, G. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J. Int. Soc. Prev. Community Dent. 2016, 6, 97–104. [Google Scholar] [PubMed]
- Hendaus, M.A.; Jomha, F.A.; Ehlayel, M. Allergic diseases among children: Nutritional prevention and intervention. Ther. Clin. Risk Manag. 2016, 12, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Mattozzi, C.; Paolino, G.; Richetta, A.G.; Calvieri, S. Psoriasis, Vitamin D and the importance of the cutaneous barrier’s integrity: An update. J. Dermatol. 2016, 43, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Stokes, P.J.; Rimmer, J. The relationship between serum Vitamin D and chronic rhinosinusitis: A systematic review. Am. J. Rhinol. Allergy 2016, 30, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Luthje, P.; Brauner, A. Novel strategies in the prevention and treatment of urinary tract infections. Pathogens 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Domingues-Faria, C.; Vasson, M.P.; Goncalves-Mendes, N.; Boirie, Y.; Walrand, S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res. Rev. 2016, 26, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, I.; Pawliczak, R. The active metabolite of Vitamin D3 as a potential immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Hewison, M.; Studzinski, G.P.; Li, Y.C.; Kalia, V. Role of Vitamin D in cytotoxic t lymphocyte immunity to pathogens and cancer. Crit. Rev. Clin. Lab. Sci. 2016, 53, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Randle, H.W. Suntanning: Differences in perceptions throughout history. Mayo Clin. Proc. 1997, 72, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Howell, J. Niels Ryberg Finsen, 1903. In Nobel Laureates in Medicine or Physiology: A Biographical Dictionary; Fox, D.M., Meldrum, M., Rezak, I., Eds.; Garland Publishing: New York, NY, USA, 1990; pp. 181–183. [Google Scholar]
- Shih, H.Y.; Sciume, G.; Mikami, Y.; Guo, L.; Sun, H.W.; Brooks, S.R.; Urban, J.F., Jr.; Davis, F.P.; Kanno, Y.; O’Shea, J.J. Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell 2016, 165, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M. Vitamin D effects on lung immunity and respiratory diseases. Vitam. Horm. 2011, 86, 217–237. [Google Scholar] [PubMed]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappa-b-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a Vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Young, G.A.; Underdahl, N.R.; Carpenter, L.E. Vitamin D intake and susceptibility of mice to experimental swine influenza virus infection. Proc. Soc. Exp. Biol. Med. 1949, 72, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of Vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Mezawa, H.; Noya, M.; Camargo, C.A., Jr. Effects of Vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014, 5, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Chadha, M.K.; Fakih, M.; Muindi, J.; Tian, L.; Mashtare, T.; Johnson, C.S.; Trump, D. Effect of 25-hydroxyVitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate 2011, 71, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.F.; Li-Ng, M. Re: Epidemic influenza and Vitamin D. Epidemiol. Infect. 2007, 135, 1095–1098. [Google Scholar] [PubMed]
- Tostmann, A.; Wielders, J.P.; Kibiki, G.S.; Verhoef, H.; Boeree, M.J.; van der Ven, A.J. Serum 25-hydroxy-Vitamin D3 concentrations increase during tuberculosis treatment in Tanzania. Int. J. Tuberc. Lung Dis. 2010, 14, 1147–1152. [Google Scholar] [PubMed]
- Belderbos, M.E.; Houben, M.L.; Wilbrink, B.; Lentjes, E.; Bloemen, E.M.; Kimpen, J.L.; Rovers, M.; Bont, L. Cord blood Vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Hesketh, K.; Power, C.; Hypponen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Li-Ng, M.; Aloia, J.F.; Pollack, S.; Cunha, B.A.; Mikhail, M.; Yeh, J.; Berbari, N. A randomized controlled trial of Vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009, 137, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Vitamin D, respiratory infections, and asthma. Curr. Allergy Asthma Rep. 2009, 9, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Scragg, R. Relationship between serum 25-hydroxyVitamin D and pulmonary function in the third national health and nutrition examination survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Wayse, V.; Yousafzai, A.; Mogale, K.; Filteau, S. Association of subclinical Vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur. J. Clin. Nutr. 2004, 58, 563–567. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Leis, K.; Matheson, L.A.; Karuananyake, C.; Sankaran, K.; Rosenberg, A.M. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr. Pulmonol. 2009, 44, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Wallace, J.M.; Horigan, G.; Hill, T.R.; Barnes, M.S.; Lucey, A.J.; Bonham, M.P.; Taylor, N.; Duffy, E.M.; Seamans, K.; et al. Estimation of the dietary requirement for Vitamin D in free-living adults >=64 y of age. Am. J. Clin. Nutr. 2009, 89, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, B.; Plantalech, L.; Bagur, A.; Wittich, A.C.; Rovai, G.; Pusiol, E.; Lopez Giovanelli, J.; Ponce, G.; Nieva, A.; Chaperon, A.; et al. High prevalence of Vitamin D insufficiency in healthy elderly people living at home in Argentina. Eur. J. Clin. Nutr. 2004, 58, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association between serum 25-hydroxyVitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Jones, A.B.; Prosser, C.; Robinson, J.L.; Vohra, S. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J. Infect. Dis. 2008, 197, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Peng, X.; Murillo, G.; Mehta, R.G. Functional significance of Vitamin D receptor Fokl polymorphism in human breast cancer cells. PLoS ONE 2011, 6, e16024. [Google Scholar] [CrossRef] [PubMed]
- Ogunkolade, B.W.; Boucher, B.J.; Prahl, J.M.; Bustin, S.A.; Burrin, J.M.; Noonan, K.; North, B.V.; Mannan, N.; McDermott, M.F.; DeLuca, H.F.; et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to Vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 2002, 51, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Salimpour, R. Rickets in Tehran. Study of 200 cases. Arch. Dis. Child. 1975, 50, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Lubani, M.M.; al-Shab, T.S.; al-Saleh, Q.A.; Sharda, D.C.; Quattawi, S.A.; Ahmed, S.A.; Moussa, M.A.; Reavey, P.C. Vitamin-D-deficiency rickets in Kuwait: The prevalence of a preventable disease. Ann. Trop. Paediatr. 1989, 9, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.E.; Cole, T.J.; Salem, S.I.; Galal, O.M.; el-Meligy, R.; Abdel-Azim, S.; Paul, A.A.; el-Husseini, S. Etiology of rickets in Egyptian children. Hum. Nutr. Clin. Nutr. 1987, 41, 199–208. [Google Scholar] [PubMed]
- Kriesel, J.D.; Spruance, J. Calcitriol (1,25-dihydroxy-Vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine 1999, 17, 1883–1888. [Google Scholar] [CrossRef]
- Cooper, C.; Thorne, A.; Canadian HIV Trials Network (CTN) Influenza Vaccine Research, G. Vitamin D supplementation does not increase immunogenicity of seasonal influenza vaccine in HIV-infected adults. HIV Clin. Trials 2011, 12, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Avenell, A.; Cook, J.A.; Maclennan, G.S.; Macpherson, G.C. Vitamin D supplementation to prevent infections: A sub-study of a randomised placebo-controlled trial in older people (record trial, isrctn 51647438). Age Ageing 2007, 36, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Yamshchikov, A.V.; Desai, N.S.; Blumberg, H.M.; Ziegler, T.R.; Tangpricha, V. Vitamin D for treatment and prevention of infectious diseases: A systematic review of randomized controlled trials. Endocr. Pract. 2009, 15, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Bivins, R. Ideology and disease identity: The politics of rickets, 1929–1982. Med. Humanit. 2014, 40, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bivins, R. “The English disease” or “Asian rickets”? Medical responses to postcolonial immigration. Bull. Hist. Med. 2007, 81, 533–568. [Google Scholar] [CrossRef] [PubMed]
- Webster, C. The Health Services since the War. Problems of Health Care: The National Health Service before 1957; TSO: London, UK, 1988; Volume 1. [Google Scholar]
- MacNalty, A. The public health in war time. Br. Med. J. 1940, 1, 333–336. [Google Scholar] [CrossRef]
- Magee, H.E. Application of nutrition to public health: Some lessons of the war. Br. Med. J. 1946, 1, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Medical Advances. “Not due to Flash of Genius”: Sir Henry Dale Confident of Progress. Manchester Guardian, 8 October 1957; 3. [Google Scholar]
- Noble, M.R. House of Commons Official Report; Hansard: London, UK, 1964. [Google Scholar]
- Apple, R. Vitamania: Vitamins in American Culture; Rutgers University Press: New Brunswick, NJ, USA, 1996. [Google Scholar]
- Arneil, G.C.; Crosbie, J.C. Infantile rickets returns to Glasgow. Lancet 1963, 2, 423–425. [Google Scholar] [CrossRef]
- Maggie, Z.; He, G.; Wang, H. Effects of nutritional supplementation on children with HIV/aids in China. J. Cent. South Univ. (Med. Ed.) 2012, 37, 305–310. [Google Scholar]
- Tien, H.Y. Demography in China: From zero to now. Popul. Index 1981, 47, 683–710. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.; Chan, E.W.; Chui, C.S.; Sutcliffe, A.G.; Wong, I.C. The phenomenon of micronutrient deficiency among children in China: A systematic review of the literature. Public Health Nutr. 2014, 17, 2605–2618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Stoecklin, E.; Eggersdorfer, M. A glimpse of Vitamin D status in mainland China. Nutrition 2013, 29, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.Z.; Lanham, J.S. Failure to thrive: An update. Am. Fam. Physician 2011, 83, 829–834. [Google Scholar] [PubMed]
- Agarwal, A.; Talwar, J. Radiographic changes in nutritional ricket hips in children in response to treatment. J. Orthop. Surg. 2014, 22, 368–373. [Google Scholar] [CrossRef]
- Astrup, A.; Dyerberg, J.; Selleck, M.; Stender, S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes. Rev. 2008, 9, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Chan, W. Nutrition and chronic renal disease in children. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1996, 37, 244–247. [Google Scholar] [PubMed]
- Evans, C.E.; Albar, S.A.; Vargas-Garcia, E.J.; Xu, F. School-based interventions to reduce obesity risk in children in high- and middle-income countries. Adv. Food Nutr. Res. 2015, 76, 29–77. [Google Scholar] [PubMed]
- Feng, A.; Wang, L.; Chen, X.; Liu, X.; Li, L.; Wang, B.; Luo, H.; Mo, X.; Tobe, R.G. Developmental origins of health and disease (DOHaD): Implications for health and nutritional issues among rural children in China. Biosci. Trends 2015, 9, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Mu, Z.; Nwaru, B.I.; Gu, G.; Meng, W.; Wu, Z. Child neglect in one-child families from Suzhou city of mainland China. BMC Int. Health Hum. Rights 2014, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiong, Y.L. Technologies and mechanisms for safety control of ready-to-eat muscle foods: An updated review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1886–1901. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; van der Beek, E.M.; Chan, M.Y.; Zhao, X.; Stevenson, L. Health claims on food products in southeast Asia: Regulatory frameworks, barriers, and opportunities. Nutr. Rev. 2015, 73, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wen, W.; Deng, Y.; Tian, Y.; Sun, H.; Sun, Q. Chinese ethnic meat products: Continuity and development. Meat Sci. 2016, 120, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.Y.; Du, S.F.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Popkin, B.M. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes. Rev. 2014, 15, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H. Nutritional situation of Beijing residents. Southeast Asian J. Trop. Med. Public Health 1992, 23, 65–68. [Google Scholar] [PubMed]
- Beck-Nielsen, S.S.; Brock-Jacobsen, B.; Gram, J.; Brixen, K.; Jensen, T.K. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur. J. Endocrinol. 2009, 160, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Beck-Nielsen, S.S.; Jensen, T.K.; Gram, J.; Brixen, K.; Brock-Jacobsen, B. Nutritional rickets in Denmark: A retrospective review of children’s medical records from 1985 to 2005. Eur. J. Pediatr. 2009, 168, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Fuhua, Z.; Qin, G. Child maltreatment among Asian Americans: Characteristics and explanatory framework. Child Maltreat. 2009, 14, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Dietz, W.H., Jr.; Hass, G.; Suskind, R. Risk of nutritional rickets among vegetarian children. Am. J. Dis. Child. 1979, 133, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, P.C.; Vergote, F.J.; van Staveren, W.A.; van den Berg, H.; Dingjan, P.G.; Hautvast, J.G. High prevalence of rickets in infants on macrobiotic diets. Am. J. Clin. Nutr. 1990, 51, 202–208. [Google Scholar] [PubMed]
- Green, T.J.; Li, W.; Barr, S.I.; Jahani, M.; Chapman, G.E. Vitamin D supplementation is associated with higher serum 25OHD in Asian and white infants living in Vancouver, Canada. Matern. Child Nutr. 2015, 11, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.C.; Burmeister, W. Vitamin D status of children and adolescents of African and Asian diplomats in Germany. Klin. Padiatrie 1993, 205, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Boyle, I.T. Bones for the future. Acta Paediatr. Scand. Suppl. 1991, 373, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Heald, F.P.; Rosebrough, R.H.; Jacobson, M.S. Nutrition and the adolescent: An update. J. Adolesc. Health Care 1980, 1, 142–151. [Google Scholar] [CrossRef]
- Goel, K.M.; Sweet, E.M.; Logan, R.W.; Warren, J.M.; Arneil, G.C.; Shanks, R.A. Florid and subclinical rickets among immigrant children in Glasgow. Lancet 1976, 1, 1141–1145. [Google Scholar] [CrossRef]
- Ford, J.A.; Davidson, D.C.; McIntosh, W.B.; Fyfe, W.M.; Dunnigan, M.G. Neonatal rickets in Asian immigrant population. Br. Med. J. 1973, 3, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.E.B.; Taylor, J.L.; Jones, M.E. Occult rickets and osteomalacia amongst the Asian immigrant population. Q. J. Med. 1973, 42, 125–149. [Google Scholar]
- Burrell, T.; Opfer, E.; Berglund, L.; Lowe, L.H.; Anderst, J. A witnessed case of a classic metaphyseal fracture caused during IV line placement in a child: Insight into mechanism of injury. J. Forensic. Leg. Med. 2015, 35, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Cundiff, D.K.; Harris, W. Case report of 5 siblings: Malnutrition? Rickets? Di George syndrome? Developmental delay? Nutr. J. 2006, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Trube-Becker, E. The neglected child. Monatsschr. Kinderheilkd. 1986, 134, 315–318. [Google Scholar] [PubMed]
- Feldman, K.W.; Brewer, D.K. Child abuse, cardiopulmonary resuscitation, and rib fractures. Pediatrics 1984, 73, 339–342. [Google Scholar] [PubMed]
- Trube-Becker, E. The death of children following negligence: Social aspects. Forensic. Sci 1977, 9, 111–115. [Google Scholar] [CrossRef]
- Weigel, W.; Kaufmann, H.J. Der verschleierte Pflegeschaden. Rontgenblatter 1975, 28, 463–470. [Google Scholar] [PubMed]
- DeRusso, P.A.; Spevak, M.R.; Schwarz, K.B. Fractures in biliary atresia misinterpreted as child abuse. Pediatrics 2003, 112, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Guigne, F.; Duke, P.; Rourke, L. Is Vitamin D deficiency an underreported issue in refugee health? Two cases of infants presenting with Vitamin D-deficiency rickets. Can. Fam. Physician 2013, 59, 641–643. [Google Scholar] [PubMed]
- Guigne, F.; Duke, P.; Rourke, L. Looking beyond literacy: Understanding and approaching barriers to refugee health in 2 cases of Vitamin D-deficiency rickets. Can. Fam. Physician 2013, 59, 607–608. [Google Scholar] [PubMed]
- Munns, C.F.; Simm, P.J.; Rodda, C.P.; Garnett, S.P.; Zacharin, M.R.; Ward, L.M.; Geddes, J.; Cherian, S.; Zurynski, Y.; Cowell, C.T.; et al. Incidence of Vitamin D deficiency rickets among Australian children: An Australian paediatric surveillance unit study. Med. J. Aust. 2012, 196, 466–468. [Google Scholar] [PubMed]
- Sheikh, M.; Wang, S.; Pal, A.; MacIntyre, C.R.; Wood, N.; Gunesekera, H. Vitamin D deficiency in refugee children from conflict zones. J. Immigr. Minor. Health 2011, 13, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Brunvand, L.; Brunvatne, R. Health problems among immigrant children in Norway. Tidsskr. Nor Laegeforen. 2001, 121, 715–718. [Google Scholar] [PubMed]
- Neuhaus, T.J.; Smaadahl, F.; Losa, M.; Largo, R.H. New faces, forgotten diseases: Border medical examination of asylum seekers’ children 1990–1991. Schweiz. Med. Wochenschr. 1992, 122, 1838–1842. [Google Scholar] [PubMed]
- Caldwell, J.P.; Kain, B.F.; McDonald, R.C. A Canadian medical team in Ethiopia. Can. Fam. Physician 1985, 31, 2115–2117. [Google Scholar] [PubMed]
- Agnew-Blais, J.; Danese, A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: A systematic review and meta-analysis. Lancet Psychiatry 2016, 3, 342–349. [Google Scholar] [CrossRef]
- Friedman, E.; Billick, S.B. Unintentional child neglect: Literature review and observational study. Psychiatr. Q. 2015, 86, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, A.R.; Dietl, M.F.; Muenzenmaier, K.H.; Huber, C.G.; Lang, U.E. Stressful childhood experiences and health outcomes in sexual minority populations: A systematic review. Soc. Psychiatry Psychiatr. Epidemiol. 2014, 49, 1427–1445. [Google Scholar] [CrossRef] [PubMed]
- Plotka, J.; Narkowicz, S.; Polkowska, Z.; Biziuk, M.; Namiesnik, J. Effects of addictive substances during pregnancy and infancy and their analysis in biological materials. Rev. Environ. Contam. Toxicol. 2014, 227, 55–77. [Google Scholar] [PubMed]
- Danese, A.; Tan, M. Childhood maltreatment and obesity: Systematic review and meta-analysis. Mol. Psychiatry 2014, 19, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Morantz, G.; Cole, D.; Vreeman, R.; Ayaya, S.; Ayuku, D.; Braitstein, P. Child abuse and neglect among orphaned children and youth living in extended families in sub-Saharan Africa: What have we learned from qualitative inquiry? Vulnerable Child. Youth Stud. 2013, 8, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Shield, K.; Rehm, J.; Popova, S. Prevalence of fetal alcohol spectrum disorders in child care settings: A meta-analysis. Pediatrics 2013, 132, e980–e995. [Google Scholar] [CrossRef] [PubMed]
- Teeuw, A.H.; Derkx, B.H.; Koster, W.A.; van Rijn, R.R. Educational paper: Detection of child abuse and neglect at the emergency room. Eur. J. Pediatr. 2012, 171, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Eckenrode, J.; Campa, M.; Luckey, D.W.; Henderson, C.R., Jr.; Cole, R.; Kitzman, H.; Anson, E.; Sidora-Arcoleo, K.; Powers, J.; Olds, D. Long-term effects of prenatal and infancy nurse home visitation on the life course of youths: 19-year follow-up of a randomized trial. Arch. Pediatr. Adolesc. Med. 2010, 164, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Eckenrode, J.; Ganzel, B.; Henderson, C.R., Jr.; Smith, E.; Olds, D.L.; Powers, J.; Cole, R.; Kitzman, H.; Sidora, K. Preventing child abuse and neglect with a program of nurse home visitation: The limiting effects of domestic violence. JAMA 2000, 284, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Shen, F.; Petryk, A.; Tang, J.; Chen, X.; Sergi, C. “English Disease”: Historical Notes on Rickets, the Bone–Lung Link and Child Neglect Issues. Nutrients 2016, 8, 722. https://doi.org/10.3390/nu8110722
Zhang M, Shen F, Petryk A, Tang J, Chen X, Sergi C. “English Disease”: Historical Notes on Rickets, the Bone–Lung Link and Child Neglect Issues. Nutrients. 2016; 8(11):722. https://doi.org/10.3390/nu8110722
Chicago/Turabian StyleZhang, Mingyong, Fan Shen, Anna Petryk, Jingfeng Tang, Xingzhen Chen, and Consolato Sergi. 2016. "“English Disease”: Historical Notes on Rickets, the Bone–Lung Link and Child Neglect Issues" Nutrients 8, no. 11: 722. https://doi.org/10.3390/nu8110722
APA StyleZhang, M., Shen, F., Petryk, A., Tang, J., Chen, X., & Sergi, C. (2016). “English Disease”: Historical Notes on Rickets, the Bone–Lung Link and Child Neglect Issues. Nutrients, 8(11), 722. https://doi.org/10.3390/nu8110722





