The Effect of Vegan Protein-Based Diets on Metabolic Parameters, Expressions of Adiponectin and Its Receptors in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Biochemical Measurement
2.3. Semi-Quantitative Reverse Transcriptase Polymerase Chain Reaction
2.4. Western Blot
2.5. Statistical Analysis
3. Results
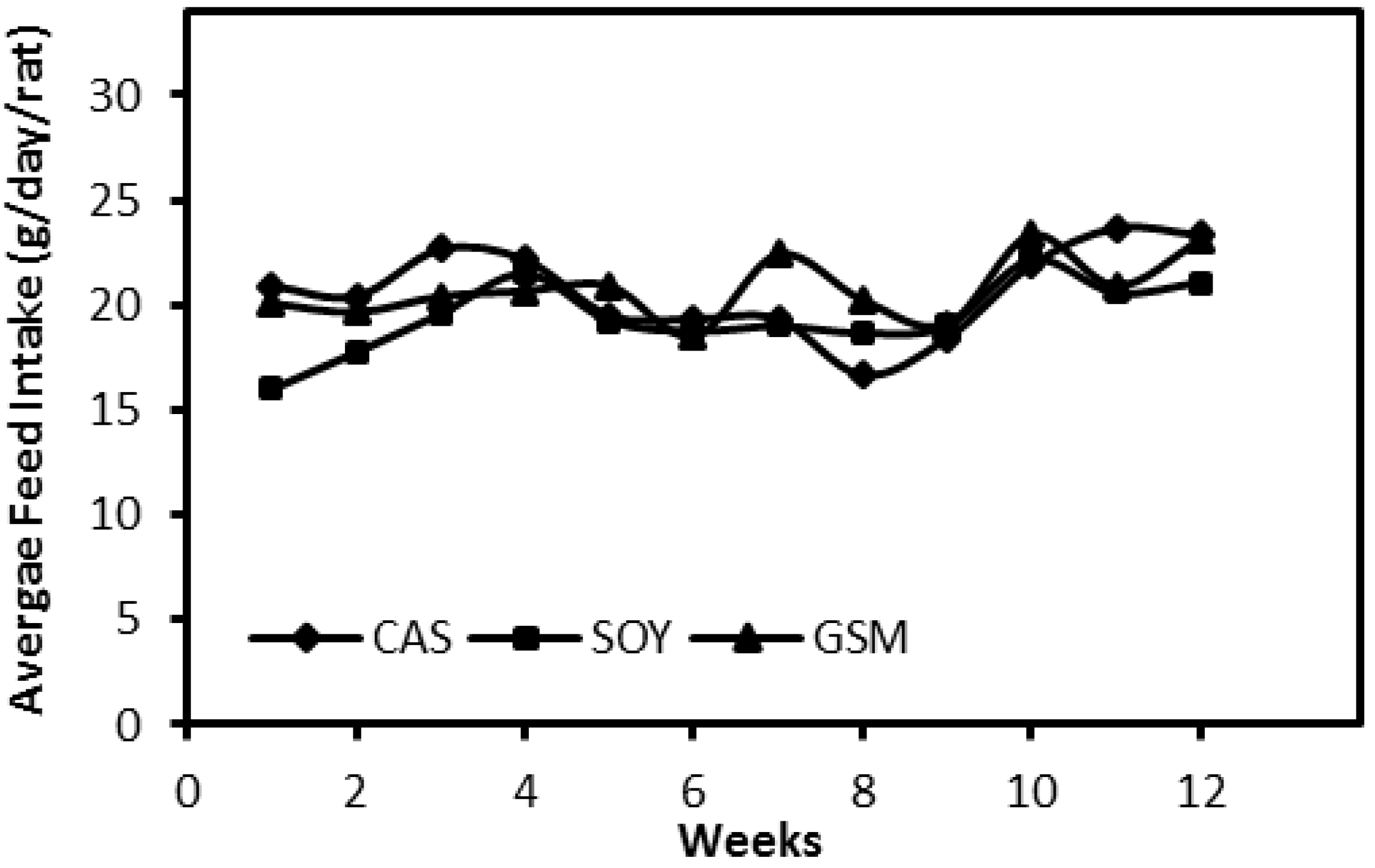
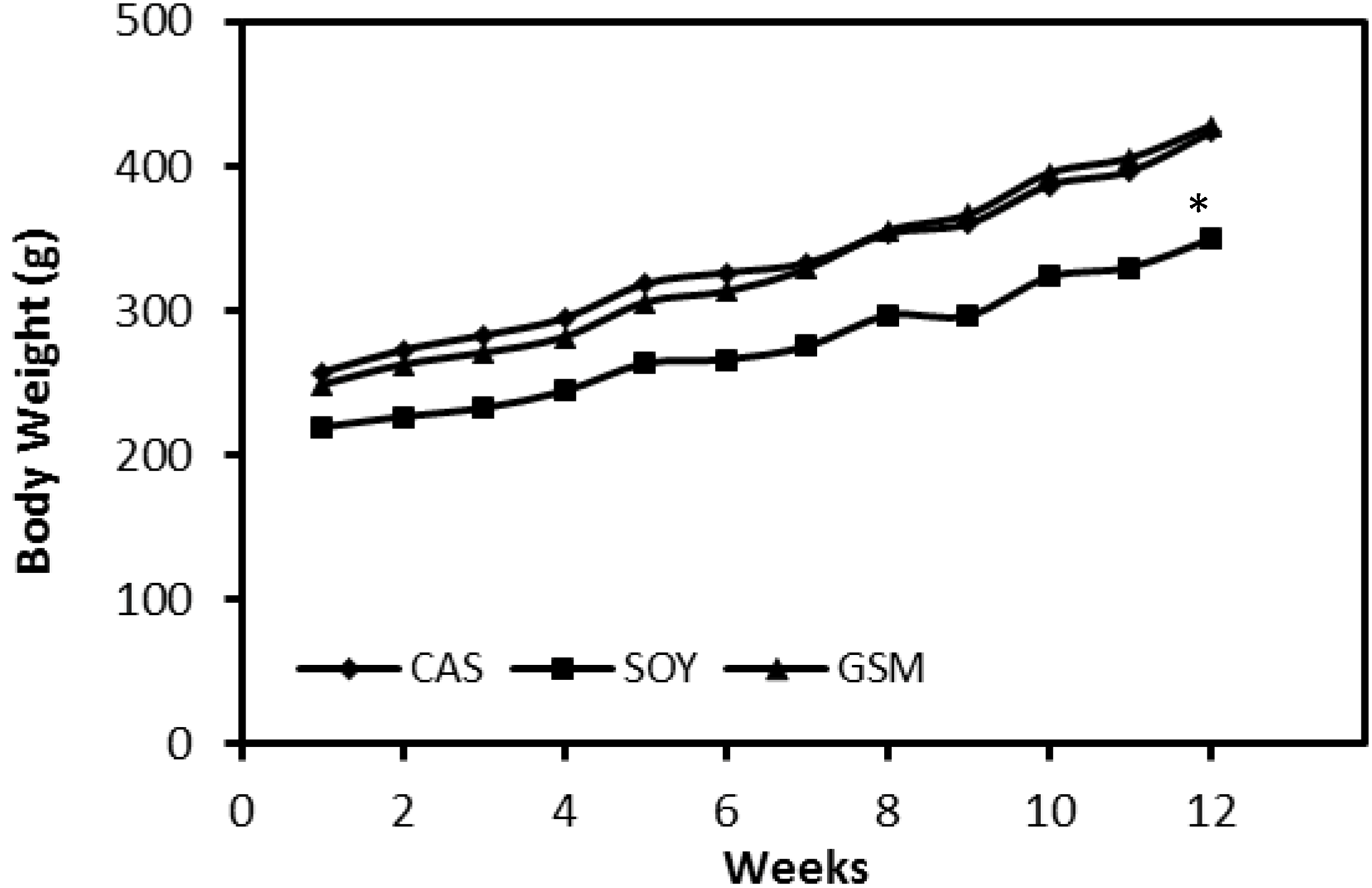
3.1. Feed Intake and Body Weight
3.2. Changes in Metabolic Parameters
3.3. Gene Expression
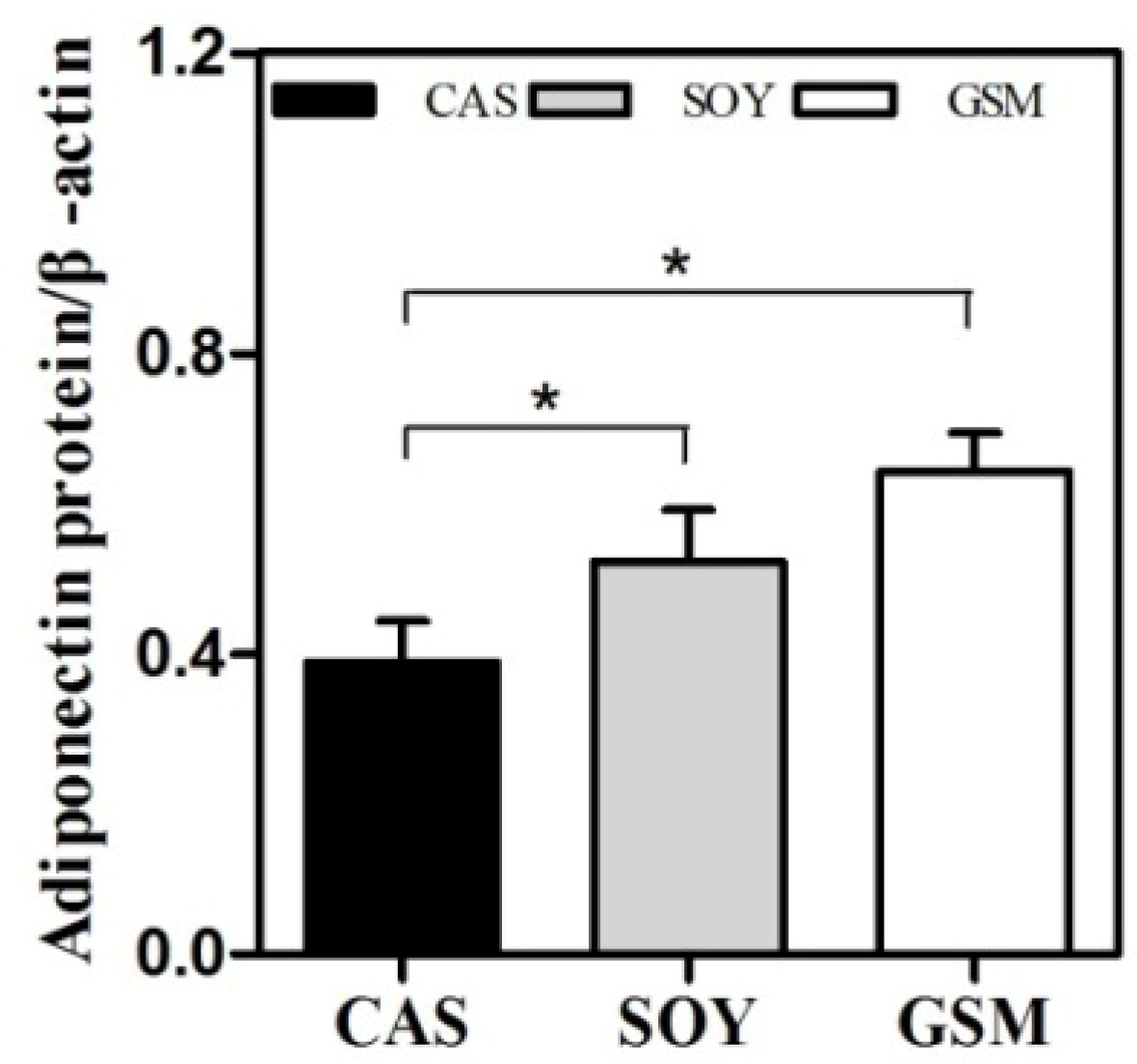
3.4. Adiponectin Protein Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, S.; Quon, M.J.; Koh, K.K. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis 2014, 233, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related disease. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bullen, J.W., Jr.; Bluher, S.; Kelesidis, T.; Mantzoros, C.S. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1079–E1086. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Iida, K.T.; Sone, H.; Yokoo, T.; Yamada, N.; Ajisaka, R. The effect of exercise training on adiponectin receptor expression in kkay obese/diabetic mice. J. Endocrinol. 2006, 189, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Hoehn, K.L.; Lawrence, R.T.; Sawbridge, L.; Talbot, N.A.; Tomsig, J.L.; Turner, N.; Cooney, G.J.; Whitehead, J.P.; Kraegen, E.W.; et al. Overexpression of the adiponectin receptor adipor1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology 2012, 153, 5231–5246. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, C.; Shukla, A.; Hirche, F.; Stangl, G.I.; Eder, K. Effect of proteins from beef, pork, and turkey meat on plasma and liver lipids of rats compared with casein and soy protein. Nutrition 2006, 22, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Vegan proteins may reduce risk of cancer, obesity, and cardiovascular disease by promoting increased glucagon activity. Med. Hypotheses 1999, 53, 459–485. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.D.; Zhang, L.; Shadoan, M.K.; Kavanagh, K.; Chen, H.; Tresnasari, K.; Kaplan, J.R.; Adams, M.R. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism 2008, 57, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Bressani, R. Protein supplementation and complementation. In Evaluation of Proteins for Humans; Bodwell, C., Ed.; AVI: Westport, Connecticut, CT, USA, 1977; pp. 204–232. [Google Scholar]
- Sacks, F.M.; Wood, P.G.; Kass, E.H. Stability of blood pressure in vegetarians receiving dietary protein supplements. Hypertension 1984, 6, 199–201. [Google Scholar] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Chen, J.H.; Ouyang, C.; Ding, Q.; Song, J.; Cao, W.; Mao, L. A moderate low-carbohydrate low-calorie diet improves lipid profile, insulin sensitivity and adiponectin expression in rats. Nutrients 2015, 7, 4724–4738. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gerbaix, M.; Metz, L.; Ringot, E.; Courteix, D. Visceral fat mass determination in rodent: Validation of dual-energy X-ray absorptiometry and anthropometric techniques in fat and lean rats. Lipids Health Dis. 2010, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. The origins of western obesity: A role for animal protein? Med. Hypotheses 2000, 54, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, A.; Koba, K.; Ichinose, F.; Kaneko, M.; Shimoda, A.; Nonaka, K.; Yamasaki, M.; Iwata, T.; Yamauchi, Y.; Tsutsumi, K.; et al. Dietary protein modulates the effect of cla on lipid metabolism in rats. Lipids 2004, 39, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Fukui, K.; Takamatsu, K.; Hashimoto, Y.; Yamamoto, T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow kk). Nutrition 2000, 16, 349–354. [Google Scholar] [CrossRef]
- Oliva, M.E.; Selenscig, D.; D'Alessandro, M.E.; Chicco, A.; Lombardo, Y.B. Soya protein ameliorates the metabolic abnormalities of dysfunctional adipose tissue of dyslipidaemic rats fed a sucrose-rich diet. Br. J. Nutr. 2011, 105, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the framingham heart study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Freedland, E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Sasakabe, T.; Haimoto, H.; Umegaki, H.; Wakai, K. Effects of a moderate low-carbohydrate diet on preferential abdominal fat loss and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab. Syndr. Obes. 2011, 4, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.K.; Kurowska, E.M. Soy consumption and cholesterol reduction: Review of animal and human studies. J. Nutr. 1995, 125, 594S–597S. [Google Scholar] [PubMed]
- Sanchez, A.; Hubbard, R.W. Plasma amino acids and the insulin/glucagon ratio as an explanation for the dietary protein modulation of atherosclerosis. Med. Hypotheses 1991, 36, 27–32. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Lin, J.; Pen, A.; Ying, C.; Cao, W.; Mao, L. A lower proportion of dietary saturated/monounsaturated/polyunsaturated fatty acids reduces the expression of adiponectin in rats fed a high-fat diet. Nutr. Res. 2012, 32, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Fritsche, A.; Thamer, C.; Haap, M.; Shirkavand, F.; Rahe, S.; Staiger, H.; Maerker, E.; Haring, H.; Stumvoll, M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 2003, 52, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Fukui, K.; Kojima, M.; Kishida, K.; Maeda, N.; Nagaretani, H.; Hibuse, T.; Nishizawa, H.; Kihara, S.; Waki, M.; et al. Divergent effects of soy protein diet on the expression of adipocytokines. Biochem. Biophys. Res. Commun. 2003, 311, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Bjursell, M.; Ahnmark, A.; Bohlooly, Y.M.; William-Olsson, L.; Rhedin, M.; Peng, X.R.; Ploj, K.; Gerdin, A.K.; Arnerup, G.; Elmgren, A.; et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 2007, 56, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of adipor1 and adipor2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]


| Components | CAS Group | SOY Group | GSM Group |
|---|---|---|---|
| Casein a (g) | 200.0 | 0.0 | 0.0 |
| Soy protein isolates b (g) | 0.0 | 400.0 | 200.0 |
| Wheat Gluten isolates c (g) | 0.0 | 0.0 | 142.8 |
| Vitamin mix ** (g) | 10.0 | 10.0 | 10.0 |
| Mineral mix ** (g) | 35.0 | 35.0 | 35.0 |
| Sucrose (g) | 50.0 | 50.0 | 50.0 |
| Corn starch (g) | 585.0 | 385.0 | 442.2 |
| Fiber (g) | 50.0 | 50.0 | 50.0 |
| Lipid (g) | 70.0 | 70.0 | 70.0 |
| Total amount (g) | 1000.0 | 1000.0 | 1000.0 |
| Sense Primer | Antisense Primer | |
|---|---|---|
| Adiponectin | 5′-TCACTCAGCATTCAGCGTAG-3′ | 5′-CTGATACTGGTCGTAGGTGAAG-3′ |
| AdipoR1 | 5′-ACTGGACTATTCAGGGATTGC-3′ | 5′-CCATAGAAGTGGACGAAAGC-3′ |
| AdipoR2 | 5′-TTGTGTATTCTTCCTGTGCC-3′ | 5′-CAGCACACAGATGACAATCA-3′ |
| β-actin | 5′-CATCACTATCGGCAATGAGC-3′ | 5′-GACAGCACTGTGTTGGCATA-3′ |
| mmol/L | Insulin (IU/mL) | HOMA-IR # | ||||
|---|---|---|---|---|---|---|
| Triglyceride | Total Cholesterol | HDL-C | Glucose | |||
| 0-week | ||||||
| CAS | 0.95 ± 0.28 | 1.99 ± 0.05 | 0.56 ± 0.11 | 7.54 ± 0.96 | 7.69 ± 1.79 | 2.56 ± 0.61 |
| SOY | 1.00 ± 0.21 | 2.14 ± 0.13 | 0.54 ± 0.10 | 7.53 ± 0.66 | 8.06 ± 1.66 | 2.37 ± 0.70 |
| GSM | 0.90 ± 0.19 | 2.14 ± 0.20 | 0.52 ± 0.07 | 7.07 ± 0.74 | 7.58 ± 1.99 | 2.39 ± 0.68 |
| 12-week | ||||||
| CAS | 1.55 ± 0.23 | 2.20 ± 0.24 | 0.62 ± 0.05 | 4.55 ± 0.77 | 9.21 ± 1.57 | 1.83 ± 0.51 |
| SOY | 0.79 ± 0.33 a | 1.77 ± 0.38 a | 0.69 ± 0.08 | 4.37 ± 0.83 | 7.45 ± 1.92 a | 1.31 ± 0.42 a,c |
| GSM | 0.67 ± 0.24 a,b | 1.63 ± 0.49 a | 0.79 ± 0.06 a | 4.82 ± 0.65 | 8.23 ± 1.86 | 1.77 ± 0.57 |
| Change (%) † | ||||||
| CAS | 48.7 ± 20.2 | 13.2 ± 10.2 | −9.5 ± 20.8 | −40.7 ± 15.0 | 16.4 ± 24.1 | −32.1 ± 19.5 |
| SOY | −19.5 ± 11.9 a | −15.0 ± 9.0 a | 19.5 ± 15.2 a | −39.7 ± 15.7 | −4.0 ± 23.6 | −42.3 ± 21.5 |
| GSM | −46.4 ± 16.9 a,b | −19.1 ± 11.8 a,b | 45.1 ± 18.1 a,b | −32.9 ± 11.2 | 16.1 ± 30.7 | −21.9 ± 26.5 |
| Group | Visceral Fat Mass (g) #, * | Visceral Fat Mass (%) *, Δ |
|---|---|---|
| CAS | 11.57 ± 1.67 | 2.62 ± 0.33 |
| SOY | 7.15 ± 1.71 a, b | 1.94 ± 0.46 a |
| GSM | 9.95 ± 1.08 | 2.13 ± 0.21 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-H.; Song, J.; Chen, Y.; Ding, Q.; Peng, A.; Mao, L. The Effect of Vegan Protein-Based Diets on Metabolic Parameters, Expressions of Adiponectin and Its Receptors in Wistar Rats. Nutrients 2016, 8, 643. https://doi.org/10.3390/nu8100643
Chen J-H, Song J, Chen Y, Ding Q, Peng A, Mao L. The Effect of Vegan Protein-Based Diets on Metabolic Parameters, Expressions of Adiponectin and Its Receptors in Wistar Rats. Nutrients. 2016; 8(10):643. https://doi.org/10.3390/nu8100643
Chicago/Turabian StyleChen, Jie-Hua, Jia Song, Yan Chen, Qiang Ding, Anfang Peng, and Limei Mao. 2016. "The Effect of Vegan Protein-Based Diets on Metabolic Parameters, Expressions of Adiponectin and Its Receptors in Wistar Rats" Nutrients 8, no. 10: 643. https://doi.org/10.3390/nu8100643
APA StyleChen, J.-H., Song, J., Chen, Y., Ding, Q., Peng, A., & Mao, L. (2016). The Effect of Vegan Protein-Based Diets on Metabolic Parameters, Expressions of Adiponectin and Its Receptors in Wistar Rats. Nutrients, 8(10), 643. https://doi.org/10.3390/nu8100643





