Association between Serum Copper Status and Working Memory in Schoolchildren
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.2.1. Collection and Analyses of Blood Copper Samples
2.2.2. Working Memory Tasks
2.2.3. Covariates
2.3. Statistical Analysis
3. Results
Sample Characteristics and Working Memory
| n (%)/Mean ± Standard Deviation | |
|---|---|
| Sex | |
| Girls | 387 (46.8) |
| Boys | 439 (53.2) |
| Age | |
| 10–11 years | 437 (52.9) |
| 12–14 years | 389 (47.1) |
| Mother’s education # | |
| 1 Middle school or less | 345 (42.2) |
| 2 High school | 190 (23.2) |
| 3 College or higher | 283 (34.6) |
| Father’s education # | |
| 1 Middle school or less | 247 (3.2) |
| 2 High school | 238 (29.1) |
| 3 College or higher | 334 (40.8) |
| Categorical serum copper | |
| 1 Q1: ≤84.3 μg/dL | 208 (25.6) |
| 2 Q1–Q3: >84.3, ≤110.4 μg/dL | 413 (5.8) |
| 3 Q3: >110.4 μg/dL | 191 (23.5) |
| Serum Iron | 116.90 ± 50.04 |
| Serum Zinc | 88.87 ± 16.01 |
| Working memory test score | |
| Dot trajectory | 8.29 ± 2.12 |
| Digit span | 6.25 ± 1.5 |
| Dot memory | 5.93 ± 1.90 |
| Box transform memory | 4.77 ± 1.18 |

| Latent Working Memory | t/F | Kendall’s Correlation | |
|---|---|---|---|
| Sex | 0.2751 | ||
| Girls | 0.01 ± 0.67 | ||
| Boys | 0.001 ± 0.69 | ||
| Age | 0.84 | ||
| 10–11 years | −0.01 ± 0.69 | ||
| 12–14 years | 0.03 ± 0.67 | ||
| Mother’s education # | 2.64 *** (Post hoc Sheffe test: 3 > 1 ***, 3 > 2 **) | ||
| 1 Middle school or less | −0.13 ± 0.63 | ||
| 2 High school | −0.03 ± 0.59 | ||
| 3 College or higher | 0.21 ± 0.75 | ||
| Father’s education # | 1.64 *** (Post hoc Sheffe test 3 > 1 ***, 3 > 2 *) | ||
| 1 Middle school or less | −0.11 ± 0.61 | ||
| 2 High school | −0.03 ± 0.63 | ||
| 3 College or higher | 0.13 ± 0.75 | ||
| Categorical serum copper | 1.74 | ||
| 1 Q1: ≤84.3 μg/dL | 0.01 ± 0.69 | ||
| 2 Q1–Q3: 84.3, 110.4 μg/dL | 0.05 ± 0.71 | ||
| 3 Q3: >110.4 μg/dL | −0.06 ± 0.62 | ||
| Serum Iron | 0.011 | ||
| Serum Zinc | −0.003 |
| Latent Working Memory | b (Robust s.e.) | p Values | 95% CI |
|---|---|---|---|
| Categorical serum copper | |||
| Q1: ≤84.3 μg/dL | 0.097 (0.053) | 0.067 | (−0.007, 0.201) |
| Q1–Q3: >84.3, ≤110.4 μg/dL | 0.099 (0.009) | <0.001 | (0.082, 0.117) |
| Q3: >110.4 μg/dL | Ref. | ||
| Sex | |||
| Girls | 0.013 (0.019) | 0.490 | (−0.024, 0.049) |
| Boys | Ref. | ||
| Age | |||
| 10–11 years | −0.073 (0.032) | 0.023 | (−0.137, −0.010) |
| 12–14 years | Ref. | ||
| Mother’s education | |||
| Middle school or less | −0.307 (0.057) | <0.001 | (−0.551, −0.062) |
| High school | −0.208 (0.051) | <0.001 | (−0.429, 0.013) |
| College or higher | Ref. | ||
| Father’s education | |||
| Middle school or less | −0.087 (0.046) | 0.056 | (−0.285, 0.111) |
| High school | −0.070 (0.046) | 0.120 | (−0.268, 0.126) |
| College or higher | Ref. | ||
| Serum Iron | 0.00003 (0.00004) | 0.451 | (−0.00005, 0.0001) |
| Serum Zinc | 0.00008 (0.0002) | 0.603 | (−0.0002, 0.0004) |
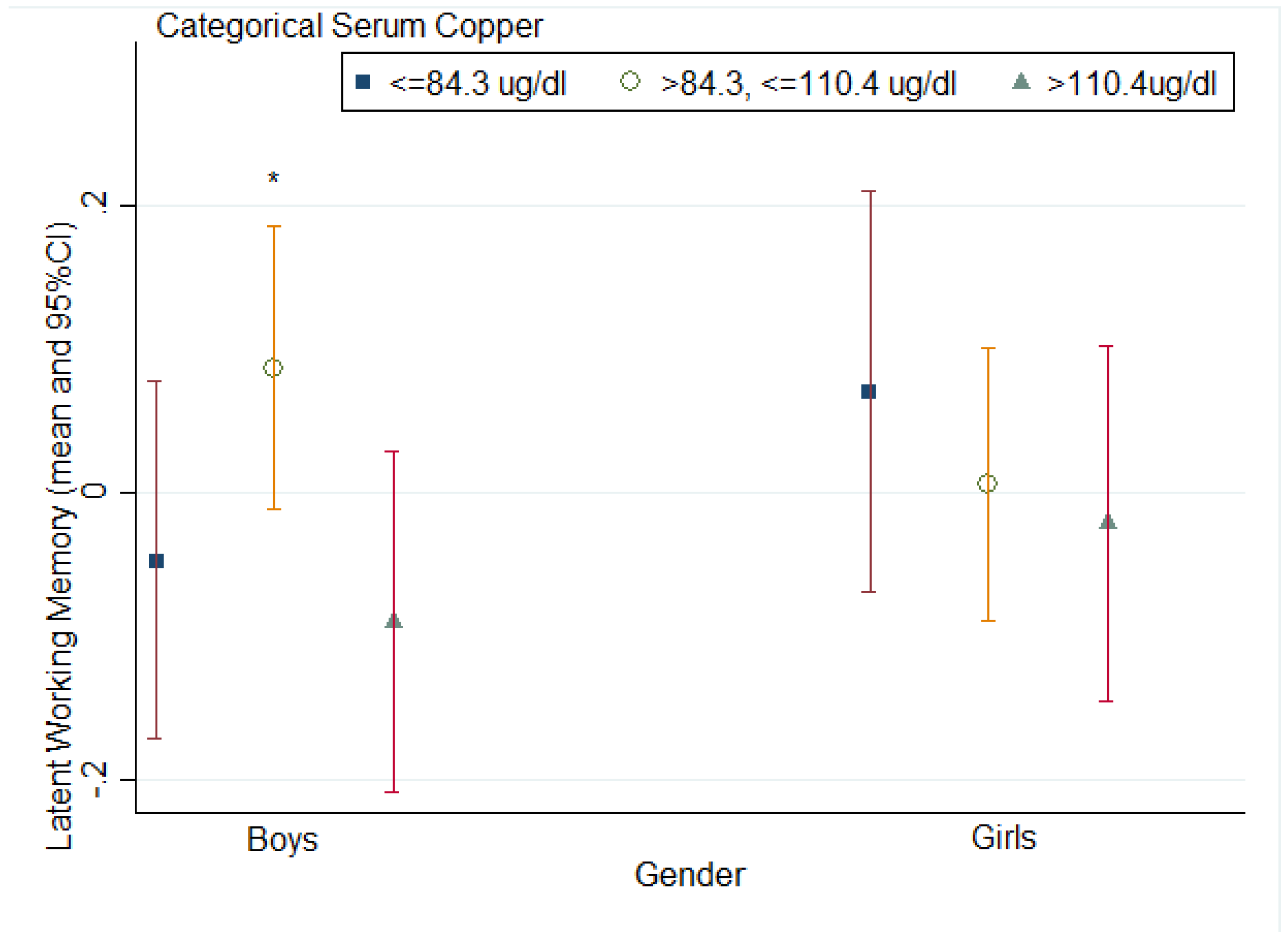
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, M. Role of micronutrients for physical growth and mental development. Indian J. Pediatr. 2004, 71, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Osendarp, S.; Hughes, D.; Calvaresi, E.; Baghurst, K.; van Klinken, J.W. Nutrients for cognitive development in school-aged children. Nutr. Rev. 2004, 62, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S. [Google Scholar] [PubMed]
- Sandstead, H.H. Subclinical zinc deficiency impairs human brain function. J. Trace Elem. Med. Biol. 2012, 26, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L. Nutrition in Clinical Practice: A Comprehensive, Evidence-Based Manual for the Practitioner; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 37–38. [Google Scholar]
- Multhaup, G.; Schlicksupp, A.; Hesse, L.; Beher, D.; Ruppert, T.; Masters, C.L.; Beyreuther, K. The amyloid precursor protein of Alzheimer’s Disease in the reduction of copper (II) to copper (I). Science 1996, 271, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.K.; Kritz-Silverstein, D.; Barrett-Connor, E.; Milne, D.; Nielsen, F.; Gamst, A.; Morton, D.; Wingard, D. Plasma trace elements and cognitive function in older men and women: The Rancho Bernardo study. J. Nutr. Health Aging 2008, 12, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Kanzer, S.H.; Zimmerman, E.A.; Celmins, D.F.; Heckman, S.M.; Dick, R. Copper and ceruloplasmin abnormalities in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2010, 25, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Oregon State University Micronutrient Information Center. Available online: http://lpi.oregonstate.edu/mic/minerals/copper#RDA (accessed on 25 July 2015).
- Pala, A.; Siottob, M.; Prasada, R.; Squittic, R. Towards a unified vision of copper involvement in Alzheimer’s Disease: A review connecting basic, experimental, and clinical research. J. Alzheimer’s Dis. 2015, 44, 343–354. [Google Scholar]
- Squitti, R.; Siotto, M.; Polimanti, R. Low-copper diet as a preventive strategy for Alzheimer’s Disease. Neurobiol. Aging 2014, 35, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Simonelli, I.; Ventriglia, M.; Siotto, M.; Pasqualetti, P.; Rembach, A.; Doecke, J.; Bush, A.I. Meta-analysis of serum non-ceruloplasmin copper in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 38, 809–822. [Google Scholar]
- Salustri, C.; Barbati, G.; Ghidoni, R.; Quintiliani, L.; Ciappina, S.; Binetti, G.; Squitti, R. Is cognitive function linked to serum free copper levels? A cohort study in a normal population. Clin. Neurophysiol. 2010, 121, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Schneider, J.A.; Wilson, R.S.; Scherr, P.A. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch. Neurol. 2006, 63, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cognit. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Alloway, T.P.; Kirkwood, H.J.; Elliott, J.G.; Holmes, J.; Hilton, K.A. Attentional and executive function behaviours in children with poor working memory. Learn. Individ. Differ. 2008, 18, 214–223. [Google Scholar] [CrossRef]
- Holmes, J.; Hilton, K.A.; Place, M.; Alloway, T.P.; Elliott, J.G.; Gathercole, S.E. Children with low working memory and children with ADHD: Same or different? Front. Hum. Neurosci. 2014, 8, 976. [Google Scholar] [CrossRef] [PubMed]
- Alloway, T.P.; Gathercole, S.E.; Kirkwood, H.; Elliott, J. The cognitive and behavioral characteristics of children with low working memory. Child Dev. 2009, 80, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; McCauley, L.A.; Zhao, Y.; Zhang, H.; Pinto-Martin, J. Cohort Profile: The China Jintan Child Cohort Study. Int. J. Epidemiol. 2010, 39, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ai, Y.X.; Hanlon, A.; Shi, Z.; Dickerman, B.; Compher, C. Micronutrients deficiency and associated sociodemographic factors in Chinese children. World J. Pediatr. 2011, 7, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Working Memory and Processing Speed in Children with Arithmetical Difficulties. J. Nanjing Norm. Univ. (Soc. Sci.) 2004, 3, 81–103. [Google Scholar]
- Dang, C.P.; Braeken, J.; Ferrer, E.; Liu, C. Unitary or non-unitary nature of working memory? Evidence from tis relation to general fluid and crystallized intelligence. Intelligence 2012, 40, 499–508. [Google Scholar] [CrossRef]
- Benton, D. Micronutrient status, cognition and behavioral problems in childhood. Eur. J. Nutr. 2008, 47, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Kanarek, R.B.; Prasad, C. Nutritional Neuroscience; CRC Press: Boca Raton, FL, US, 2010. [Google Scholar]
- Gao, S.; Jin, Y.; Unverzagt, F.W.; Ma, F.; Hall, K.S.; Murrell, J.R.; Cheng, Y.; Shen, J.; Ying, B.; Ji, R. Trace element levels and cognitive function in rural elderly Chinese. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 635–641. [Google Scholar] [CrossRef]
- Squitti, R. Copper dysfunction in Alzheimer’s Disease: From meta-analysis of biochemical studies to new insight into genetics. J. Trace Elem. Med. Biol. 2012, 26, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cuajungco, M.P.; Atwood, C.S.; Hartshorn, M.A.; Tyndall, J.D.; Hanson, G.R.; Stokes, K.C.; Leopold, M.; Multhaup, G.; Goldstein, L.E. Cu (II) potentiation of Alzheimer Aβ neurotoxicity correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999, 274, 37111–37116. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Moir, R.D.; Tanzi, R.E.; Bush, A.I.; Rogers, J.T. Redox-active metals, oxidative stress, and Alzheimer’s Disease pathology. Ann. N. Y. Acad. Sci. 2004, 1012, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Stroppolo, A.; Gatti, A.; Tampellini, D.; Toscani, M.; Gallorini, M.; Giaveri, G.; Arosio, P.; Santambrogio, P.; Fariello, R.G. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. USA 2004, 101, 9843–9848. [Google Scholar] [CrossRef] [PubMed]
- Awh, E.; Vogel, E.; Oh, S.H. Interactions between attention and working memory. Neuroscience 2006, 139, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.D. The precautionary principle also applies to public health actions. Am. J. Public Health 2001, 91, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Ji, X.; Cui, N.; Cao, S.; Liu, C.; Liu, J. Association between Serum Copper Status and Working Memory in Schoolchildren. Nutrients 2015, 7, 7185-7196. https://doi.org/10.3390/nu7095331
Zhou G, Ji X, Cui N, Cao S, Liu C, Liu J. Association between Serum Copper Status and Working Memory in Schoolchildren. Nutrients. 2015; 7(9):7185-7196. https://doi.org/10.3390/nu7095331
Chicago/Turabian StyleZhou, Guoping, Xiaopeng Ji, Naixue Cui, Siyuan Cao, Chang Liu, and Jianghong Liu. 2015. "Association between Serum Copper Status and Working Memory in Schoolchildren" Nutrients 7, no. 9: 7185-7196. https://doi.org/10.3390/nu7095331
APA StyleZhou, G., Ji, X., Cui, N., Cao, S., Liu, C., & Liu, J. (2015). Association between Serum Copper Status and Working Memory in Schoolchildren. Nutrients, 7(9), 7185-7196. https://doi.org/10.3390/nu7095331





