Black Beans, Fiber, and Antioxidant Capacity Pilot Study: Examination of Whole Foods vs. Functional Components on Postprandial Metabolic, Oxidative Stress, and Inflammation in Adults with Metabolic Syndrome
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Participants
2.2. Study Design
2.3. Meal Composition
| Nutrient | Units | BB Meal | FM Meal | AM Meal |
|---|---|---|---|---|
| Calories | kj | 3845 | 3904 | 3862 |
| kcals | 919 | 933 | 923 | |
| Fat | g | 25 | 25 | 25 |
| Saturated fat | g | 10 | 10 | 10 |
| Monounsaturated fat | g | 4 | 4 | 4 |
| Polyunsaturated fat | g | 1 | 1 | 1 |
| Carbohydrate | g | 129 | 135 | 133 |
| Fiber | g | 18 | 17 | 4 |
| Insoluble fiber | g | 4 | 2 | 0 |
| Soluble fiber | g | 10 | 8 | 0 |
| Protein | g | 42 | 39 | 39 |
| Total ORAC | μmol/L trolox equivalents | 4499 | 580 | 5758 |
2.4. Food Records
2.5. Biochemical Measurements
2.6. Statistical Analysis
3. Results
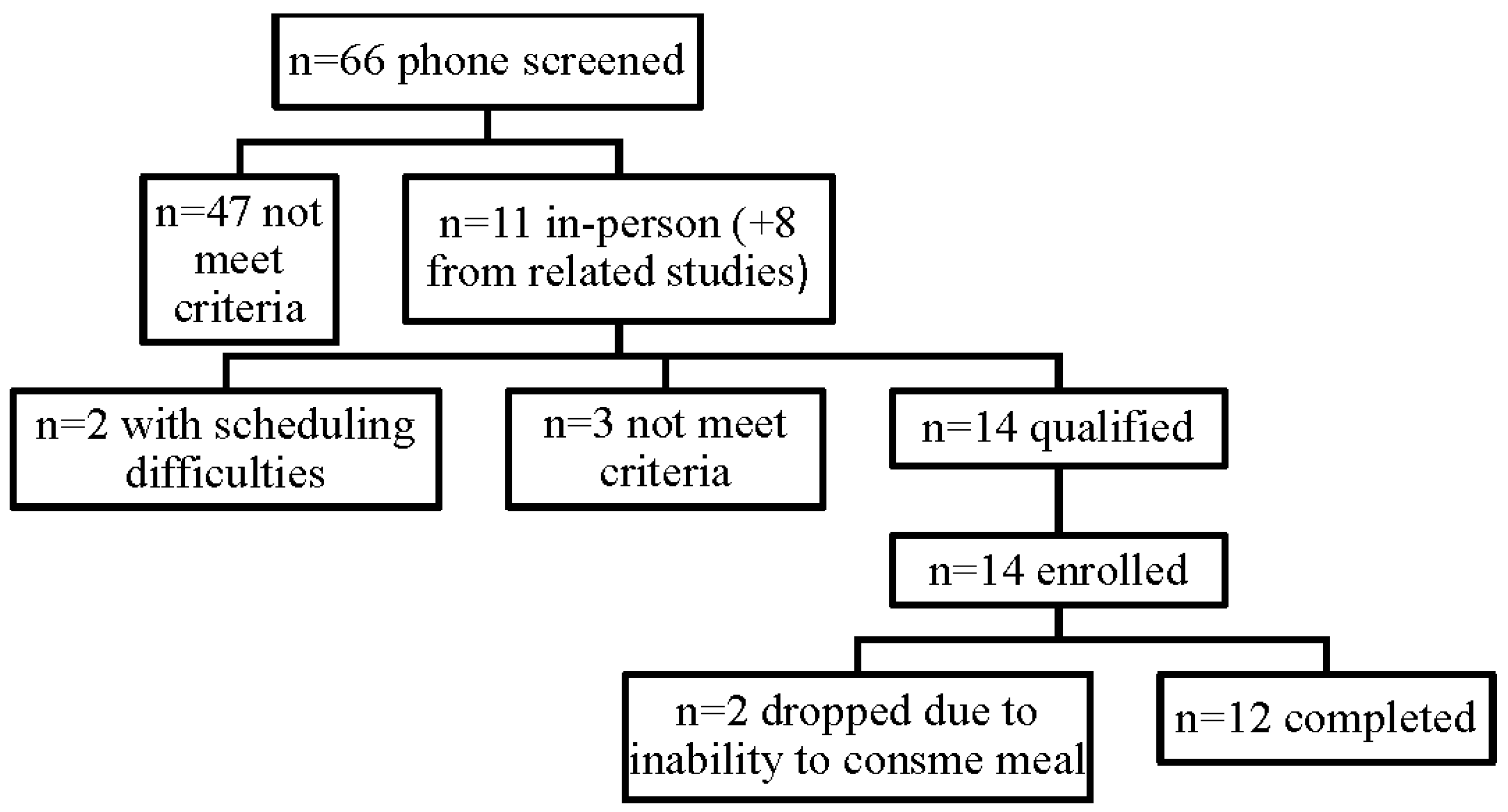
| Baseline Values | Units | Mean | SD |
|---|---|---|---|
| Lipids | |||
| Triglycerides | mmol/L | 1.5 | 0.7 |
| Total-cholesterol | mmol/L | 4.6 | 0.8 |
| Inflammation | |||
| hs-CRP b | mg/dL | 4.0 | 4.2 |
| IL-6 | pg/mL | 2.2 | 1.3 |
| IL-1β c | pg/mL | ND | ND |
| sICAM1 | ng/mL | 202.5 | 3.1 |
| sVCAM1 | ng/mL | 484.3 | 21.1 |
| Glycemia | |||
| Glucose | mmol/L | 5.6 | 0.4 |
| Insulin | pmol/L | 68.2 | 25.1 |
| HOMA-insulin resistance d | ratio | 2.4 | 1.0 |
| HOMA-β cell function e | % | 92.6 | 32.0 |
| Quantitative insulin sensitivity check index | ratio | 0.34 | 0.02 |
| Glucose:insulin ratio | ratio | 11.5 | 2.8 |
| Oxidative stress | |||
| OxLDL | mU/L | 49.8 | 11.4 |
| Total ORAC f | μmol/L trolox equivalents | 18.6 | 12.7 |
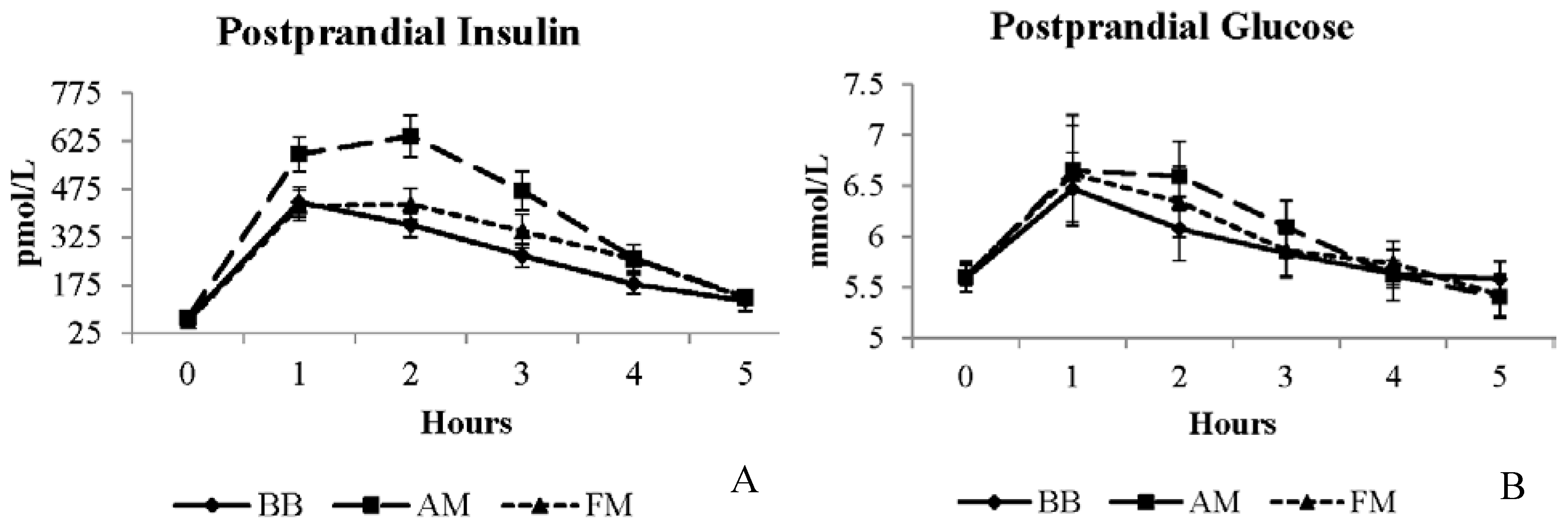
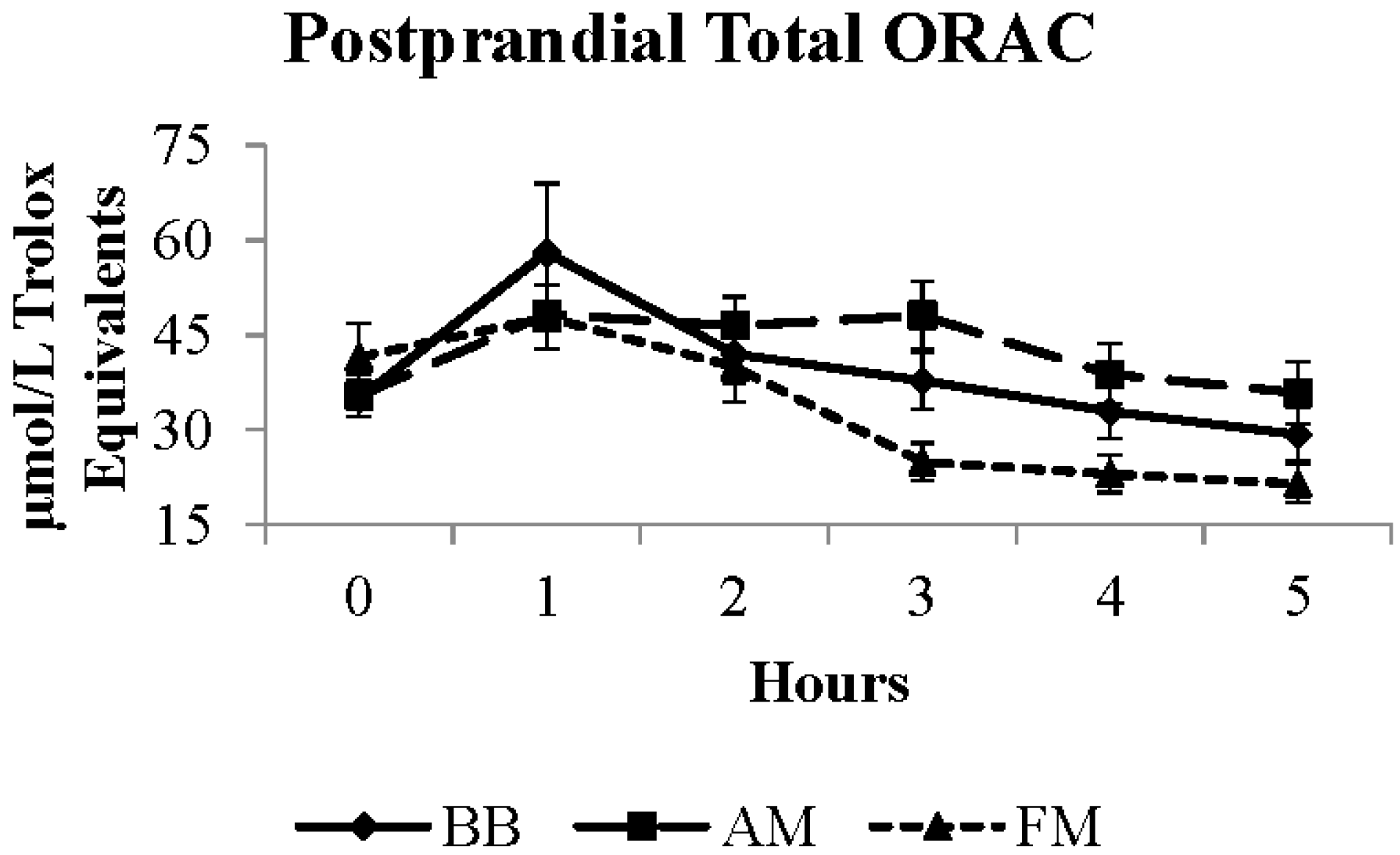
| Biochemical Measurement | BB Meal LSM | FM Meal LSM | AM Meal LSM | SE | Units |
|---|---|---|---|---|---|
| Triglycerides b | 1.8 | 2.0 | 2.0 | 0.2 | mmol/L |
| Glucose b | 5.9 | 5.9 | 6.0 | 0.2 | mmol/L |
| Insulin a,b | 240.4 | 275.5 | 360.9 * | 30.9 | pmol/L |
| IL-6 b | 3.5 | 3.4 | 3.2 | 0.5 | pg/mL |
| sICAM1 | 204.2 | 189.7 * | 198.4 | 15 | ng/mL |
| sVCAM1 | 492.7 * | 449.5 | 458.6 | 30.9 | ng/mL |
| Total ORAC a,b | 37.1 | 33.2 * | 42.2 | 3.2 | μmol/L TE |
| OxLDL | 46.7 | 49.5 | 49.1 | 2.5 | U/L |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome: The Atherosclerosis Risk in Communities study. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Poppitt, S.D.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 220, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Lindman, A.S.; Veierod, M.B.; Tverdal, A.; Pedersen, J.I.; Selmer, R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian Counties Study. Eur. J. Epidemiol. 2010, 25, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D.; Lopez-Miranda, J.; Williams, C. Methodology for studying postprandial lipid metabolism. Eur. J. Clin. Nutr. 2007, 61, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D.; Play, B.; Jourdheuil-Rahmani, D. Digestible and indigestible carbohydrates: Interactions with postprandial lipid metabolism. J. Nutr. Biochem. 2007, 18, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Randolph, J.; Cheema, M.; Tadapaneni, R.; Park, E.; Burton-Freeman, B.; Kappagoda, T. Effect of grape seed extract on postprandial oxidative status and metabolic responses in men and women with the metabolic syndrome—Randomized, cross-over, placebo-controlled study. Funct. Foods Health Dis. 2012, 2, 508–521. [Google Scholar]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 437S–442S. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, I.; Olson, B.; Backus, R.; Richter, B.D.; Davis, P.A.; Schneeman, B.O. Beans, as a source of dietary fiber, increase cholecystokinin and apolipoprotein b48 response to test meals in men. J. Nutr. 2001, 131, 1485–1490. [Google Scholar] [PubMed]
- Nilsson, A.; Johansson, E.; Ekstrom, L.; Bjorck, I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: A randomized cross-over study. PLoS ONE 2013, 8, e59985. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Pedrosa, M.M.; Cuadrado, C.; Brito, M.; Asensio, S.M.C.; Asensio-Vegas, C. Composition of two Spanish common dry beans (Phaseolus vulgaris), ‘Almonga’ and ‘Curruquilla’, and their postprandial effect in type 2 diabetics. J. Sci. Food Agric. 2013, 93, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Spadafranca, A.; Rinelli, S.; Riva, A.; Morazzoni, P.; Magni, P.; Bertoli, S.; Battezzati, A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br. J. Nutr. 2013, 109, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Winham, D.M.; Hutchins, A.M.; Johnston, C.S. Pinto bean consumption reduces biomarkers for heart disease risk. J. Am. Coll. Nutr. 2007, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.V.; Winham, D.M.; Hutchins, A.M. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr. J. 2012, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Winham, D.M.; Thompson, S.V. Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br. J. Nutr. 2012, 108 (Suppl. S1), S52–S65. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, S.K.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.A.; Rajalakshmi, V.; Jamdar, S.N.; Sharma, A. Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- USDA Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods. Available online: http://www.ars.usda.gov/services/docs.htm?docid=15866 (accessed on 26 April 2015).
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; deVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Okuma, K. Determination of insoluble, soluble, and total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: Collaborative study. J. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Nappo, F.; Esposito, K.; Cioffi, M.; Giugliano, G.; Molinari, A.M.; Paolisso, G.; Marfella, R.; Giugliano, D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002, 39, 1145–1150. [Google Scholar] [CrossRef]
- Neri, S.; Calvagno, S.; Mauceri, B.; Misseri, M.; Tsami, A.; Vecchio, C.; Mastrosimone, G.; Di Pino, A.; Maiorca, D.; Judica, A.; et al. Effects of antioxidants on postprandial oxidative stress and endothelial dysfunction in subjects with impaired glucose tolerance and type 2 diabetes. Eur. J. Nutr. 2010, 49, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Cortes, B.; Nunez, I.; Cofan, M.; Gilabert, R.; Perez-Heras, A.; Casals, E.; Deulofeu, R.; Ros, E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J. Am. Coll. Cardiol. 2006, 48, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; Lopez, S.; Bermudez, B.; Abia, R.; Villar, J.; Muriana, F.J. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J. Nutr. Biochem. 2008, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Lopez-Miranda, J.; Perez-Martinez, P.; Jimenez, Y.; Marin, C.; Gomez, P.; Fernandez, J.M.; Caballero, J.; Delgado-Lista, J.; Perez-Jimenez, F. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with alpha-linolenic acid on postprandial endothelial function in healthy men. Br. J. Nutr. 2008, 100, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, P.; Moreno-Conde, M.; Cruz-Teno, C.; Ruano, J.; Fuentes, F.; Delgado-Lista, J.; Garcia-Rios, A.; Marin, C.; Gomez-Luna, M.J.; Perez-Jimenez, F.; et al. Dietary fat differentially influences regulatory endothelial function during the postprandial state in patients with metabolic syndrome: From the LIPGENE study. Atherosclerosis 2010, 209, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Herieka, M.; Erridge, C. High-fat meal induced postprandial inflammation. Mol. Nutr. Food Res. 2014, 58, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Wang-Polagruto, J.; Polagruto, J.; Keen, C.L.; Jialal, I. High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism 2008, 57, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Miglio, C.; Peluso, I.; Raguzzini, A.; Villano, D.V.; Cesqui, E.; Catasta, G.; Toti, E.; Serafini, M. Antioxidant and inflammatory response following high-fat meal consumption in overweight subjects. Eur. J. Nutr. 2013, 52, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Talbot, J.; Park, E.; Krishnankutty, S.; Edirisinghe, I. Protective activity of processed tomato products on postprandial oxidation and inflammation: A clinical trial in healthy weight men and women. Mol. Nutr. Food Res. 2012, 56, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Nappo, F.; Giugliano, F.; di Palo, C.; Ciotola, M.; Barbieri, M.; Paolisso, G.; Giugliano, D. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2003, 78, 1135–1140. [Google Scholar] [PubMed]
- Troseid, M.; Seljeflot, I.; Arnesen, H. The role of interleukin-18 in the metabolic syndrome. Cardiovasc. Diabetol. 2010, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, B.J.; Papacosta, O.; Owen, C.G.; Wannamethee, S.G.; Humphries, S.E.; Woodward, M.; Lennon, L.T.; Thomson, A.; Welsh, P.; Rumley, A.; et al. Interleukin 18 and coronary heart disease: Prospective study and systematic review. Atherosclerosis 2011, 217, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabate, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Hudthagosol, C.; Haddad, E.H.; McCarthy, K.; Wang, P.; Oda, K.; Sabate, J. Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. J. Nutr. 2011, 141, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Teeter, D.L.; Chen, C.Y.; Vanden Heuvel, J.P.; West, S.G. A high antioxidant spice blend attenuates postprandial insulin and triglyceride responses and increases some plasma measures of antioxidant activity in healthy, overweight men. J. Nutr. 2011, 141, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Gu, L.; Wu, X.; Jacob, R.A.; Sotoudeh, G.; Kader, A.A.; Cook, R.A. Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status. J. Am. Coll. Nutr. 2007, 26, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Chusak, C.; Thilavech, T.; Adisakwattana, S. Consumption of Mesona chinensis attenuates postprandial glucose and improves antioxidant status induced by a high carbohydrate meal in overweight subjects. Am. J. Chin. Med. 2014, 42, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Blacker, B.C.; Snyder, S.M.; Eggett, D.L.; Parker, T.L. Consumption of blueberries with a high-carbohydrate, low-fat breakfast decreases postprandial serum markers of oxidation. Br. J. Nutr. 2013, 109, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Holub, B.J. The effect of wild blueberry (Vaccinium angustifolium) consumption on postprandial serum antioxidant status in human subjects. Br. J. Nutr. 2002, 88, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.M.; Reber, J.D.; Freeman, B.L.; Orgad, K.; Eggett, D.L.; Parker, T.L. Controlling for sugar and ascorbic acid, a mixture of flavonoids matching navel oranges significantly increases human postprandial serum antioxidant capacity. Nutr. Res. 2011, 31, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Oda, E. Metabolic syndrome: Its history, mechanisms, and limitations. Acta Diabetol. 2012, 49, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cox-York, K.A.; Sharp, T.A.; Stotz, S.A.; Bessesen, D.H.; Pagliassotti, M.J.; Horton, T.J. The effects of sex, metabolic syndrome and exercise on postprandial lipemia. Metabolism 2013, 62, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Pavlidis, A.N.; Salpea, K.D.; Iraklianou, S.A.; Hoursalas, I.S.; Mikhailidis, D.P.; Cokkinos, D.V. Metabolic syndrome and gender differences in postprandial lipaemia. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Payette, C.; Blackburn, P.; Lamarche, B.; Tremblay, A.; Bergeron, J.; Lemieux, I.; Despres, J.P.; Couillard, C. Sex differences in postprandial plasma tumor necrosis factor-alpha, interleukin-6, and C-reactive protein concentrations. Metabolism 2009, 58, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reverri, E.J.; Randolph, J.M.; Steinberg, F.M.; Kappagoda, C.T.; Edirisinghe, I.; Burton-Freeman, B.M. Black Beans, Fiber, and Antioxidant Capacity Pilot Study: Examination of Whole Foods vs. Functional Components on Postprandial Metabolic, Oxidative Stress, and Inflammation in Adults with Metabolic Syndrome. Nutrients 2015, 7, 6139-6154. https://doi.org/10.3390/nu7085273
Reverri EJ, Randolph JM, Steinberg FM, Kappagoda CT, Edirisinghe I, Burton-Freeman BM. Black Beans, Fiber, and Antioxidant Capacity Pilot Study: Examination of Whole Foods vs. Functional Components on Postprandial Metabolic, Oxidative Stress, and Inflammation in Adults with Metabolic Syndrome. Nutrients. 2015; 7(8):6139-6154. https://doi.org/10.3390/nu7085273
Chicago/Turabian StyleReverri, Elizabeth J., Jody M. Randolph, Francene M. Steinberg, C. Tissa Kappagoda, Indika Edirisinghe, and Britt M. Burton-Freeman. 2015. "Black Beans, Fiber, and Antioxidant Capacity Pilot Study: Examination of Whole Foods vs. Functional Components on Postprandial Metabolic, Oxidative Stress, and Inflammation in Adults with Metabolic Syndrome" Nutrients 7, no. 8: 6139-6154. https://doi.org/10.3390/nu7085273
APA StyleReverri, E. J., Randolph, J. M., Steinberg, F. M., Kappagoda, C. T., Edirisinghe, I., & Burton-Freeman, B. M. (2015). Black Beans, Fiber, and Antioxidant Capacity Pilot Study: Examination of Whole Foods vs. Functional Components on Postprandial Metabolic, Oxidative Stress, and Inflammation in Adults with Metabolic Syndrome. Nutrients, 7(8), 6139-6154. https://doi.org/10.3390/nu7085273




