Oral Zinc Supplementation Reduces the Erythropoietin Responsiveness Index in Patients on Hemodialysis
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Participants
2.2. Study Evaluations
2.3. Anemia Management Protocol
2.4. HD Procedure
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Data
| Variables | Polaprezinc Group | Control Group | p value |
|---|---|---|---|
| n (male/female) | 35 (21/14) | 35 (22/13) | 0.811 |
| Age (years) | 69 ± 10 | 69 ± 10 | 0.797 |
| Hemodialysis duration (mo) | 66 ± 45 | 64 ± 43 | 0.361 |
| Diabetes mellitus (%) | 16 (45.7) | 15 (42.8) | 0.813 |
| Cardiovascular comorbidity (%) | 6 (17.1) | 5 (14.2) | 0.746 |
| Systolic blood pressure (mmHg) | 143 ± 15 | 144 ± 19 | 0.210 |
| Diastolic blood pressure (mmHg) | 77 ± 14 | 75 ± 13 | 0.818 |
| Heart rate (bpm) | 74 ± 10 | 76 ± 10 | 0.713 |
| Medications (%) | |||
| Active vitamin D therapy | 48.5 | 45.7 | 0.814 |
| Angiotensin receptor blockers | 57.1 | 60.0 | 0.811 |
| ACE inhibitors | 8.5 | 5.7 | 0.648 |
| Calcium channel blockers | 51.4 | 54.2 | 0.814 |
| Phosphate binders | 100 | 100 | 1.000 |
| Proton pump inhibitors | 85.7 | 88.5 | 0.725 |
| Statins | 42.8 | 37.1 | 0.631 |
| Kt/V | 1.33 ± 0.06 | 1.34 ± 0.06 | 0.520 |
| Body mass index (kg/m2) | 21.5 ± 3.5 | 21.8 ± 3.2 | 0.715 |
| Hemoglobin (g/dL) | 10.8 ± 0.7 | 11.1 ± 0.8 | 0.240 |
| Erythropoietin responsiveness index | 10.5 ± 5.2 | 10.2 ± 8.0 | 0.851 |
| Serum zinc (mg/dL) | 53 ± 6 | 55 ± 5 | 0.427 |
| Serum creatinine (mg/dL) | 8.2 ± 1.3 | 8.3 ± 1.2 | 0.831 |
| Serum urea nitrogen (mg/dL) | 67 ± 9 | 78 ± 9 | 0.376 |
| Serum albumin (g/dL) | 3.7 ± 0.3 | 3.7 ± 0.3 | 0.623 |
| Total cholesterol (mg/dL) | 150 ± 28 | 151 ± 26 | 0.492 |
| HDL cholesterol (mg/dL) | 49 ± 10 | 50 ± 11 | 0.427 |
| Triglycerides (mg/dL) | 110 ± 52 | 112 ± 49 | 0.422 |
| High sensitivity-CRP (mg/dL) | 0.17 ± 0.17 | 0.18 ± 0.17 | 0.561 |
| Corrected calcium (mg/dL) | 8.7 ± 0.5 | 8.6 ± 0.4 | 0.362 |
| Phosphate (mg/dL) | 5.7 ± 0.5 | 5.6 ± 0.5 | 0.860 |
| Intact PTH (pg/mL) | 111 ± 63 | 92 ± 61 | 0.104 |
3.2. Effects of Polaprezinc Supplementation on Anemia Management
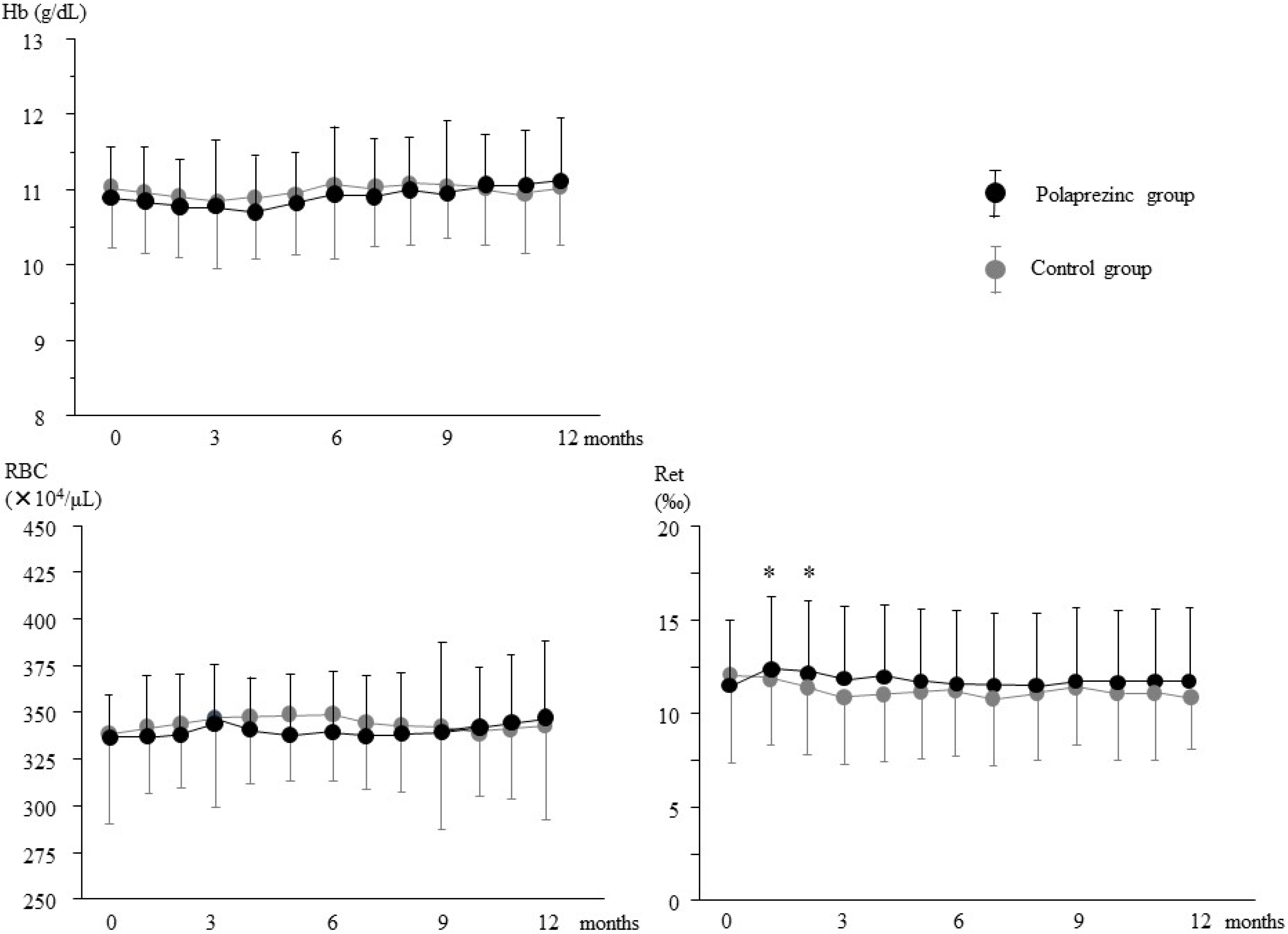
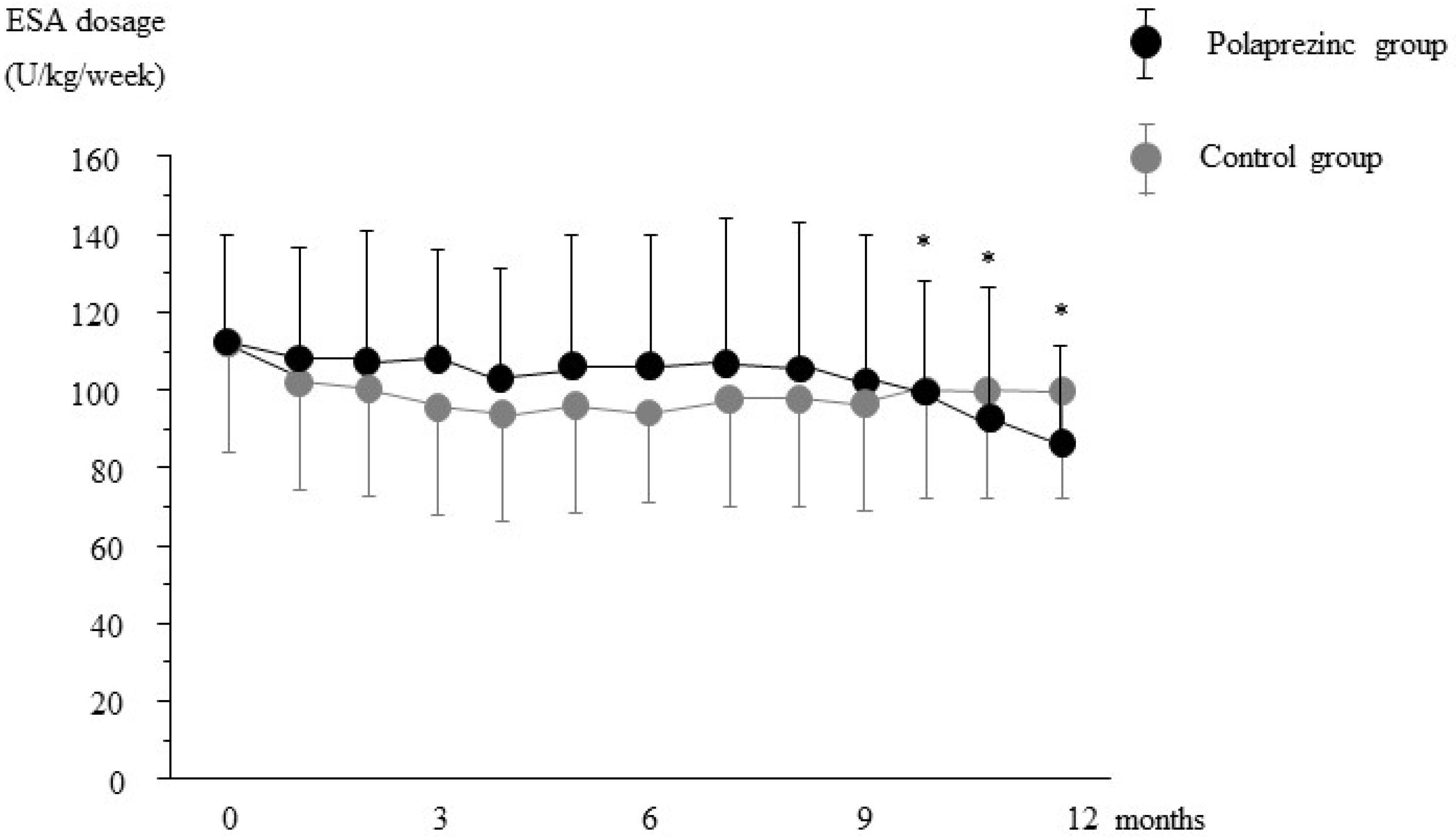

3.3. Effects of Polaprezinc Supplementation on Mineral Parameters
| Variables | Group | Baseline | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|---|
| Serum zinc | Polaprezinc | 53 ± 6 | 67 ± 12 †,*** | 73 ± 21 ‡,*** | 83 ± 27 ‡,*** | 80 ± 18 ‡,*** |
| Control | 55 ± 5 | 54 ± 6 | 56 ± 7 | 56 ± 7 | 56 ± 10 | |
| Serum copper | Polaprezinc | 89 ± 17 | 83 ± 13 * | 82 ± 14 * | 79 ± 11 ** | 79 ± 12 *** |
| Control | 88 ± 17 | 86 ± 17 | 86 ± 17 | 85 ± 17 | 85 ± 23 | |
| Corrected calcium | Polaprezinc | 8.7 ± 0.5 | 8.6 ± 0.7 | 8.7 ± 0.6 | 8.8 ± 0.6 | 8.8 ± 0.5 |
| Control | 8.6 ± 0.4 | 8.7 ± 0.6 | 8.6 ± 0.4 | 8.8 ± 0.6 | 8.8 ± 0.6 | |
| Serum iron | Polaprezinc | 59 ± 16 | 53 ± 19 | 60 ± 21 | 62 ± 21 | 57 ± 24 |
| Control | 64 ± 30 | 57 ± 34 | 62 ± 24 | 57 ± 18 | 56 ± 23 | |
| TSAT (%) | Polaprezinc | 28 ± 8 | 26 ± 9 | 28 ± 8 | 30 ± 15 | 30 ± 15 |
| Control | 29 ± 14 | 28 ± 18 | 30 ± 13 | 29 ± 12 | 27 ± 14 | |
| Serum ferritin | Polaprezinc | 142 ± 74 | 128 ± 53 * | 121 ± 38 * | 115 ± 32 * | 108 ± 30 ** |
| Control | 145 ± 85 | 129 ± 83 | 133 ± 93 | 141 ± 83 | 141 ± 83 |
3.4. Multivariate Regression Analysis
| Variables | β | SE | 95% CI | p value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 0.0070 | 0.0224 | −0.0398 | 0.0546 | 0.7466 |
| Hemodialysis duration | 0.0010 | 0.0118 | −0.0238 | 0.0259 | 0.9327 |
| Male sex | −0.0450 | 0.1946 | −0.4542 | 0.3637 | 0.8188 |
| Diabetes | 0.3140 | 0.3530 | −0.4268 | 1.0657 | 0.3842 |
| Kt/V | 3.3530 | 2.7444 | −2.4126 | 9.1190 | 0.2375 |
| Baseline hemoglobin | −0.1080 | 0.3230 | −0.7873 | 0.5700 | 0.7045 |
| Serum albumin | −0.3800 | 0.6987 | −1.8489 | 1.0872 | 0.5924 |
| Change in iron | 0.0001 | 0.0077 | −0.0163 | 0.0163 | 0.9976 |
| Change in TSAT | 0.0086 | 0.0155 | −0.0240 | 0.0412 | 0.5860 |
| Change in ferritin | 0.0011 | 0.0027 | −0.0046 | 0.0070 | 0.6801 |
| Change in copper | −0.0027 | 0.0131 | −0.0303 | 0.0249 | 0.8375 |
| Change in zinc | −0.0479 | 0.0120 | −0.0740 | −0.0219 | 0.0011 |
| Calcium | −0.3040 | 0.3562 | −1.0524 | 0.4443 | 0.4046 |
| Phosphate | −0.4355 | 0.6372 | −1.7743 | 0.9032 | 0.5030 |
| Intact PTH | 0.0050 | 0.0026 | −0.0005 | 0.0106 | 0.0720 |
| High sensitivity-CRP | 1.7622 | 1.1604 | −0.6758 | 4.2002 | 0.1462 |
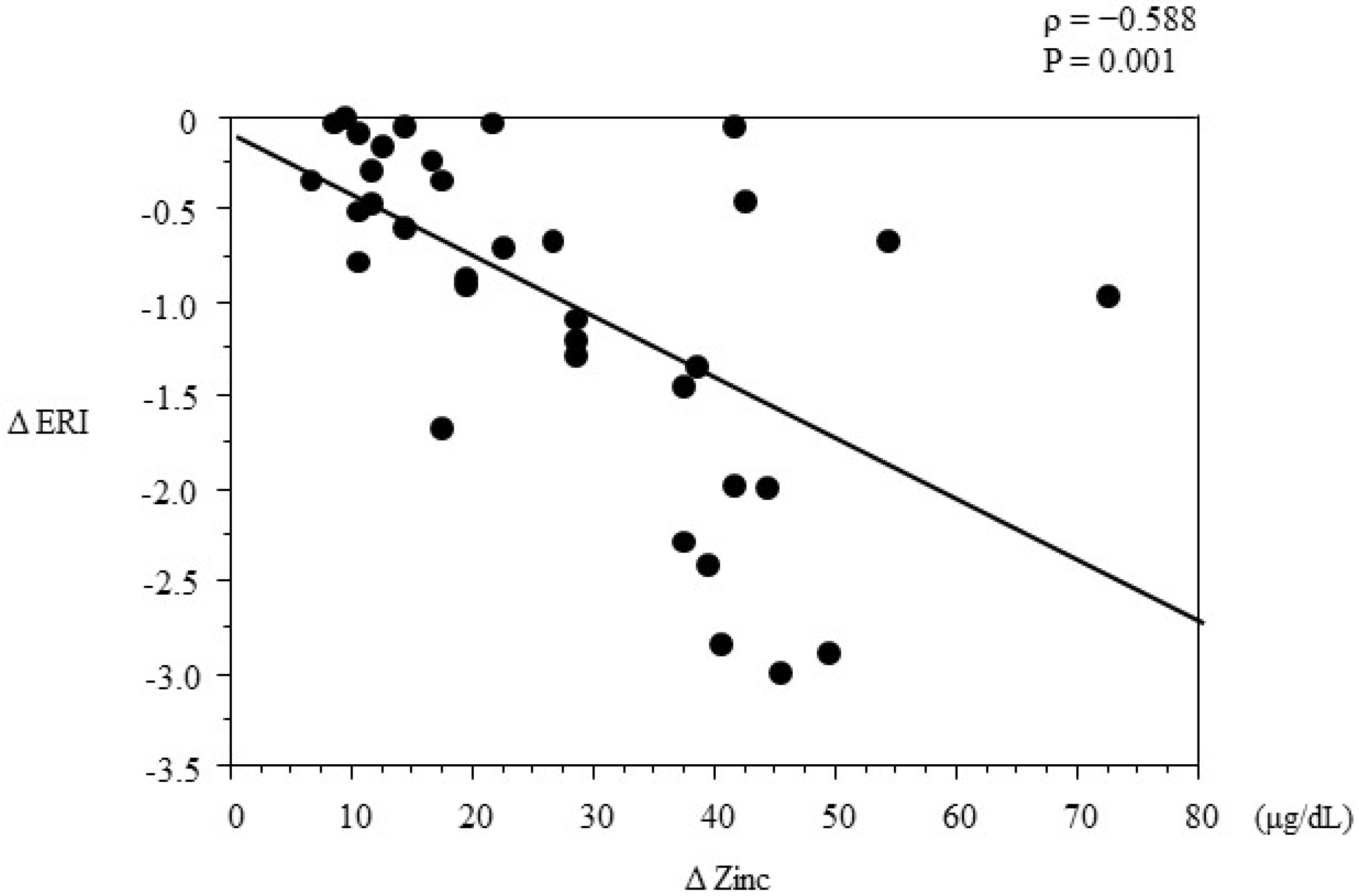
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Japanese Society for Dialysis Therapy. 2008 Japanese Society for Dialysis Therapy: Guidelines for renal anemia for chronic kidney disease. Ther. Apher. Dail. 2010, 14, 240–275. [Google Scholar]
- KDIGO. Clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 299–310. [Google Scholar]
- Hörl, W.H.; Jacobs, C.; Macdougall, I.C.; Valderrábano, F.; Parrondo, I.; Thompson, K.; Carveth, B.G. European best practice guidelines 14–16: Inadequate response to epoetin. Nephrol. Dial. Transplant. 2000, 15, 43–50. [Google Scholar] [CrossRef]
- Abe, M.; Okada, K.; Soma, M. Mineral metabolic abnormalities and mortality in dialysis patients. Nutrients 2013, 5, 1002–1023. [Google Scholar] [CrossRef] [PubMed]
- Zumkley, H.; Bertram, H.P.; Lison, A.; Knoll, O.; Losse, H. Aluminum, zinc and copper concentration in plasma in chronic renal insufficiency. Clin. Nephrol. 1979, 12, 18–21. [Google Scholar] [PubMed]
- Hosokawa, S.; Yoshida, O. Effect of erythropoietin on trace elements in patients with chronic renal failure undergoing hemodialysis. Nephron 1993, 65, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef]
- Reuter, S.; Faull, R.J.; Ranieri, E.; Evans, A.M. Endogenous plasma carnitine pool composition and response to erythropoietin treatment in chronic haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Abe, M.; Yamazaki, T.; Mizuno, M.; Okawa, E.; Ando, H.; Oikawa, O.; Okada, K.; Kikuchi, F.; Soma, M. Effects of levocarnitine on brachial-ankle pulse wave velocity in hemodialysis patients: A randomized controlled trial. Nutrients 2014, 6, 5992–6004. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Tighiouart, H.; Manjunath, G.; MacLeod, B.; Griffith, J.; Salem, D.; Levey, A.S. Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (ARIC) study. J. Am. Coll. Cardiol. 2002, 40, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jurkovitz, C.T.; Abramson, J.L.; Vaccarino, L.V.; Weintraub, W.S.; McClellan, W.M. Association of high serum creatinine and anemia increases the risk of coronary events: Results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 2003, 14, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Ma, J.Z.; Ebben, J. Impact of hematocrit on morbidity and mortality. Semin. Nephrol. 2000, 20, 345–349. [Google Scholar] [PubMed]
- Moura, A.; Madureira, J.; Alija, P.; Fernandes, J.C.; Oliveira, J.G.; Lopez, M.; Filgueiras, M.; Amado, L.; Sameiro-Faria, M.; Miranda, V.; et al. Predictors of health-related quality of life perceived by end-stage renal disease patients under online hemodiafiltration. Qual. Life Res. 2014. [Google Scholar] [CrossRef]
- Stenvinkel, P. The role of inflammation in the anaemia of end-stage renal disease. Nephrol. Dial. Transplant. 2001, 16, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Maruyama, T.; Maruyama, N.; Matsumoto, K.; Soma, M. Relationship between erythropoietin responsiveness, insulin resistance, and malnutrition-inflammation-atherosclerosis (MIA) syndrome in hemodialysis patients with diabetes. Int. J. Artif. Organs 2011, 34, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Okada, K.; Soma, M.; Matsumoto, K. Relationship between insulin resistance and erythropoietin responsiveness in hemodialysis patients. Clin. Nephrol. 2011, 75, 49–58. [Google Scholar] [PubMed]
- Randrianarison-Huetz, V.; Laurent, B.; Bardet, V.; Blobe, G.C.; Huetz, F.; Duménil, D. Gfi-1B controls human erythroid and megakaryocytic differentiation by regulating TGF-beta signaling at the bipotent erythro-megakaryocytic progenitor stage. Blood 2010, 115, 2784–2795. [Google Scholar] [CrossRef]
- Osawa, M.; Yamaguchi, T.; Nakamura, Y.; Kaneko, S.; Onodera, M.; Sawada, K.; Jegalian, A.; Wu, H.; Nakauchi, H.; Iwama, A. Erythroid expansion mediated by the Gfi-1B zinc finger protein: Role in normal hematopoiesis. Blood 2002, 100, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Horike, H.; Fujiki, S.; Kitada, S.; Sasaki, T.; Kashihara, N. Zinc deficiency anemia and effects of zinc therapy in maintenance hemodialysis patients. Ther. Apher. Dial. 2009, 13, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.P.; Morgan, M.; Rudolph, D.S.; Hwang, A.; Albert, N.E.; Valenzano, M.C.; Wang, X.; Mercogliano, G.; Mullin, J.M. Proton pump inhibitors interfere with zinc absorption and zinc body stores. Gastroenterol. Res. 2011, 4, 243–251. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron sequestration and anemia of inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.K. The many faces of the octahedral ferritin protein. Biometals 2011, 24, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Flounders, C.J.; Mullin, J.M. Zinc enhancement of LLC-PK(1) renal epithelial barrier function. Clin. Nutr. 2014, 33, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Garrick, M.D.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. DMT1: A mammalian transporter for multiple metals. Biometals 2003, 16, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Herzog, C.; Chavers, B.; Gilbertson, D.; Herzog, C.; Ishani, A.; Johansen, K.; Kasiske, B.; Kutner, N.; et al. US renal data system 2012 annual data report. Am. J. Kidney Dis. 2013, 61 (Suppl. 1), 1–476. [Google Scholar] [CrossRef] [PubMed]
- Vergnano, A.M.; Rebola, N.; Savtchenko, L.P.; Pinheiro, P.S.; Casado, M.; Kieffer, B.L.; Rusakov, D.A.; Mulle, C.; Paoletti, P. Zinc dynamics and action at excitatory synapses. Neuron 2014, 82, 1101–1114. [Google Scholar]
- Wan, C.; Zhang, M.; Fang, Q.; Xiong, L.; Zhao, X.; Hasunuma, T.; Bai, F.; Kondo, A. The impact of zinc sulfate addition on the dynamic metabolic profiling of Saccharomyces cerevisiae subjected to long term acetic acid stress treatment and identification of key metabolites involved in the antioxidant effect of zinc. Metallomics 2015, 7, 322–332. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral Zinc Supplementation Reduces the Erythropoietin Responsiveness Index in Patients on Hemodialysis. Nutrients 2015, 7, 3783-3795. https://doi.org/10.3390/nu7053783
Kobayashi H, Abe M, Okada K, Tei R, Maruyama N, Kikuchi F, Higuchi T, Soma M. Oral Zinc Supplementation Reduces the Erythropoietin Responsiveness Index in Patients on Hemodialysis. Nutrients. 2015; 7(5):3783-3795. https://doi.org/10.3390/nu7053783
Chicago/Turabian StyleKobayashi, Hiroki, Masanori Abe, Kazuyoshi Okada, Ritsukou Tei, Noriaki Maruyama, Fumito Kikuchi, Terumi Higuchi, and Masayoshi Soma. 2015. "Oral Zinc Supplementation Reduces the Erythropoietin Responsiveness Index in Patients on Hemodialysis" Nutrients 7, no. 5: 3783-3795. https://doi.org/10.3390/nu7053783
APA StyleKobayashi, H., Abe, M., Okada, K., Tei, R., Maruyama, N., Kikuchi, F., Higuchi, T., & Soma, M. (2015). Oral Zinc Supplementation Reduces the Erythropoietin Responsiveness Index in Patients on Hemodialysis. Nutrients, 7(5), 3783-3795. https://doi.org/10.3390/nu7053783




