Sourdough Fermentation of Wheat Flour does not Prevent the Interaction of Transglutaminase 2 with α2-Gliadin or Gluten
Abstract
:1. Introduction
1.1. Celiac Disease
1.2. Deamidation of Gluten Initiates Celiac Disease
1.3. Potential Use of Sourdough in Bread Making to Decrease the Celiac Response
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Strains and Culture Conditions
2.1.2. Lactic Fermentation of Wheat Flour
2.1.3. Sourdough Bread Making
2.1.4. In Vitro Digestion of Wheat Flour and Sourdough Bread
2.2. Detection of the TG2 Binding Motif QLP in α2-Gliadin
2.3. TG2-Mediated Transamidation Assay
2.4. Statistics
3. Results
3.1. The Fermentation Process
3.2. Fermentation with L. plantarum Significantly Increases Available QLP Motives on α2-Gliadin
3.3. Fermentation with L. plantarum Slightly Decreased TG2-Mediated Transamidation of Gluten
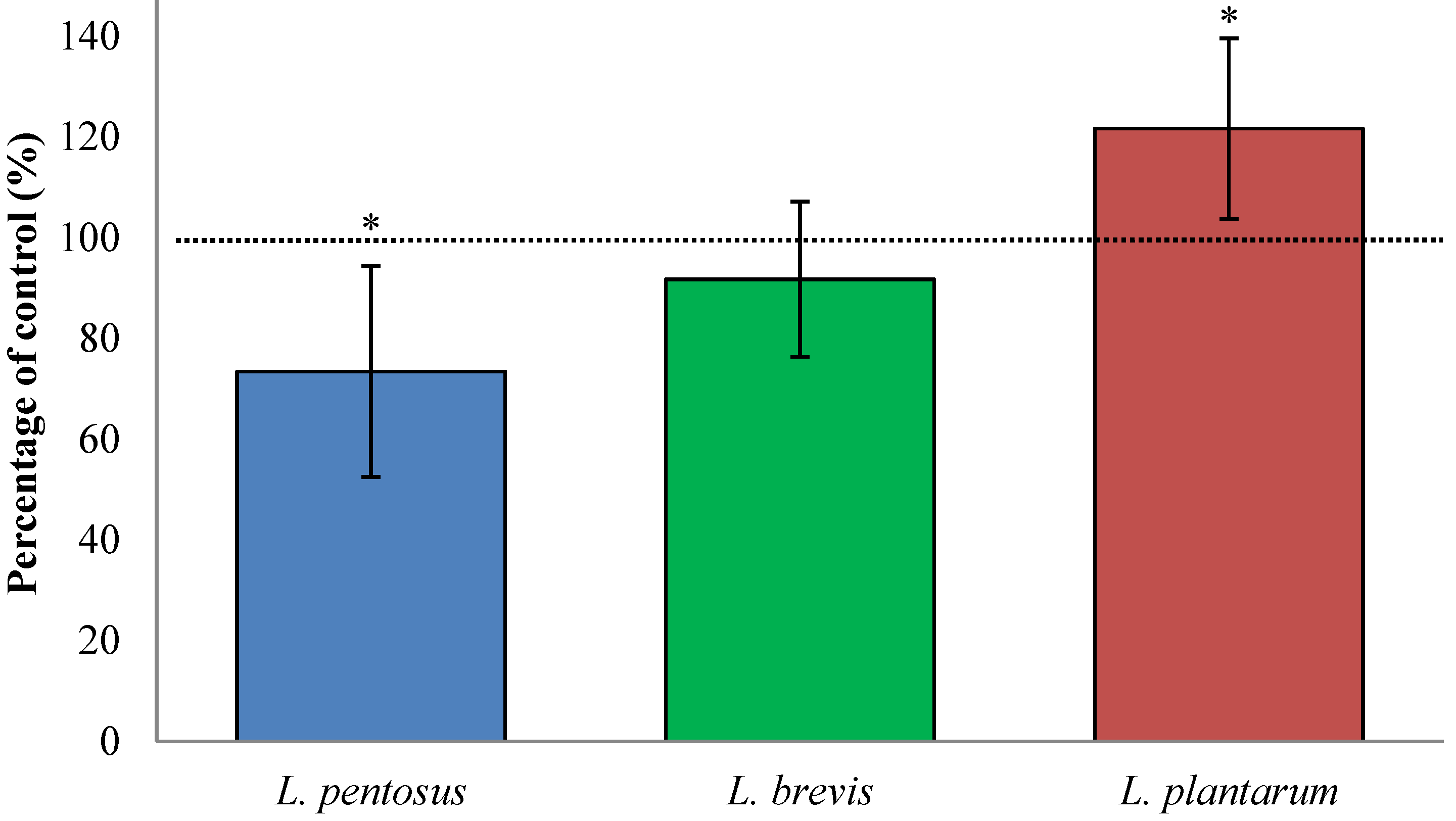
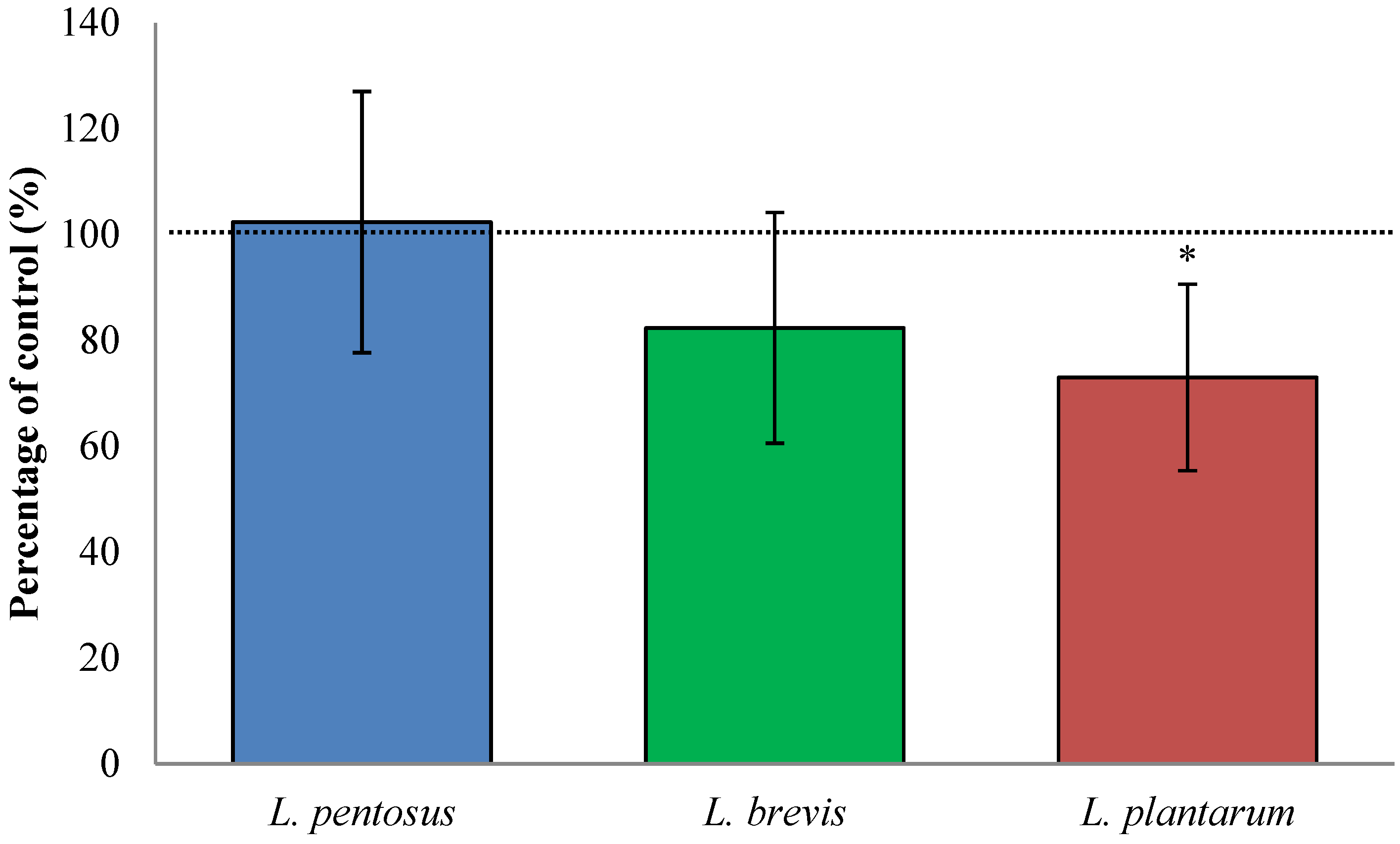
3.4. Sourdough Fermentation with L. plantarum Decreased Available QLP Motives in α2-Gliadin
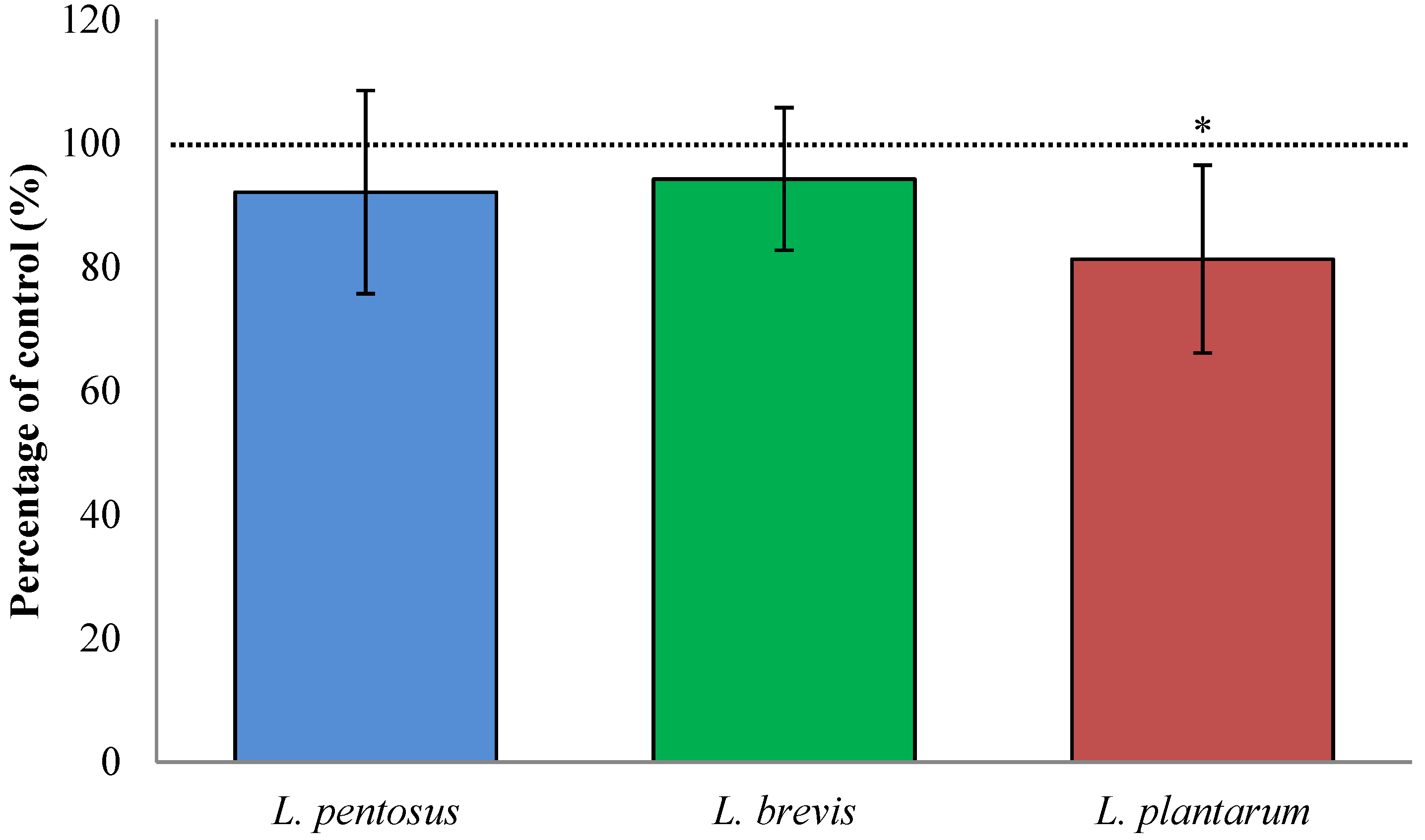
4. Discussion
4.1. Is It Beneficial to Degrade Gluten?
4.2. Evaluation of Methods for TG2 and Gluten Interactions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, E.; Catassi, C.; Villari, A.; Gismondi, P.; Pierdomenico, R.; Ratsch, I.; Coppa, G.; Giorgi, P. Dietary complience in screening-detected coeliac disease adolescents. Acta Paediatr. 1996, 85, 65–67. [Google Scholar] [CrossRef]
- Sollid, L.M.; Markussen, G.; Ek, J.; Gjerde, H.; Vartdal, F.; Thorsby, E. Evidence for a primary association of celiac disease to a particular hla-dq alpha/beta heterodimer. J. Exp. Med. 1989, 169, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Spurkland, A.; Sollid, L.M.; Polanco, I.; Vartdal, F.; Thorsby, E. Hla-dr and -dq genotypes of celiac disease patients serologically typed to be non-dr3 or non-dr5/7. Hum. Immunol. 1992, 35, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Corazza, G. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the espghan committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef]
- Sandberg-Bennich, S.; Dahlquist, G.; Källén, B. Coeliac disease is associated with intrauterine growth and neonatal infections. Acta Pædiatr. 2002, 91, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Plot, L.; Amital, H. Infectious associations of celiac disease. Autoimmun. Rev. 2009, 8, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Molberg, O.; McAdam, S.N.; Korner, R.; Quarsten, H.; Kristiansen, C.; Madsen, L.; Fugger, L.; Scott, H.; Noren, O.; Roepstorff, P.; et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived t cells in celiac disease. Nat. Med. 1998, 4, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Tack, G.; Verbeek, W.; Schreurs, M.; Mulder, C. The spectrum of coeliac disease: Epidemiology, clinical aspects and treatment. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Sollid, L.; Barreiro, L.; Jabri, B. Integration of genetic and immunlogical insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 2011, 29, 493–525. [Google Scholar] [CrossRef] [PubMed]
- Van de Wal, Y.; Kooy, Y.; van Veelen, P.; Peña, S.; Mearin, L.; Papadopoulos, G.; Koning, F. Cutting edge: Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific t cell reactivity. J. Immunol. 1998, 161, 1585–1588. [Google Scholar]
- Arentz-Hansen, H.; Körner, R.; Molberg, Ø.; Quarsten, H.; Vader, W.; Kooy, Y.M.C.; Lundin, K.E.A.; Koning, F.; Roepstorff, P.; Sollid, L.M.; et al. The intestinal T cell response to α-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 2000, 191, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, S.; Ha, K.-S. Transglutaminase 2: A multi-functional protein in multiple subcellular compartments. Amino Acids 2010, 39, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Brusco, G.; Muzi, P.; Ciccocioppo, R.; Biagi, F.; Cifone, M.G.; Corazza, G.R. Transglutaminase and coeliac disease: Endomysial reactivity and small bowel expression. Clin. Exp. Immunol. 1999, 118, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.-W.; Iversen, R.; Ráki, M.; Sollid, L. The adaptive immune response in celiac disease. Semin. Immunopathol. 2012, 34, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Skovbjerg, H.; Koch, C.; Anthonsen, D.; Sjöström, H. Deamidation and cross-linking of gliadin peptides by transglutaminases and the relation to celiac disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2004, 1690, 220–230. [Google Scholar] [CrossRef]
- Sugimura, Y.; Hosono, M.; Wada, F.; Yoshimura, T.; Maki, M.; Hitomi, K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: Identification of peptide substrates for tgase 2 and factor xiiia. J. Biol. Chem. 2006, 281, 17699–17706. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Vanoli, A.; Giuffrida, P.; Luinetti, O.; Solcia, E.; Corazza, G.R. The function of tissue transglutaminase in celiac disease. Autoimmun. Rev. 2012, 11, 746–753. [Google Scholar]
- Di Cagno, R.; Barbato, M.; Di Camillo, C.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; De Vincenzi, M.; Gobbetti, M.; Cucchiara, S. Gluten-free sourdough wheat baked goods appear safe for young celiac patients: A pilot study. J. Pediatric. Gastroenterol. Nutr. 2010, 51, 777–783. [Google Scholar]
- De Angelis, M.; Cassone, A.; Rizzello, C.G.; Gagliardi, F.; Minervini, F.; Calasso, M.; Di Cagno, R.; Francavilla, R.; Gobbetti, M. Mechanism of degradation of immunogenic gluten epitopes from triticum turgidum l. Var. Durum by sourdough lactobacilli and fungal proteases. Appl. Environ. Microbiol. 2010, 76, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; De Vincenzi, M.; De Bari, M.D.; Palmisano, F.; Maurano, F.; et al. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Loponen, J.; Sontag-Strohm, T.; Venäläinen, J.; Salovaara, H. Prolamin hydrolysis in wheat sourdoughs with differing proteolytic activities. J. Agric. Food Chem. 2007, 55, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.L.; Dallagnol, A.; Rollán, G.; Font de Valdez, G. A combination of two lactic acid bacteria improves the hydrolysis of gliadin during wheat dough fermentation. Food Microbiol. 2012, 32, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; Giovannini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Morón, B.; Bethune, M.T.; Comino, I.; Manyani, H.; Ferragud, M.; López, M.C.; Cebolla, Á.; Khosla, C.; Sousa, C. Toward the assessment of food toxicity for celiac patients: Characterization of monoclonal antibodies to a main immunogenic gluten peptide. PLoS ONE 2008, 3, e2294. [Google Scholar] [CrossRef] [PubMed]
- Skovbjerg, H.; Norén, O.; Anthonsen, D.; Moller, J.; Sjöström, H. Coeliac disease gliadin is a good substrate of several transglutaminases: Possible implication in the pathogenesis of coeliac disease. Scand. J. Gastroenterol. 2002, 37, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Tye-Din, J.A.; Anderson, R.P.; Ffrench, R.A.; Brown, G.J.; Hodsman, P.; Siegel, M.; Botwick, W.; Shreeniwas, R. The effects of alv003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin. Immunol. 2010, 134, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Koehler, P.; Wieser, H. Rapid degradation of gliadin peptides toxic for coeliac disease patients by proteases from germinating cereals. J. Cereal Sci. 2006, 44, 368–371. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engström, N.; Sandberg, A.-S.; Scheers, N. Sourdough Fermentation of Wheat Flour does not Prevent the Interaction of Transglutaminase 2 with α2-Gliadin or Gluten. Nutrients 2015, 7, 2134-2144. https://doi.org/10.3390/nu7042134
Engström N, Sandberg A-S, Scheers N. Sourdough Fermentation of Wheat Flour does not Prevent the Interaction of Transglutaminase 2 with α2-Gliadin or Gluten. Nutrients. 2015; 7(4):2134-2144. https://doi.org/10.3390/nu7042134
Chicago/Turabian StyleEngström, Niklas, Ann-Sofie Sandberg, and Nathalie Scheers. 2015. "Sourdough Fermentation of Wheat Flour does not Prevent the Interaction of Transglutaminase 2 with α2-Gliadin or Gluten" Nutrients 7, no. 4: 2134-2144. https://doi.org/10.3390/nu7042134
APA StyleEngström, N., Sandberg, A.-S., & Scheers, N. (2015). Sourdough Fermentation of Wheat Flour does not Prevent the Interaction of Transglutaminase 2 with α2-Gliadin or Gluten. Nutrients, 7(4), 2134-2144. https://doi.org/10.3390/nu7042134




