Black Tea Lowers Blood Pressure and Wave Reflections in Fasted and Postprandial Conditions in Hypertensive Patients: A Randomised Study
Abstract
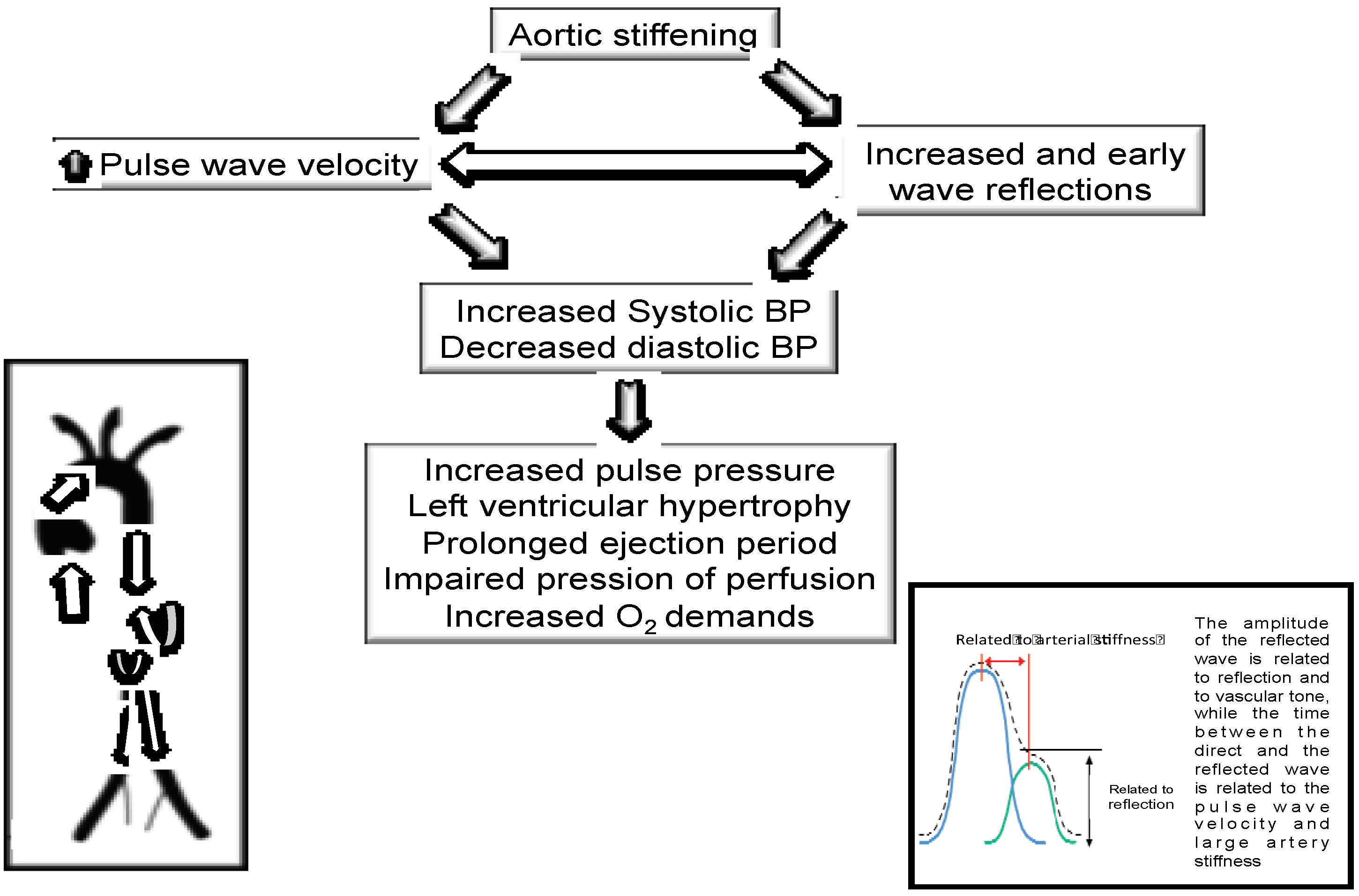
:1. Introduction
2. Methods
2.1. Subjects
2.2. Diagnosis of EH
2.3. Study Design
| Placebo | Active Treatment | |
|---|---|---|
| Polyphenols | 0 | 150 |
| Total Caffeine | 37.3 | 37.3 |
| Tea solids | - | 497.5 |
| Caffeine added | 37.3 | - |
| Caramel colour | 90 | - |
| Tea flavour | 10 | - |
| Sucrose | 1362.7 | 1402.5 |
| Total weight of sachet | 1500 | 1900 |
2.4. Digital Volume Pulse Measurements
2.5. Blood Pressure Measurements
2.6. Statistical Evaluation
2.6.1. Size of The Study Population
3. Results
| Characteristic | Value |
|---|---|
| Number of subjects (total/males) | 19 / 5M |
| Age (years) | 51.3 ± 8.2 |
| BMI (Kg/m2) | 27.1 ± 1.2 |
| Body Weight (Kg) | 73.7 ± 7.2 |
| LDL-cholesterol (mg/dL) | 141.1 ± 27.3 |
| HDL-cholesterol (mg/dL) | 45.7 ± 8.2 |
| Triglycerides (mg/dL) | 116.8 ± 38.1 |
| Plasma glucose (mg/dL) | 86.8 ± 7.3 |
| Plasma insulin (μU/mL) | 11.3 ± 5.4 |
3.1. Wave Reflections Analysis
3.2. Blood Pressure
| parameter | placebo | black tea | difference (95% CI) | Adj P-value |
|---|---|---|---|---|
| Systolic BP (mmHg) | 144.5 ± 0.2 | 141.1 ± 0.2 | −3.3 (−4.3, −2.3) | <0.0001 |
| Diastolic BP (mmHg) | 90.6 ± 0.2 | 88.1 ± 0.2 | −2.5 (−3.3, −1.8) | <0.0001 |
| Heart rate (bpm) | 72.0 ± 0.3 | 70.8 ± 0.3 | −1.2 (−2.3, 0.0) | <0.05 |
| Pulse Pressure (mmHg) | 53.9 ± 0.2 | 53.6 ± 0.2 | −0.3 (−1.3, 0.7) | n.s. |
| Peak to peak time (ms) | 198.9 ± 2.2 | 224.1 ± 2.3 | 25.1 (15.6, 34.6) | <0.0001 |
| Reflection Index (%) | 69.9 ± 0.7 | 64.6 ± 0.6 | −5.2 (−7.9, −2.6) | <0.001 |
| Stiffness Index (m/s) | 8.2 ± 0.1 | 7.5 ± 0.1 | −0.7 (−1.0, −0.5) | <0.0001 |
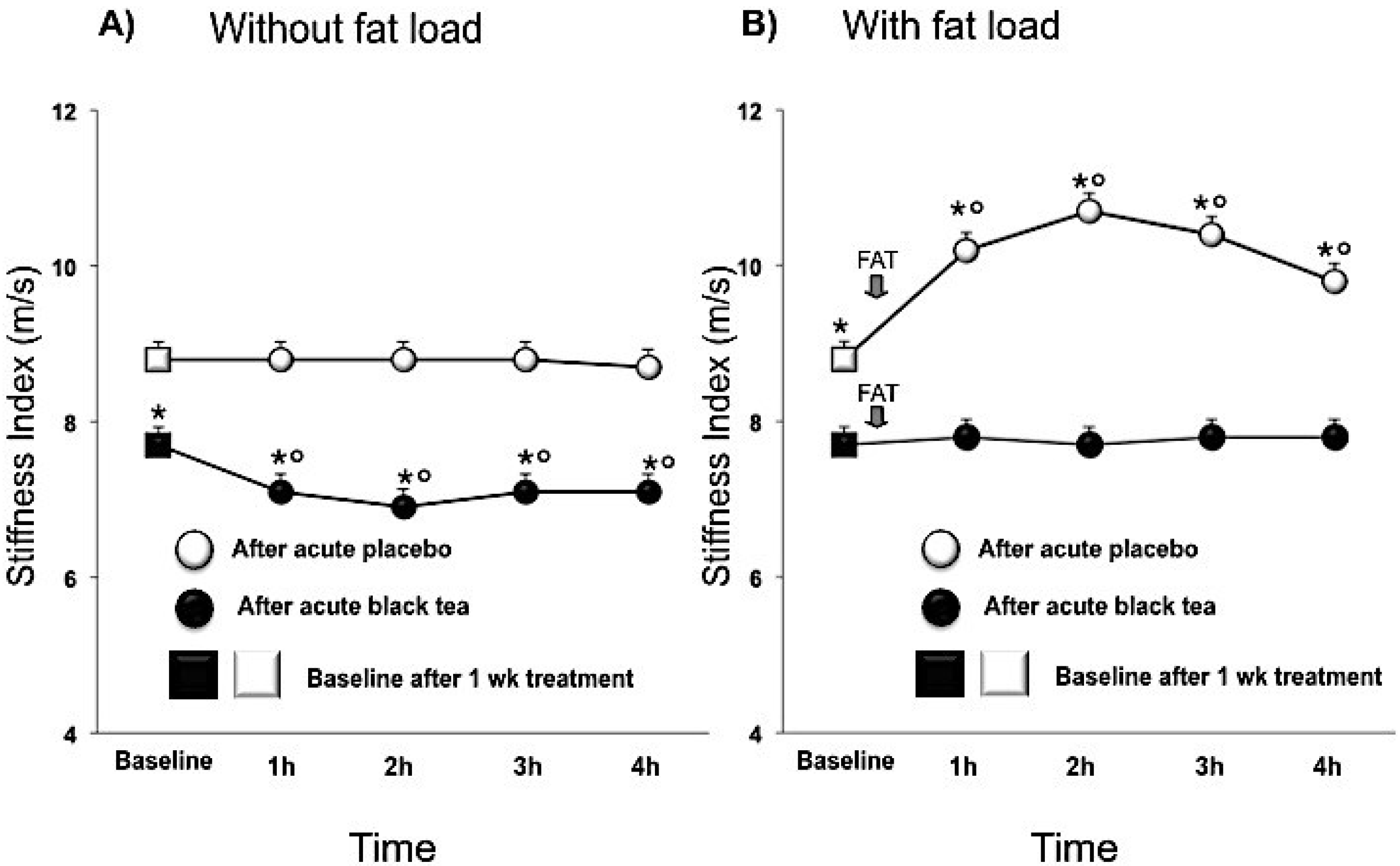
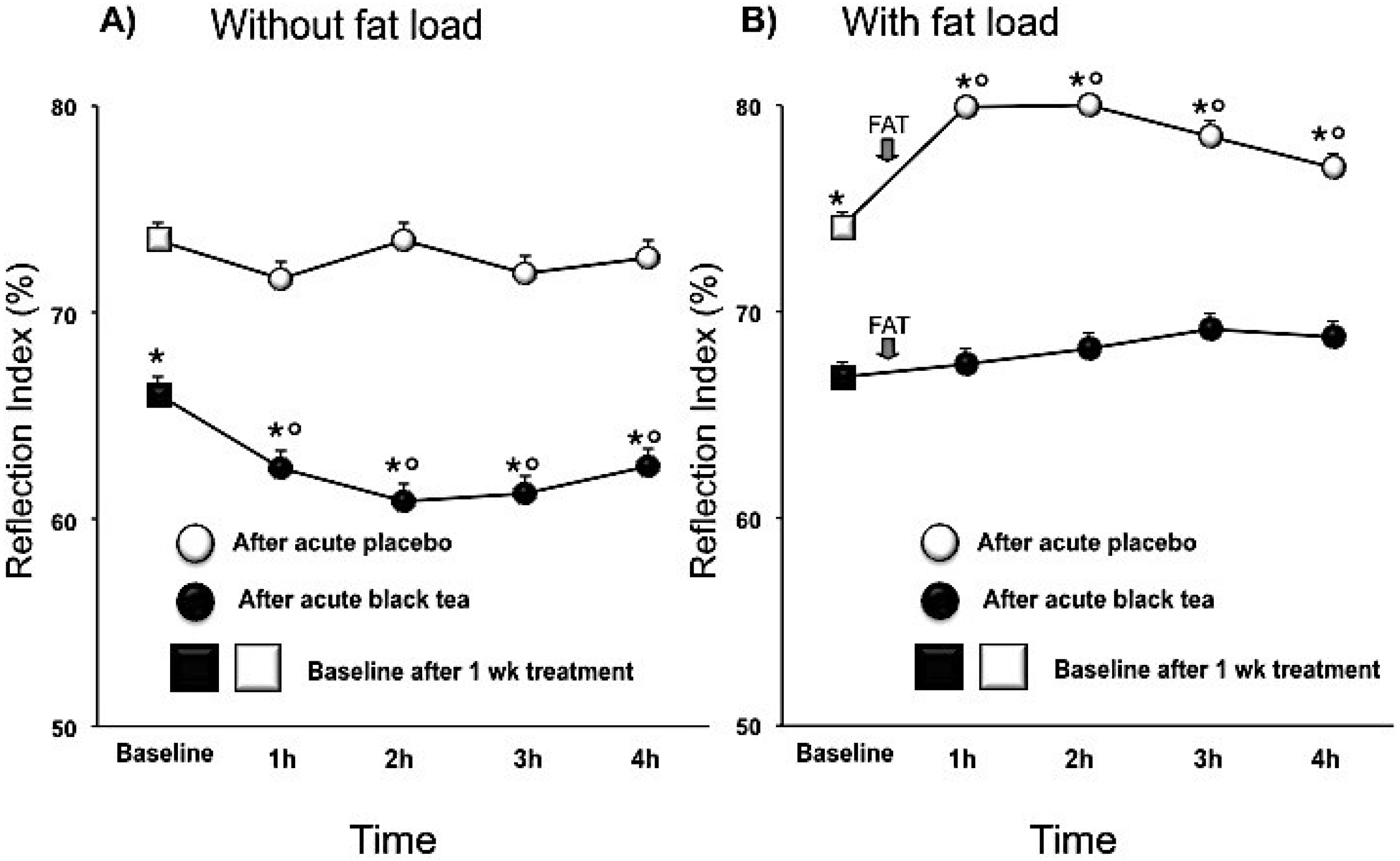
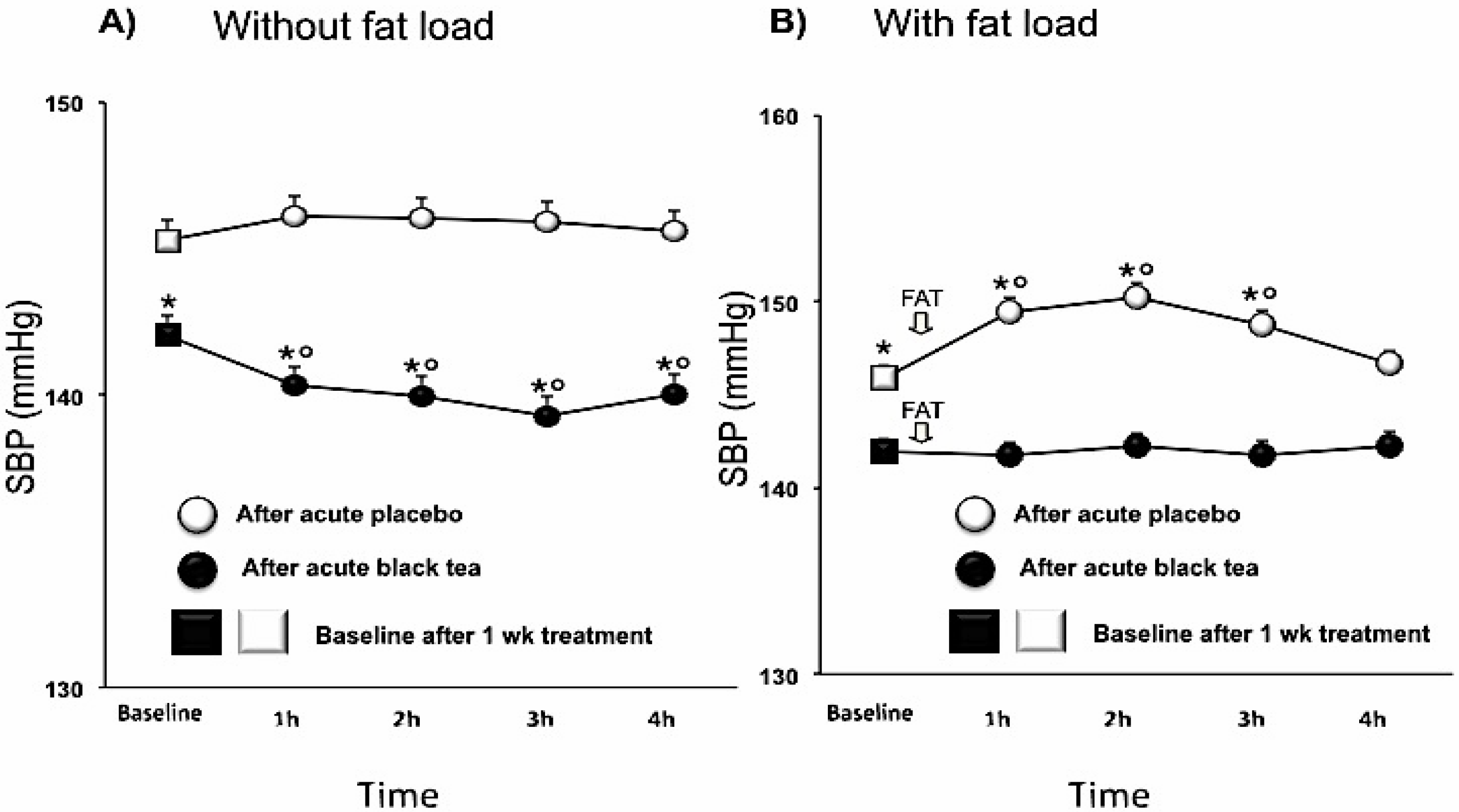
4. Discussion
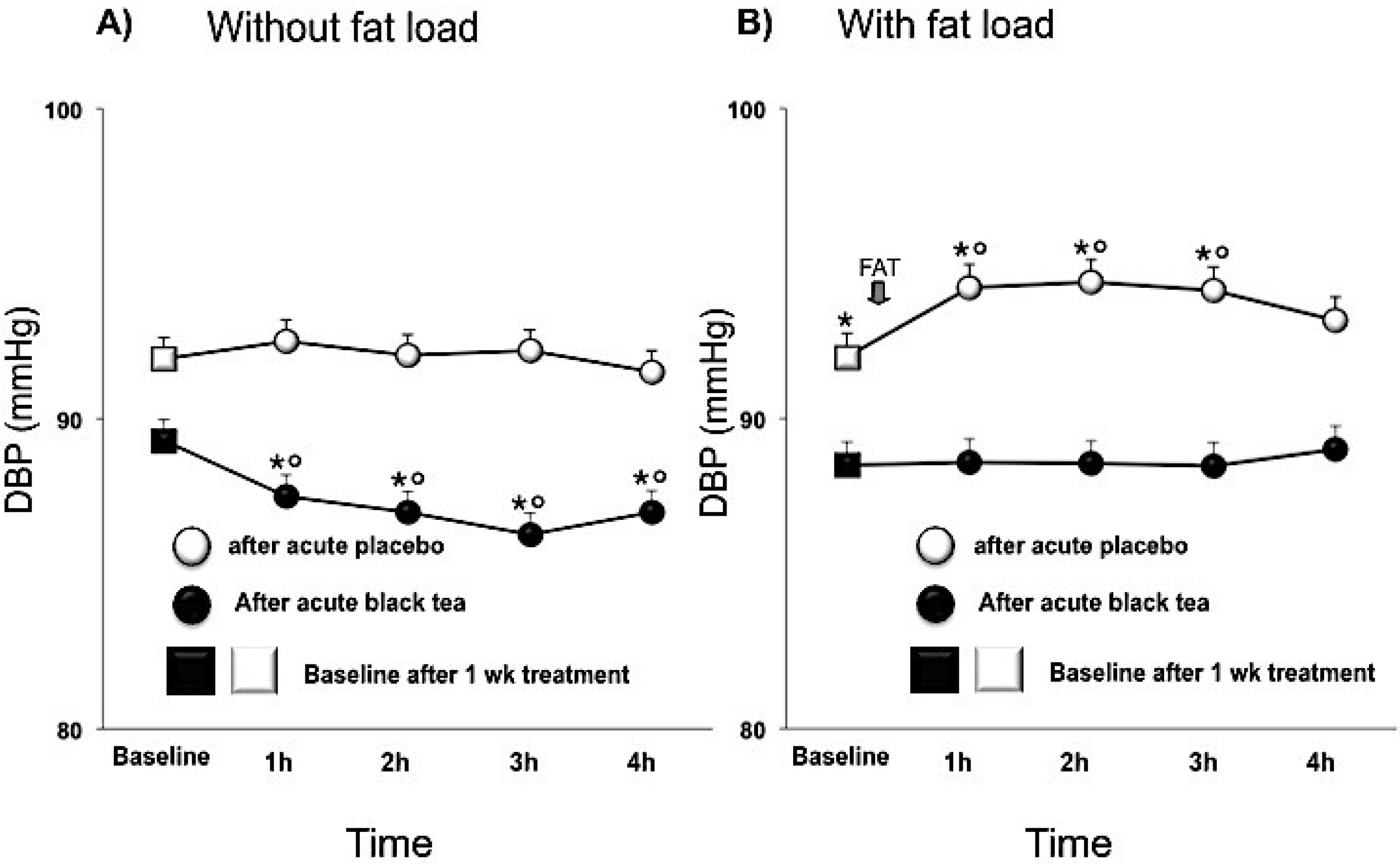
| parameter | placebo | black tea | difference (95% CI) | Adj P-value |
|---|---|---|---|---|
| SBP (mmHg) | 146.7 ± 0.2 | 143.5 ± 0.2 | −3.2 (−4.2, −2.2) | <0.0001 |
| DBP (mmHg) | 92.5 ± 0.2 | 90.5 ± 0.2 | −2.0 (−2.7,−1.2) | <0.0001 |
| HR (bpm) | 71.4 ± 0.3 | 71.4 ± 0.3 | −0.0 (−1.2, 1.1) | n.s. |
| PP (mmHg) | 54.3 ± 0.2 | 53.4 ± 0.2 | −1.0 (−2.0, 0.0) | n.s. |
| PPT (ms) | 170 ± 2.3 | 203 ± 2.2 | 33.9 (24.6, 43.3) | <0.0001 |
| RI (%) | 76.3 ± 0.7 | 70.4 ± 0.6 | −5.9 (−8.5, −3.3) | <0.0001 |
| SI (m/s) | 9.8 ± 0.1 | 8.3 ± 0.1 | −1.5 (−1.8, −1.2) | <0.0001 |
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Ross Anderson, H.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Boudier, H.A.S.; Cohuet, G.M.S.; Baumann, M.; Safar, M.E. The heart, macrocirculation and microcirculation in hypertension: A unifying hypothesis. J. Hypertens. Suppl. 2003, 21, 19–23. [Google Scholar]
- Nicols, W.W.; Denardo, S.J.; Wilkinson, I.B.; McEniery, C.M.; Cockcroft, J.; O’Rourke, M.F. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J. Clin. Hypertens. 2008, 10, 295–303. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.Z.; Takei, Y.; Hasegawa, T.; Koshaka, S.; Palmieri, V.; Elkind, M.S.V.; Homma, S.; Sacco, R.L.; Tullio, M.R.D. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J. Hypertens. 2011, 29, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Gautier, S.; Labat, C.; Salvi, P.; Valbusa, F.; Marino, F.; Toulza, O.; Agnoletti, D.; Zamboni, M.; Dubail, D.; et al. Mortality and cardiovascular events are best predicted by low central/peripheral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects: The partage (predictive values of blood pressure and arterial stiffness in institutionalized very aged population) study. J. Am. Coll. Cardiol. 2012, 60, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Aggio, A.; Onori, L.; Croce, G.; Tiberti, S.; Ferri, C.; Ferri, L.; Desideri, G. Tea, flavonoids, and nitric oxide-mediated vascular reactivity. J. Nutr. 2008, 138, 1554–1560. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Hjartåker, A.; Engeset, D.; Skeie, G.; Overvad, K.; et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2013, 109, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Puddey, I.B.; Woodman, R.J.; Mulder, T.P.; Fuchs, D.; Scott, K.; Croft, K.D. Effects of black tea on blood pressure: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Mulder, T.P.; Draijer, R.; Desideri, G.; Molhuizen, H.O.; Ferri, C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J. Hypertens. 2009, 27, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.K.; Ruempler, K.; Schwedhelm, E.; Tan-Andresen, J.; Riederer, U.; Boger, R.H.; Maas, R. Acute effects of various fast-food meals on vascular function and cardiovascular disease risk markers: The Hamburg Burger Trial. Am. J. Clin. Nutr. 2007, 86, 334–340. [Google Scholar] [PubMed]
- Gosmanov, A.R.; Smiley, D.D.; Robalino, G.; Siquiera, J.; Khan, B.; Le, N.; Patel, R.S.; Quyyumi, A.A.; Peng, L.; Kitabchi, A.E.; et al. Effects of oral and intravenous fat load on blood pressure, endothelial function, sympathetic activity, and oxidative stress in obese healthy subjects. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 953–958. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; Backer, G.D.; Dominiczak, A.; et al. Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed]
- Lakenbrink, C.; Lapczynski, S.; Maiwald, B.; Engelhardt, U.H. Flavonoids and other polyphenols in consumer brews of tea and other caffeinated beverages. J. Agric. Food Chem. 2000, 48, 2848–2852. [Google Scholar] [CrossRef] [PubMed]
- Millasseau, S.C.; Guigui, F.G.; Kelly, R.P.; Prasad, K.; Cockcroft, J.R.; Ritter, J.M.; Chowienczyk, P.J. Noninvasive assessment of the digital volume pulse. Comparison with the peripheral pressure pulse. Hypertension 2000, 36, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Millasseau, S.C.; Kelly, R.P.; Ritter, J.M.; Chowienczyk, P.J. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin. Sci. 2002, 103, 371–377. [Google Scholar] [PubMed]
- Salvi, P.; Magnani, E.; Valbusa, F.; Agnoletti, D.; Alecu, C.; Joly, L.; Benetos, A. Comparative study of methodologies for pulse wave velocity estimation. J. Hum. Hypertens. 2008, 22, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Clarenbach, C.F.; Stoewhas, A.; Gestel, A.J.V.; Latshang, T.D.; Cascio, C.M.L.; Bloch, K.E.; Kohler, M. Comparison of photoplethysmographic and arterial tonometry-derived indices of arterial stiffness. Hypertens Res. 2012, 35, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Di, G.P.; De, F.M.; Fellini, E.; Cheli, P.; Ferri, L.; Ferri, C. Tea, flavonoids, and cardiovascular health: Endothelial protection. Am. J. Clin. Nutr. 2013, 98, 1660–1666. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Ferri, C. Cardiovascular risk and endothelial dysfunction: The preferential route for atherosclerosis. Curr. Pharm. Biotechnol. 2011, 12, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.; Taneva, E.; Kästner, S.; Martens-Lobenhoffer, J.; Bode-Böger, S.; Kropf, S.; Dierkes, J.; Luley, C. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis 2006, 185, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.X.; Peng, F.; Chai, D.J.; Lin, J.X. Effects of combined glucose and fat load on endothelium-dependent brachial artery vasodilatation in hypertensive patients. Am. J. Med. Sci. 2012, 344, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Roodenburg, A.J.; Schlatmann, A.; Dötsch-Klerk, M.; Daamen, R.; Dong, J.; Guarro, M.; Stergiou, M.; Sayed, N.; Ronoh, E.; Jansen, L.; et al. Potential effects of nutrient profiles on nutrient intakes in the Netherlands, Greece, Spain, USA, Israel, China and South-Africa. PLoS One 2011, 6, 14721. [Google Scholar] [CrossRef]
- Piernas, C.; Popkin, B.M. Increased portion sizes from energy-dense foods affect total energy intake at eating occasions in US children and adolescents: Patterns and trends by age group and sociodemographic characteristics, 1977–2006. Am. J. Clin. Nutr. 2011, 94, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Ferri, C. Aortic stiffness, blood pressure and renal dysfunction. Intern. Emerg. Med. 2011, 6, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Prospective studies collaboration. age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, D.; Draijer, R.; Desideri, G.; Mulder, T.; Ferri, C. Black Tea Lowers Blood Pressure and Wave Reflections in Fasted and Postprandial Conditions in Hypertensive Patients: A Randomised Study. Nutrients 2015, 7, 1037-1051. https://doi.org/10.3390/nu7021037
Grassi D, Draijer R, Desideri G, Mulder T, Ferri C. Black Tea Lowers Blood Pressure and Wave Reflections in Fasted and Postprandial Conditions in Hypertensive Patients: A Randomised Study. Nutrients. 2015; 7(2):1037-1051. https://doi.org/10.3390/nu7021037
Chicago/Turabian StyleGrassi, Davide, Richard Draijer, Giovambattista Desideri, Theo Mulder, and Claudio Ferri. 2015. "Black Tea Lowers Blood Pressure and Wave Reflections in Fasted and Postprandial Conditions in Hypertensive Patients: A Randomised Study" Nutrients 7, no. 2: 1037-1051. https://doi.org/10.3390/nu7021037
APA StyleGrassi, D., Draijer, R., Desideri, G., Mulder, T., & Ferri, C. (2015). Black Tea Lowers Blood Pressure and Wave Reflections in Fasted and Postprandial Conditions in Hypertensive Patients: A Randomised Study. Nutrients, 7(2), 1037-1051. https://doi.org/10.3390/nu7021037






