High Prevalence of Vitamin D Deficiency in Infertile Women Referring for Assisted Reproduction
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Variables Measured
2.3. Curves
3. Results
3.1. Seasonal Vitamin D Status in Women Attending the Infertility Center
| Parameter | Mean (SD)/Number (%) | Range |
|---|---|---|
| 25-hydroxyvitamin D (ng/mL) | 24.4 (13.0) | 2–101 |
| Age (Years) | 36.3 (4.4) | 20–47 |
| Latitude (degree) | 45.2 (1.3) | 37–47 |
| Weight (Kg) | 60.4 (10.3) | 34–110 |
| Height (cm) | 164.4 (6.2) | 143–185 |
| Body Mass Index (Kg/m2) | 22.4 (3.6) | 14–40 |
| Current smoking (No. cigarettes/day) | ||
| 0 | 836 (78.0%) | |
| 1–15 | 173 (16.1%) | |
| >15 | 63 (5.9%) | |
| FSH (IU/L) | 8.4 (6.5) | 0–41 |
| LH (IU/L) | 5.9 (4.8) | 0–21 |
| E2 (pg/mL) | 49.7 (23.2) | 9–129 |
| PRL (ng/mL) | 14.1 (7.6) | 2–66 |
| TSH (µU/mL) | 2.2 (1.2) | 0–7 |
| AMH (ng/mL) | 2.2 (2.4) | 0–15 |
| Antral follicles count (n) | 10.1 (6.1) | 0–40 |
| Type of infertility | ||
| Primary | 745 (69.5%) | |
| Secondary | 327 (30.5%) | |
| Cause of Infertility | ||
| Male factor | 175 (16.3%) | |
| Tubal factor | 66 (6.2%) | |
| Poor ovarian response | 140 (13.1%) | |
| Polycystic Ovarian Syndrome | 161 (15.0%) | |
| Endometriosis | 75 (7.0%) | |
| Idiopathic | 196 (18.2%) | |
| Mixed | 259 (24.2%) |
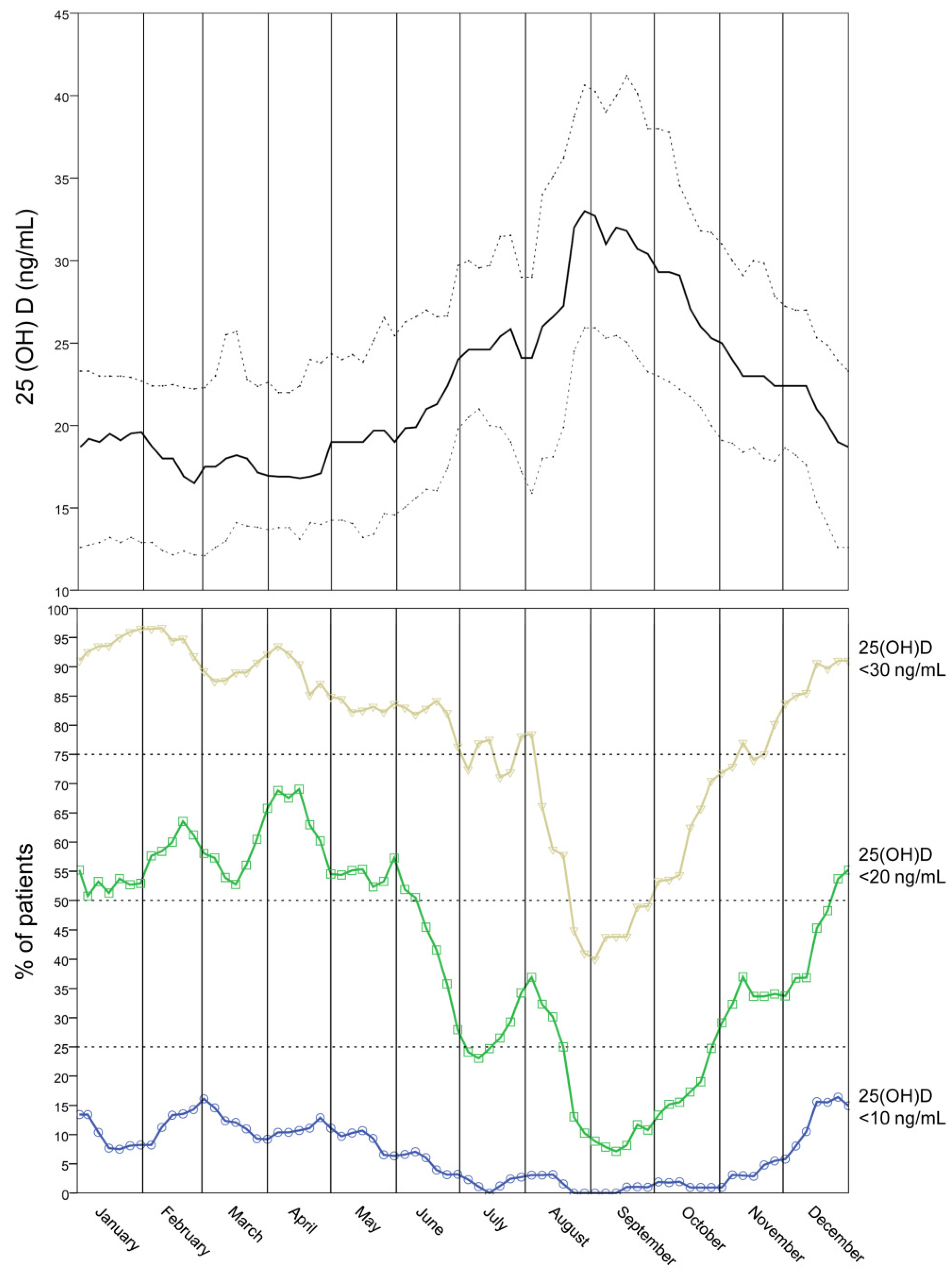
| n | Prevalence (%) | Odds Ratio | CI 95% | p-Value | |
|---|---|---|---|---|---|
| <10 ng/mL | |||||
| Total | 1072 | 6.5 | |||
| Trimester | |||||
| 1 | 256 | 10.9 | 1.00 | ||
| 2 | 292 | 8.6 | 0.76 | 0.43–1.35 | 0.38 |
| 3 | 249 | 0.8 | 0.07 | 0.02–0.28 | <0.0001 |
| 4 | 275 | 5.5 | 0.47 | 0.24–0.90 | 0.03 |
| <20 ng/mL | |||||
| Total | 1072 | 40.1 | |||
| Trimester | |||||
| 1 | 256 | 55.1 | 1.00 | ||
| 2 | 292 | 54.5 | 0.98 | 0.70–1.37 | 0.93 |
| 3 | 249 | 18.9 | 0.19 | 0.13–0.28 | <0.0001 |
| 4 | 275 | 30.2 | 0.35 | 0.25–0.50 | <0.0001 |
| <30 ng/mL | |||||
| Total | 1072 | 77.4 | |||
| Trimester | |||||
| 1 | 256 | 92.2 | 1.00 | ||
| 2 | 292 | 84.6 | 0.47 | 0.27–0.81 | 0.008 |
| 3 | 249 | 58.6 | 0.12 | 0.07–0.20 | <0.0001 |
| 4 | 275 | 73.1 | 0.23 | 0.14–0.39 | <0.0001 |
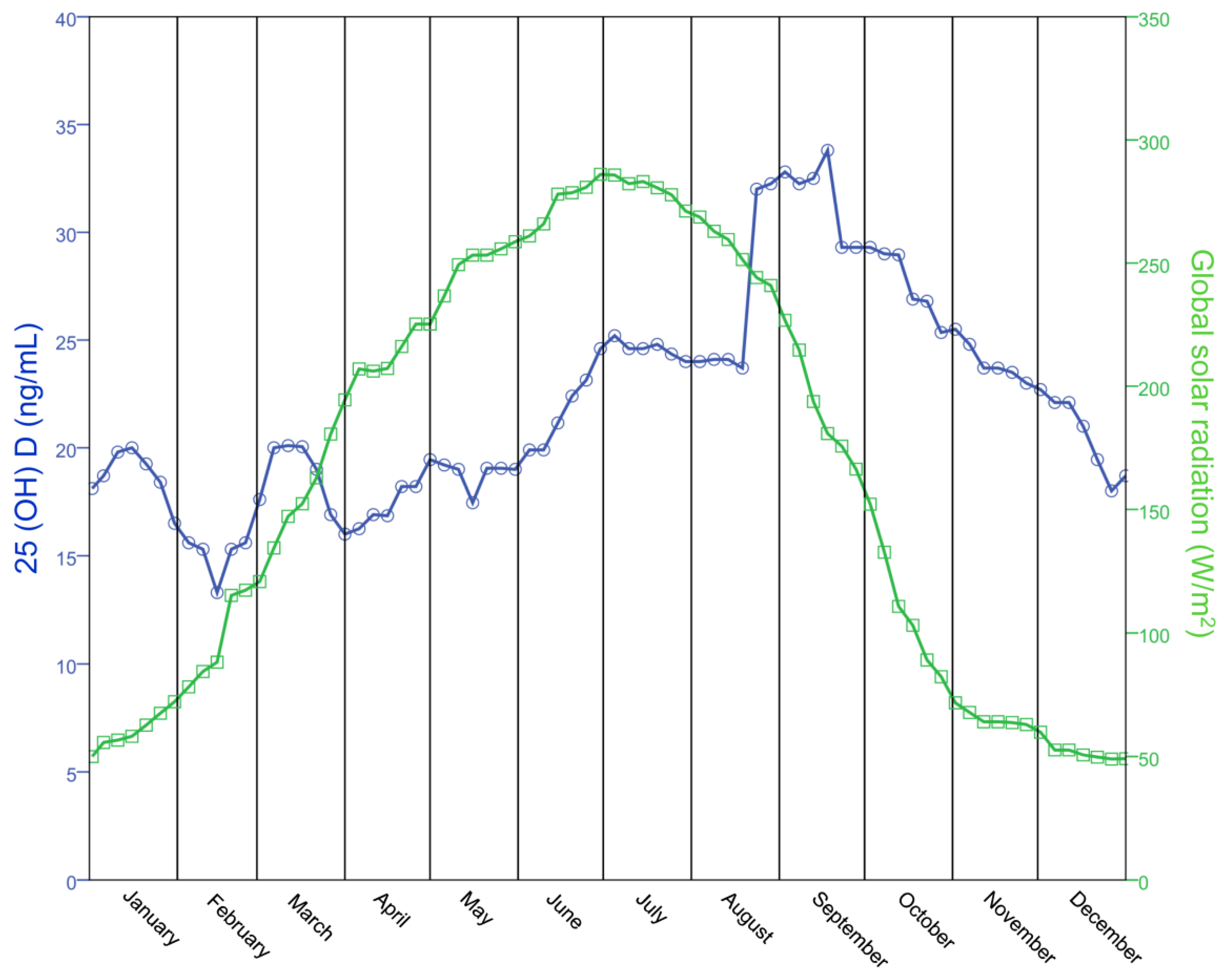
3.2. Determinants of Vitamin D Status in Women Attending the Infertility Center
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| B | B 95% CI | p-Value | B | B 95% CI | p-Value | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Age (Years) | −0.02 | −0.42 | 0.37 | 0.90 | −0.10 | −0.67 | 0.48 | 0.74 |
| Weight (Kg) | −0.33 | −0.50 | −0.16 | 0.0001 | −0.43 | −0.66 | −0.20 | 0.0003 |
| Height (cm) | 0.46 | 0.18 | 0.74 | 0.001 | 0.92 | 0.57 | 1.27 | <0.0001 |
| Current smoking (No. cigarettes/day) | ||||||||
| 0 | 0 | |||||||
| 1–15 | 2.89 | −1.85 | 7.62 | 0.23 | ||||
| >15 | −7.08 | −14.49 | 0.34 | 0.06 | ||||
| FSH (IU/L) | 0.36 | −0.06 | 0.79 | 0.09 | ||||
| LH (IU/L) | 0.39 | −0.20 | 0.98 | 0.19 | ||||
| E2 (pg/mL) | −0.11 | −0.26 | 0.04 | 0.16 | ||||
| PRL (ng/mL) | −0.11 | −0.52 | 0.30 | 0.60 | ||||
| TSH (µU/mL) | −0.11 | −2.53 | 2.32 | 0.93 | ||||
| AMH (ng/mL) | −0.02 | −0.88 | 0.83 | 0.96 | ||||
| Antral follicles count (n) | 0.23 | −0.21 | 0.67 | 0.31 | ||||
| Type of infertility | ||||||||
| Primary | 0 | |||||||
| Secondary | −2.01 | −5.82 | 1.80 | 0.30 | ||||
| Cause of Infertility | ||||||||
| Male factors | 0 | 0 | ||||||
| Tubaric factors | −4.94 | −13.43 | 3.54 | 0.25 | −6.23 | −14.75 | 2.28 | 0.15 |
| Poor ovarian response | 3.24 | −3.44 | 9.93 | 0.34 | 1.50 | −5.24 | 8.25 | 0.66 |
| Polycystic OvarianSyndrome | 4.49 | −1.95 | 10.93 | 0.17 | 3.87 | −3.37 | 11.10 | 0.29 |
| Endometriosis | 10.21 | 2.06 | 18.36 | 0.01 | 8.93 | 0.73 | 17.14 | 0.03 |
| Idiopathic | 5.18 | -0.95 | 11.31 | 0.10 | 3.24 | -2.98 | 9.45 | 0.31 |
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Paffoni, A.; Ferrari, S.; Vigano, P.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Tirelli, A.; Fedele, L.; Somigliana, E. Vitamin D deficiency and infertility: Insights from in vitro fertilization cycles. J. Clin. Endocrinol. Metab. 2014, 99, E2372–E2376. [Google Scholar] [CrossRef] [PubMed]
- Vanni, V.S.; Vigano, P.; Somigliana, E.; Papaleo, E.; Paffoni, A.; Pagliardini, L.; Candiani, M. Vitamin D and assisted reproduction technologies: Current concepts. Reprod. Biol. Endocrinol. 2014, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Rudick, B.; Ingles, S.; Chung, K.; Stanczyk, F.; Paulson, R.; Bendikson, K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum. Reprod. 2012, 27, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- Rudick, B.J.; Ingles, S.A.; Chung, K.; Stanczyk, F.Z.; Paulson, R.J.; Bendikson, K.A. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil. Steril. 2014, 101, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Viganò, P.; Lattuada, D.; Mangioni, S.; Ermellino, L.; Vignali, M.; Caporizzo, E.; Panina-Bordignon, P.; Besozzi, M.; di Blasio, A.M. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J. Mol. Endocrinol. 2006, 36, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, N.P.; Anckaert, E.; Guzman, L.; Schiettecatte, J.; van Landuyt, L.; Camus, M.; Smitz, J.; Tournaye, H. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum. Reprod. 2014, 29, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Molinaro, T.A.; Dubell, E.K.; Scott, K.L.; Ruiz, A.R.; Forman, E.J.; Werner, M.D.; Hong, K.H.; Scott, R.T., Jr. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am. J. Obstet. Gynecol. 2014, 212, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Aleyasin, A.; Hosseini, M.A.; Mahdavi, A.; Safdarian, L.; Fallahi, P.; Mohajeri, M.R.; Abbasi, M.; Esfahani, F. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, R.D.; Rahmani, E.; Rahsepar, M.; Firouzabadi, M.M. Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch. Gynecol. Obstet. 2014, 289, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Farzadi, L.; Khayatzadeh Bidgoli, H.; Ghojazadeh, M.; Bahrami, Z.; Fattahi, A.; Latifi, Z.; Shahnazi, V.; Nouri, M. Correlation between follicular fluid 25-OH vitamin D and assisted reproductive outcomes. Iran. J. Reprod. Med. 2015, 13, 361–366. [Google Scholar] [PubMed]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P.; Zosky, G.R.; Whitehouse, A.J.; Zhu, K.; Allen, K.L.; Kusel, M.M.; Anderson, D.; Mountain, J.A. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2015, 135, e167–e173. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D and pregnancy: Skeletal effects, nonskeletal effects, and birth outcomes. Calcif. Tissue Int. 2013, 92, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements and supplementation during pregnancy. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; van der Poel, S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum. Reprod. 2009, 24, 2683–2687. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; de Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Ferraretti, A.P.; la Marca, A.; Fauser, B.C.; Tarlatzis, B.; Nargund, G.; Gianaroli, L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar]
- World Health Organisation. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- ARPA Lombardia. Available online: http://ita.arpalombardia.it/ITA/index.asp (accessed on 27 November 2015).
- Carlberg, M.; Nejaty, J.; Froysa, B.; Guan, Y.M.; Soder, O.; Bergqvist, A. Elevated expression of tumour necrosis factor alpha in cultured granulosa cells from women with endometriosis. Hum. Reprod. 2000, 15, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Vigano, P.; Quattrone, F.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Panina-Bordignon, P. The WNT/beta-catenin signaling pathway and expression of survival promoting genes in luteinized granulosa cells: Endometriosis as a paradigm for a dysregulated apoptosis pathway. Fertil. Steril. 2014, 101, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Grzechocinska, B.; Dabrowski, F.A.; Cyganek, A.; Wielgos, M. The role of vitamin D in impaired fertility treatment. Neuro Endocrinol. Lett. 2013, 34, 756–762. [Google Scholar] [PubMed]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, V.; Modoni, S.; Pileri, M.; di Giorgio, A.; Chiodini, I.; Minisola, S.; Vieth, R.; Scillitani, A. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: Seasonal and gender differences. Osteoporos. Int. 2001, 12, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Disanto, G.; Chaplin, G.; Morahan, J.M.; Giovannoni, G.; Hypponen, E.; Ebers, G.C.; Ramagopalan, S.V. Month of birth, vitamin D and risk of immune-mediated disease: A case control study. BMC Med. 2012, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Torrey, E.F.; Miller, J.; Rawlings, R.; Yolken, R.H. Seasonal birth patterns of neurological disorders. Neuroepidemiol. 2000, 19, 177–185. [Google Scholar] [CrossRef]
- Eurostat. Seasonality in Tourism Demand. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Seasonality_in_tourism_demand#Residents_oo_Greece_and_Italy_spent_more_than_half_of_their_tourism_nights_in_July_and_August (accessed on 27 November 2015).
- Gill, T.K.; Hill, C.L.; Shanahan, E.M.; Taylor, A.W.; Appleton, S.L.; Grant, J.F.; Shi, Z.; dal Grande, E.; Price, K.; Adams, R.J. Vitamin D levels in an Australian population. BMC Public Health 2014, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Pittaway, J.K.; Ahuja, K.D.; Beckett, J.M.; Bird, M.L.; Robertson, I.K.; Ball, M.J. Make vitamin D while the sun shines, take supplements when it doesn’t: A longitudinal, observational study of older adults in Tasmania, Australia. PLoS ONE 2013, 8, e59063. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.; Campbell, P.P.; Reinhardt, T.; Gilsanz, V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J. Clin. Endocrinol. Metab. 2009, 94, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Hatun, S.; Islam, O.; Cizmecioglu, F.; Kara, B.; Babaoglu, K.; Berk, F.; Gokalp, A.S. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J. Nutr. 2005, 135, 218–222. [Google Scholar] [PubMed]
- Wagner, C.L.; Greer, F.R. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008, 122, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Panina-Bordignon, P.; Murone, S.; di Lucia, P.; Vercellini, P.; Vigano, P. Vitamin D reserve is higher in women with endometriosis. Hum. Reprod. 2007, 22, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Vigano, P.; Somigliana, E.; Panina, P.; Rabellotti, E.; Vercellini, P.; Candiani, M. Principles of phenomics in endometriosis. Hum. Reprod. Update 2012, 18, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; MacLaughlin, J.A.; Doppelt, S.H. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science 1981, 211, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982, 1, 74–76. [Google Scholar] [CrossRef]
- Grant, W.B. On the roles of skin type and sun exposure in the risk of endometriosis and melanoma. Int. J. Epidemiol. 2011, 40, 513–514. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliardini, L.; Vigano’, P.; Molgora, M.; Persico, P.; Salonia, A.; Vailati, S.H.; Paffoni, A.; Somigliana, E.; Papaleo, E.; Candiani, M. High Prevalence of Vitamin D Deficiency in Infertile Women Referring for Assisted Reproduction. Nutrients 2015, 7, 9972-9984. https://doi.org/10.3390/nu7125516
Pagliardini L, Vigano’ P, Molgora M, Persico P, Salonia A, Vailati SH, Paffoni A, Somigliana E, Papaleo E, Candiani M. High Prevalence of Vitamin D Deficiency in Infertile Women Referring for Assisted Reproduction. Nutrients. 2015; 7(12):9972-9984. https://doi.org/10.3390/nu7125516
Chicago/Turabian StylePagliardini, Luca, Paola Vigano’, Michela Molgora, Paola Persico, Andrea Salonia, Simona Helda Vailati, Alessio Paffoni, Edgardo Somigliana, Enrico Papaleo, and Massimo Candiani. 2015. "High Prevalence of Vitamin D Deficiency in Infertile Women Referring for Assisted Reproduction" Nutrients 7, no. 12: 9972-9984. https://doi.org/10.3390/nu7125516
APA StylePagliardini, L., Vigano’, P., Molgora, M., Persico, P., Salonia, A., Vailati, S. H., Paffoni, A., Somigliana, E., Papaleo, E., & Candiani, M. (2015). High Prevalence of Vitamin D Deficiency in Infertile Women Referring for Assisted Reproduction. Nutrients, 7(12), 9972-9984. https://doi.org/10.3390/nu7125516






