Tomato (Lycopersicon esculentum) Supplementation Induces Changes in Cardiac miRNA Expression, Reduces Oxidative Stress and Left Ventricular Mass, and Improves Diastolic Function
Abstract
:1. Introduction
2. Experimental Section
2.1. Diet Preparation
2.2. Echocardiographic Analysis
2.3. Morphometric Analysis
2.4. Myocardial Lipid Hydroperoxide and Antioxidant Enzyme Analysis
2.5. Nrf-2, Type I and III Collagen Analysis
2.6. Left Ventricular miRNA Expression
2.7. Statistical Analysis
3. Results
| Variable | Control Group (n = 16) | Tomato Group (n = 16) | p Value |
|---|---|---|---|
| BW (g) | 434 (419–444) | 456 (422–486) | 0.090 |
| LVDD (mm) | 7.87 ± 0.43 | 7.72 ± 0.38 | 0.305 |
| LA (mm) | 5.31 ± 0.30 | 5.05 ± 0.33 | 0.029 |
| LVDD/BW (mm/kg) | 18.2 ± 1.27 | 17.3 ± 1.9 | 0.154 |
| LA/BW (mm/kg) | 12.3 ± 0.84 | 11.3 ± 1.19 | 0.016 |
| PWT (mm) | 1.31 ± 0.07 | 1.31 ± 0.07 | 0.879 |
| RWT | 0.34 ± 0.02 | 0.34 ± 0.02 | 0.375 |
| HR (bpm) | 259 ± 25.0 | 265 ± 26.2 | 0.454 |
| FS (%) | 50.0 ± 4.71 | 47.9 ± 3.32 | 0.148 |
| PWSV (mm/s) | 36.9 ± 4.21 | 37.5 ± 4.48 | 0.730 |
| E wave (ms) | 77.1 ± 8.60 | 73.9 ± 5.00 | 0.219 |
| A wave (ms) | 48.4 ± 4.86 | 44.3 ± 6.72 | 0.071 |
| E/A | 1.61 ± 0.19 | 1.70 ± 0.25 | 0.243 |
| EDT (ms) | 46.9 ± 8.29 | 45.7 ± 8.13 | 0.685 |
| IRT/RR0.5 (ms) | 54.2 ± 5.91 | 48.8 ± 7.52 | 0.030 |
| Variable | Control Group (n = 15) | Tomato Group (n = 8) | p Value |
|---|---|---|---|
| RV (g) | 0.25 (0.21–0.31) | 0.17 (0.14–0.18) | <0.001 |
| RV/BW | 0.06 (0.05–0.07) | 0.04 (0.03–0.04) | <0.001 |
| LV (g) | 0.96 (0.81–1.08) | 0.90 (0.84–0.96) | 0.540 |
| LV/BW | 0.22 (0.19–0.25) | 0.20 (0.18–0.20) | 0.129 |
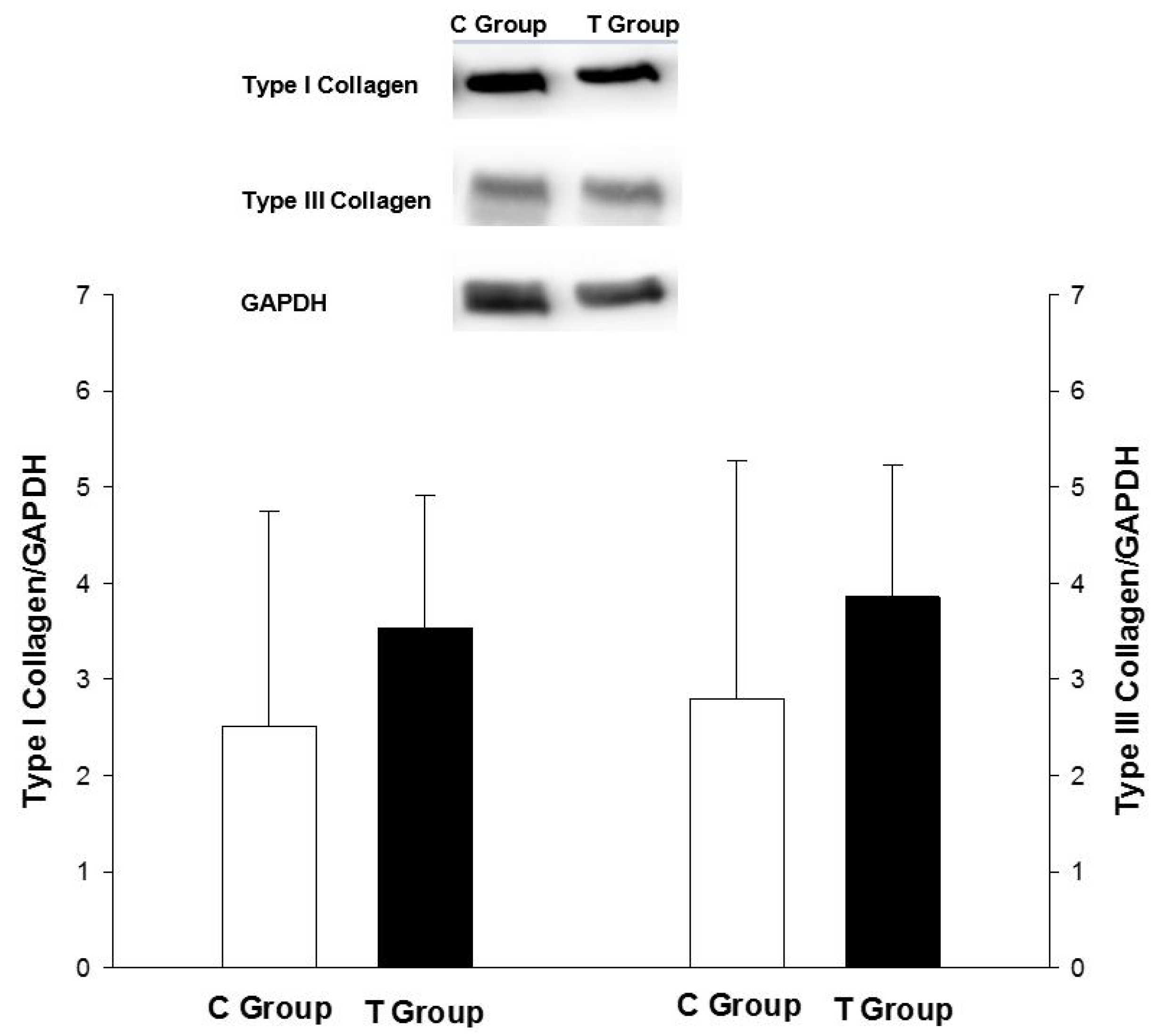
| Variable | Control Group (n = 6) | Tomato Group (n = 8) | p Value |
|---|---|---|---|
| LH (nmol/g) | 267 ± 46.7 | 219 ± 23.0 | 0.039 |
| CAT (µmol/g) | 72.8 ± 7.14 | 95.3 ± 18.3 | 0.015 |
| SOD (nmol/g) | 16.2 ± 2.19 | 12.8 ± 1.10 | 0.002 |
| GSH-Px (nmol/g) | 38.5 ± 6.25 | 91.3 ± 6.36 | <0.001 |
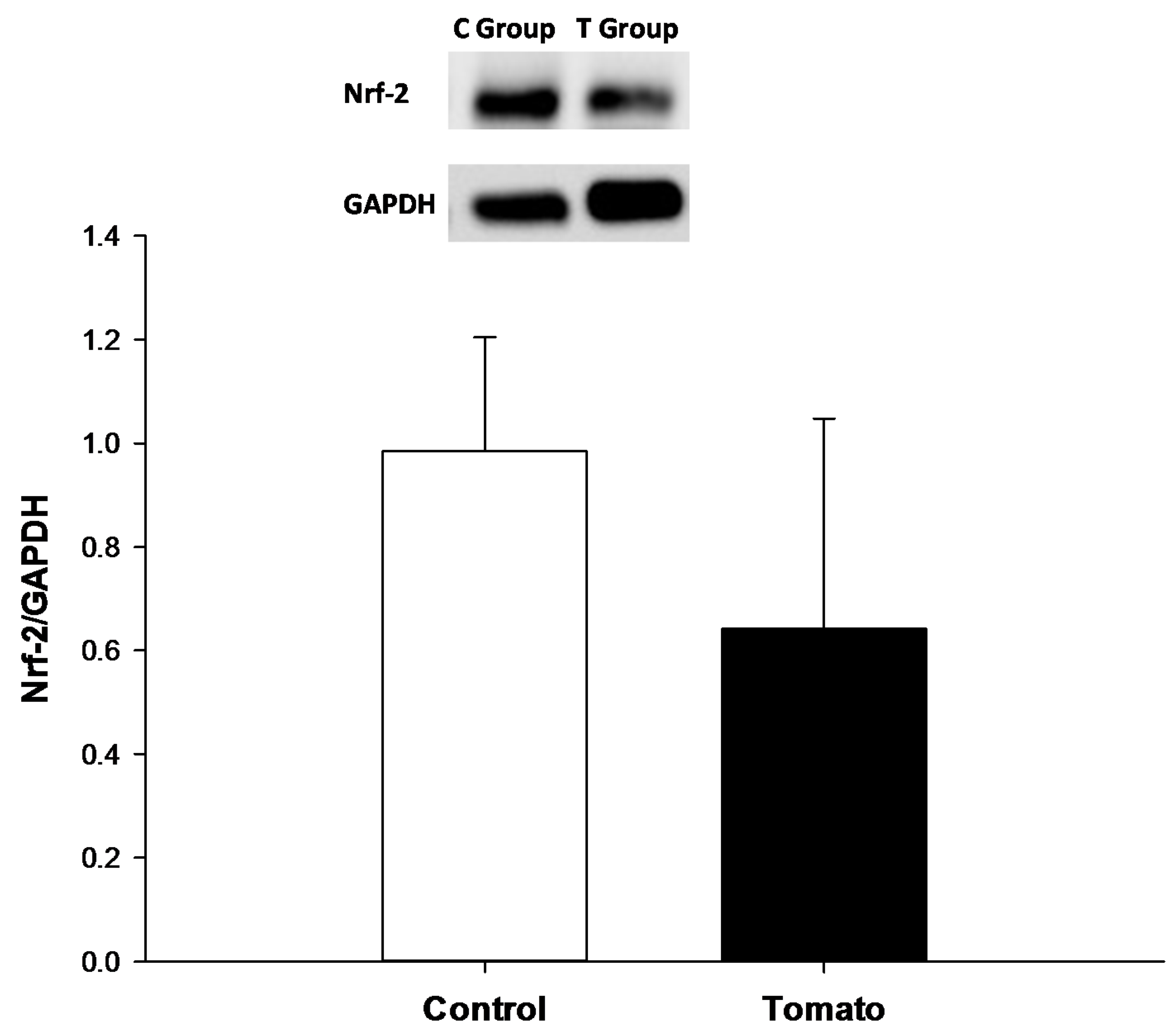
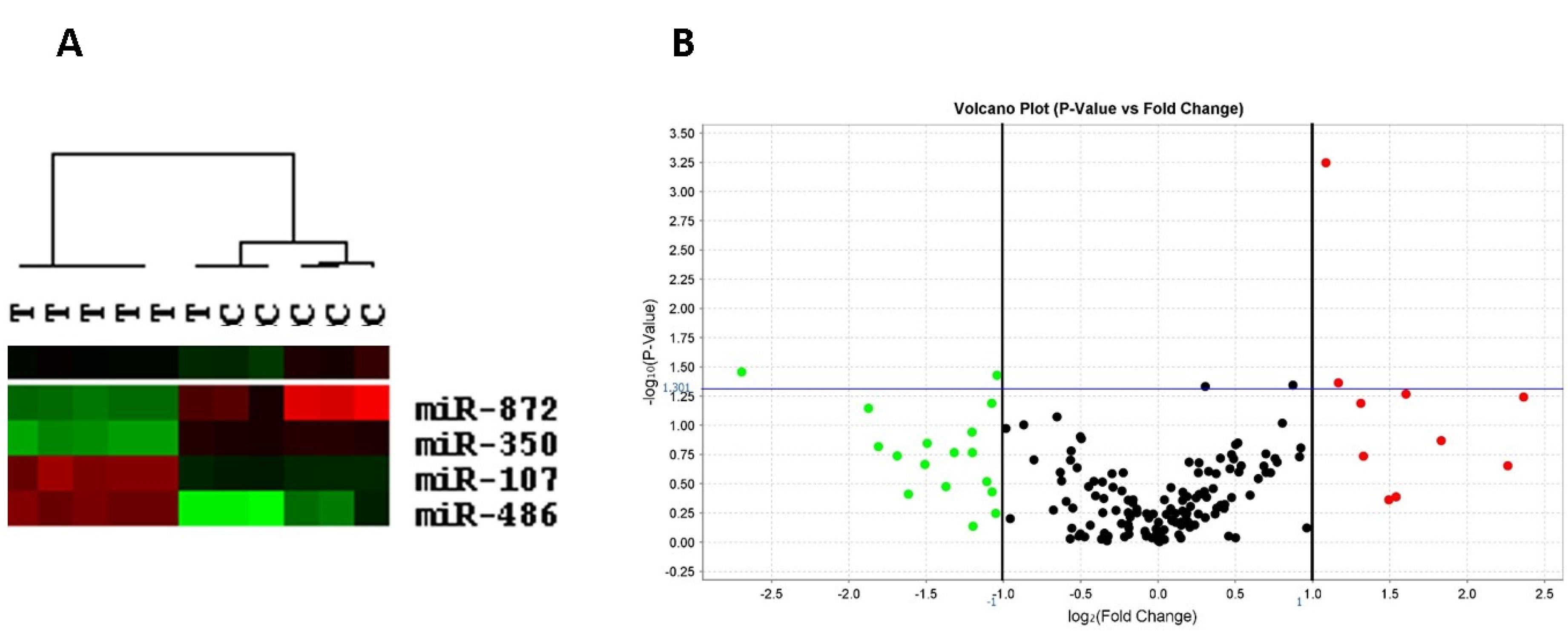
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- World Health Organization. Cardiovascular Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 14 June 2015).
- Burton-Freeman, B.M.; Sesso, H.D. Whole food versus supplement: Comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv. Nutr. 2014, 5, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Steck, S. Lycopene and cardiovascular disease. Am. J. Clin. Nutr. 2000, 71, 1691S–1695S. [Google Scholar] [PubMed]
- Riso, P.; Visioli, F.; Grande, S.; Guarnieri, S.; Gardana, C.; Simonetti, P.; Porrini, M. Effects of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J. Agric. Food Chem. 2006, 54, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.M.; Lai, C.H.; Chang, C.Y.; Fan, C.T.; Chen, C.T.; Wu, C.H. Characterizing the lipid-lowering effects and antioxidant mechanism of tomato paste. Biosci. Biotechnol. Biochem. 2008, 72, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Böhm, V. Lycopene and heart health. Mol. Nutr. Food Res. 2012, 56, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [PubMed]
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Xaplanteris, P.; Pietri, P.; Terentes-Printzios, D.; Kardara, D.; Alexopoulos, N.; Aznaouridis, K.; Miliou, A.; Stefanadis, C. Tomato paste supplementation improves endotelial dynamics and reduces plasma total oxidative status in healthy subjects. Nutr. Res. 2012, 32, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; Salvadori, D.M.; Nascimento, M.C.; Rocha, N.S.; Correa, C.R.; Pereira, E.J.; Matsubara, L.S.; Matsubara, B.B.; Ladeira, M.S. Tomato-oleoresin supplement prevents doxorubicin-induced cardiac myocyte oxidative DNA damage in rats. Mutat. Res. 2007, 631, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mohamadin, A.M.; Elberry, A.A.; Mariee, A.D.; Morsy, G.M.; al-Abbasi, F.A. Lycopene attenuates oxidative stress and heart lysosomal damage in isoproterenol induced cardiotoxicity in rats: A biochemical study. Pathophysiology 2012, 19, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Upaganlawar, A.; Patel, V.; Balaraman, R. Tomato lycopene attenuates myocardil infarction induced by isoproterenol: Electrocardiographic, biochemical and anti-apoptotic study. Asian Pac. J. Trop. Biomed. 2012, 2, 345–351. [Google Scholar] [CrossRef]
- Luvizotto, R.; Nascimento, A.; Miranda, N.; Wang, X.D.; Ferreira, A. Lycopene-rich tomato oleoresin modulates plasma adiponectin concentration and mRNA levels of adiponectin, SIRT1, and FoxO1 in adipose tissue of obese rats. Hum. Exp. Toxicol. 2015, 34, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Romaine, S.P.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Kumar, S.; Shreesh, G.; Ojha, K.; Nandave, M.; Mittal, R.; Kumari, S.; Arya, D.S. Cardioprotective effect of lycopene in the experimental model of myocardial ischemia-reperfusion injury. Mol. Cell. Biochem. 2006, 289, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.; Sùren, T. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000, 154, 201–210. [Google Scholar] [CrossRef]
- Riso, P.; Porrini, M. Determination of carotenoids in vegetable foods and plasma. Int. J. Vitam. Nutr. Res. 1997, 67, 47–54. [Google Scholar] [PubMed]
- Yeum, K.J.; Booth, S.L.; Sadowski, J.A.; Liu, C.; Tang, G.; Krinsky, N.I.; Russell, R.M. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am. J. Clin. Nutr. 1996, 64, 594–602. [Google Scholar] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Minicucci, M.F.; Azevedo, P.S.; Oliveira, S.A., Jr.; Martinez, P.F.; Chiuso-Minicucci, F.; Polegato, B.F.; Justulin, L.A.; Matsubara, L.S.; Matsubara, B.B.; Paiva, S.A.; et al. Tissue vitamin A insufficiency results in adverse ventricular remodeling after experimental myocardial infarction. Cell Physiol. Biochem. 2010, 26, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Burneiko, R.C.M.; Diniz, Y.S.; Galhardi, C.M.; Rodrigues, H.G.; Ebaid, G.M.X.; Faine, L.A.; Padovani, C.R.; Cicogna, A.C.; Novelli, E.L. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem. Toxicol. 2006, 44, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, W.; Hosoda, S.; Hayashi, K. Purification and properties of rat liver glutathione peroxidase. Biochem. Biophys. Acta 1974, 358, 251–261. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzimatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Assalin, H.B.; Rafacho, B.P.; dos Santos, P.P.; Ardisson, L.P.; Roscani, M.G.; Chiuso-Minicucci, F.; Barbisan, L.F.; Fernandes, A.A.; Azevedo, P.S.; Minicucci, M.F.; et al. Impact of the length of vitamin D deficiency on cardiac remodeling. Circ. Heart Fail. 2013, 6, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Cervigne, N.K.; Reis, P.P.; Machado, J.; Sadikovic, B.; Bradley, G.; Galloni, N.N.; Pintilie, M.; Jurisica, I.; Perez-Ordonez, B.; Gilbert, R.; et al. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum. Mol. Genet. 2009, 18, 4818–4829. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef] [PubMed]
- Turakhia, M.P.; Schiller, N.B.; Whooley, M.A. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am. J. Cardiol. 2008, 102, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Mosquera, A.; Broullón, F.J.; Álvarez-García, N.; Peteiro, J.; Mosquera, V.X.; Castro-Beiras, A. Association of left ventricular mass with all-cause mortality, myocardial infarction and stroke. PLoS ONE 2012, 7, e45570. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Rafacho, B.P.; Azevedo, P.S.; Polegato, B.F.; Fernandes, A.A.; Bertoline, M.A.; Fernandes, D.C.; Chiuso-Minicucci, F.; Roscani, M.G.; dos Santos, P.P.; Matsubara, L.S.; et al. Tobacco smoke induces ventricular remodeling associated with an increase in NADPH oxidase activity. Cell Physiol. Biochem. 2011, 27, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Jung, C.H.; Ha, T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol. Nutr. Food Res. 2012, 56, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Topkara, V.K.; Mann, D.L. Role of microRNAs in cardiac remodeling and heart failure. Cardiovac. Drugs Ther. 2011, 25, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Pan, S.; Guan, D.; Yin, H.; Fan, Y.; Liu, J.; Zhang, S.; Zhang, H.; Feng, L.; Wang, Y.; et al. MicroRNA-350 induces pathological heart hypertrophy by repressing both p38 and JNK pathways. Biochim. Biophys. Acta 2013, 1832, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Myostatin signaling regulates Akt activity via the regulation of miR-486 expression. Int. J. Biochem. Cell Biol. 2014, 47, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; de Keyzer, D.; Holvoet, P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011, 25, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.T.; Wang, L.S.; Zhou, Q.; Li, Y.; Shen, C.; Zhang, X.; Wang, C. MicroRNA 107 partly inhibits endothelial progenitor cells differentiation via HIF-1β. PLoS ONE 2012, 7, e40323. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, B.L.B.; Arruda, F.C.O.; Reis, P.P.; Felix, T.F.; Santos, P.P.; Rafacho, B.P.; Gonçalves, A.F.; Claro, R.T.; Azevedo, P.S.; Polegato, B.F.; et al. Tomato (Lycopersicon esculentum) Supplementation Induces Changes in Cardiac miRNA Expression, Reduces Oxidative Stress and Left Ventricular Mass, and Improves Diastolic Function. Nutrients 2015, 7, 9640-9649. https://doi.org/10.3390/nu7115493
Pereira BLB, Arruda FCO, Reis PP, Felix TF, Santos PP, Rafacho BP, Gonçalves AF, Claro RT, Azevedo PS, Polegato BF, et al. Tomato (Lycopersicon esculentum) Supplementation Induces Changes in Cardiac miRNA Expression, Reduces Oxidative Stress and Left Ventricular Mass, and Improves Diastolic Function. Nutrients. 2015; 7(11):9640-9649. https://doi.org/10.3390/nu7115493
Chicago/Turabian StylePereira, Bruna L. B., Fernanda C. O. Arruda, Patrícia P. Reis, Tainara F. Felix, Priscila P. Santos, Bruna P. Rafacho, Andrea F. Gonçalves, Renan T. Claro, Paula S. Azevedo, Bertha F. Polegato, and et al. 2015. "Tomato (Lycopersicon esculentum) Supplementation Induces Changes in Cardiac miRNA Expression, Reduces Oxidative Stress and Left Ventricular Mass, and Improves Diastolic Function" Nutrients 7, no. 11: 9640-9649. https://doi.org/10.3390/nu7115493
APA StylePereira, B. L. B., Arruda, F. C. O., Reis, P. P., Felix, T. F., Santos, P. P., Rafacho, B. P., Gonçalves, A. F., Claro, R. T., Azevedo, P. S., Polegato, B. F., Okoshi, K., Fernandes, A. A. H., Paiva, S. A. R., Zornoff, L. A. M., & Minicucci, M. F. (2015). Tomato (Lycopersicon esculentum) Supplementation Induces Changes in Cardiac miRNA Expression, Reduces Oxidative Stress and Left Ventricular Mass, and Improves Diastolic Function. Nutrients, 7(11), 9640-9649. https://doi.org/10.3390/nu7115493




