Nutritionally-Induced Catch-Up Growth
Abstract
:1. Introduction

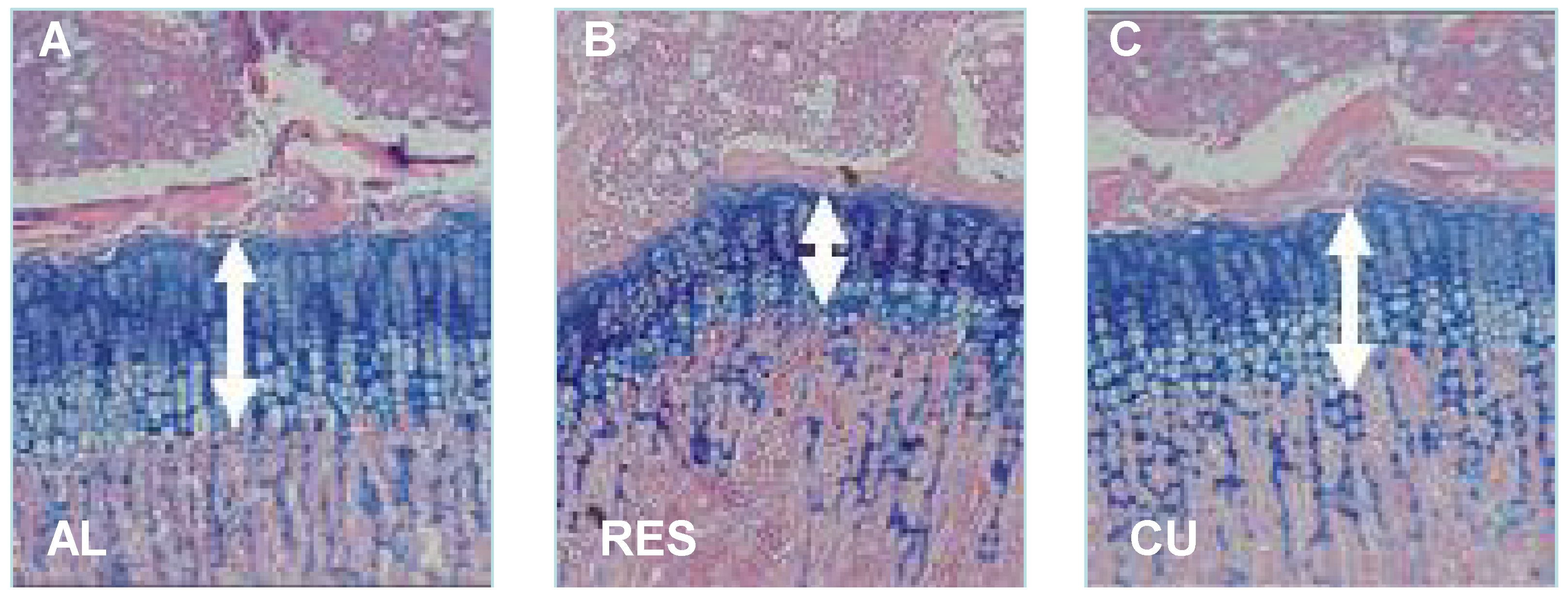
2. Systemic Factors in Malnutrition and CU Growth
2.1. Insulin
| Hormone | Affected by food restriction | Effect on growth |
|---|---|---|
| Insulin | Reduced | Stimulates growth |
| Growth Hormone | Reduced (rats and mice)/increased (humans, rabbits, sheep, cows and pigs) | Stimulates growth |
| Insulin like growth factor 1 | Reduced | Stimulates growth |
| IGFBP-1 | Increased | Inhibits growth |
| Leptin | Reduced | Stimulates growth |
| Glucocorticoids | Increased | Inhibits growth |
| Thyroid hormones | Reduced | Stimulates growth |
| FGF21 | Reduced/increased | Inhibits growth |
| Vitamin D | Reduced | Required for proper growth, inhibits chondrocyte proliferation at high concentrations |
| Sex hormones * | Reduced | Stimulates growth (testosterone), hastens EGP closure (estrogen) * |
2.2. Growth Hormone and Insulin-Like Growth Factor-1
2.3. Leptin
2.4. Glucocorticoids
2.5. Thyroid Hormone
2.6. FGF-21
2.7. Vitamin D
3. Local Molecular Mechanisms in Malnutrition and CU Growth
3.1. microRNAs
3.2. HIF1α
3.3. Autophagy (Compound Recycling)
3.4. Mammalian Target of Rapamycin (mTOR)

3.5. Epigenetics
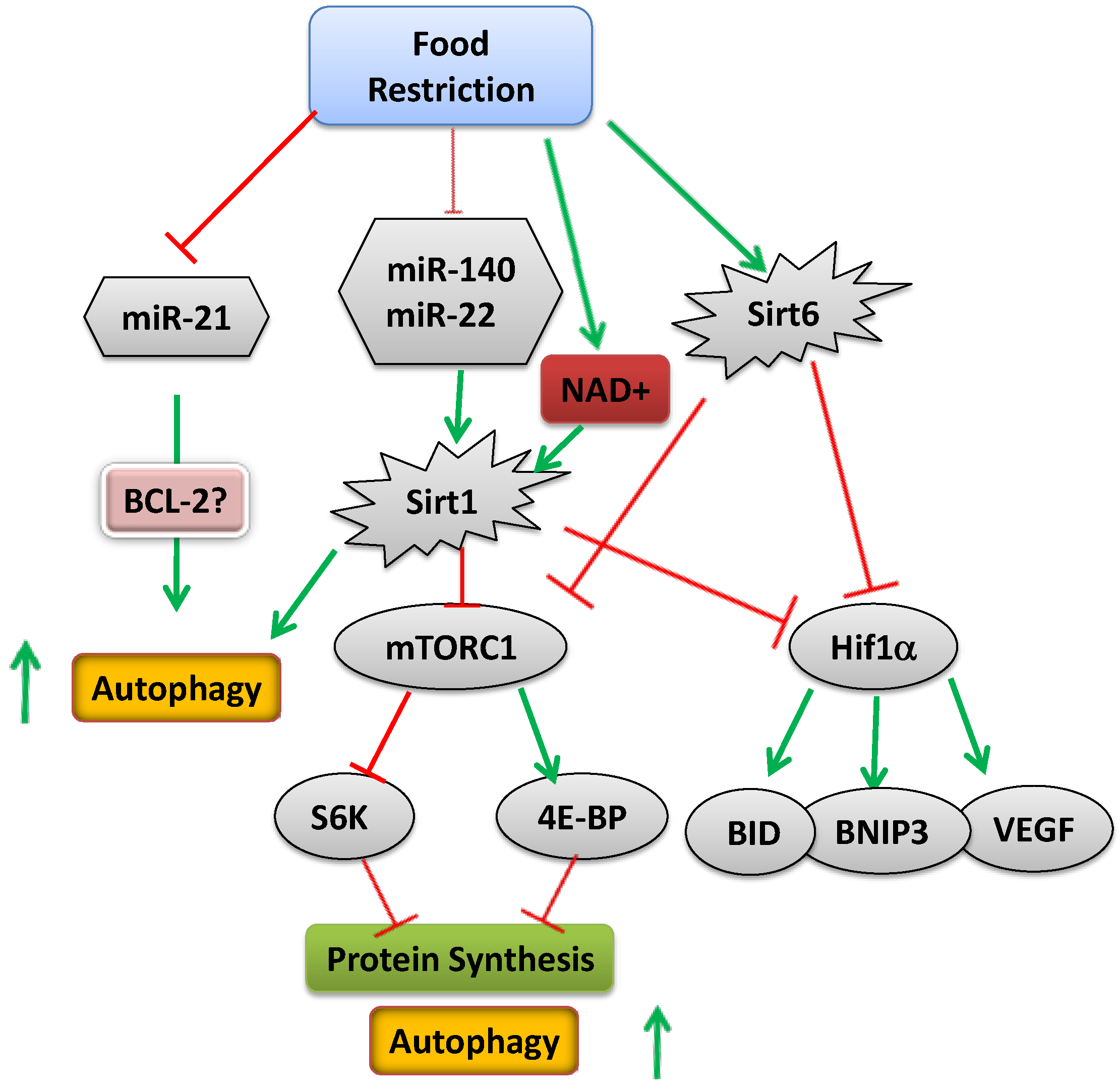
4. Conclusions
Acknowledgments
Author Contributions
Abbreviations Used in this Review (in Alphabetical Order)
| ATG | autophagy-related genes |
| BMP | bone morphogenetic protein |
| CU | catch-up |
| ECM | extracellular matrix |
| EGP | epiphyseal growth plate |
| FGF | fibroblast growth factor |
| GH | growth hormone |
| GHR | growth hormone receptor |
| HAT | histone acetyl transferase |
| HDAC | histone deacetylase |
| HIF | hypoxia inducible factor |
| IGF-1 | insulin-like growth factor 1 |
| IGF-1R | insulin like growth factor 1 receptor |
| IGFBP | insulin-like growth factor binding protein |
| Ihh | Indian hedgehog |
| IUGR | intrauterine growth retardation |
| LC3 | microtubule-associated protein light chain 3 |
| lncRNA | long non-coding RNA |
| miRNA | microRNA |
| mTOR | mammalian target of rapamycin |
| ODDD | oxygen-dependent degradation domain |
| PThrP | parathyroid hormone-related peptide |
| SGA | small for gestational age |
| UTR | untranslated region |
Conflicts of Interest
References
- Joint UNICEF–WHO–The World Bank Child Malnutrition Database: Estimates for 2012 and Launch of Interactive Data Dashboards. Available online: http://www.who.int/nutgrowthdb/jme_2012_summary_note_v2.pdf (accessed on 17 September 2014).
- Maeda, Y.; Nakamura, E.; Nguyen, M.T.; Suva, L.J.; Swain, F.L.; Razzaque, M.S.; Mackem, S.; Lanske, B. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc. Natl. Acad. Sci. USA 2007, 104, 6382–6387. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Tatarczuch, L.; Mirams, M. The skeleton: A multi-functional complex organ: The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011, 211, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Wong, K.L.; Fang, M.; Schwartz, Z. 1α,25(OH)2D3 is an autocrine regulator of extracellular matrix turnover and growth factor release via ERp60 activated matrix vesicle metalloproteinases. J. Steroid Biochem. Mol. Biol. 2007, 103, 467–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heinrichs, C.; Colli, M.; Yanovski, J.A.; Laue, L.; Gerstl, N.A.; Kramer, A.D.; Uyeda, J.A.; Baron, J. Effects of fasting on the growth plate: Systemic and local mechanisms. Endocrinology 1997, 138, 5359–5365. [Google Scholar] [PubMed]
- Farnum, C.E.; Lee, A.O.; O’Hara, K.; Wilsman, N.J. Effect of short-term fasting on bone elongation rates: An analysis of catch-up growth in young male rats. Pediatr. Res. 2003, 53, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Even-Zohar, N.; Jacob, J.; Amariglio, N.; Rechavi, G.; Potievsky, O.; Phillip, M.; Gat-Yablonski, G. Nutrition-induced catch-up growth increases hypoxia inducible factor 1α RNA levels in the growth plate. Bone 2008, 42, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Torun, B.; Chew, F. Protein energy malnutrition. In Modern Nutrition in Health and Disease, 8th ed.; Shils, M.E., Olson, J., Shike, M., Ross, C., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994; pp. 950–966. [Google Scholar]
- Soliman, A.T.; ElZalabany, M.M.; Salama, M.; Ansari, B.M. Serum leptin concentrations during severe protein-energy malnutrition: Correlation with growth parameters and endocrine function. Metabolism 2000, 49, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.; Taskin, E.; Ustundag, B.; Aygun, A.D. The evaluation of serum leptin level and other hormonal parameters in children with severe malnutrition. Clin. Biochem. 2004, 37, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Z.; Sinai, T.; Zung, A.; Reifen, R. Effect of nutrition on growth in short stature before and during growth-hormone therapy. Pediatrics 2005, 116, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Favaro, A.; Tenconi, E.; Degortes, D.; Soave, M.; Zanetti, T.; Nardi, M.T.; Caregaro, L.; Santonastaso, P. Association between low height and eating disorders: Cause or effect? Int. J. Eat. Disord. 2007, 40, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Boersma, B.; Houwen, R.H.; Blum, W.F.; van Doorn, J.; Wit, J.M. Catch-up growth and endocrine changes in childhood celiac disease. Endocrine changes during catch-up growth. Horm. Res. 2002, 58, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Nilsson, O.; Baron, J. Growth plate senescence and catch-up growth. Endocr. Dev. 2011, 21, 23–29. [Google Scholar] [PubMed]
- Prader, A.; Tanner, J.M.; von, H.G. Catch-up growth following illness or starvation. An example of developmental canalization in man. J. Pediatr. 1963, 62, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Baron, J. Impact of growth plate senescence on catch-up growth and epiphyseal fusion. Pediatr. Nephrol. 2005, 20, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; Klein, K.O.; Colli, M.J.; Yanovski, J.A.; Novosad, J.A.; Bacher, J.D.; Cutler, G.B., Jr. Catch-up growth after glucocorticoid excess: A mechanism intrinsic to the growth plate. Endocrinology 1994, 135, 1367–1371. [Google Scholar] [PubMed]
- Marino, R.; Hegde, A.; Barnes, K.M.; Schrier, L.; Emons, J.A.; Nilsson, O.; Baron, J. Catch-up growth after hypothyroidism is caused by delayed growth plate senescence. Endocrinology 2008, 149, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Gafni, R.I.; Weise, M.; Robrecht, D.T.; Meyers, J.L.; Barnes, K.M.; De-Levi, S.; Baron, J. Catch-up growth is associated with delayed senescence of the growth plate in rabbits. Pediatr. Res. 2001, 50, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; Phillip, M. Leptin and regulation of linear growth. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Pando, R.; Even-Zohar, N.; Shtaif, B.; Edry, L.; Shomron, N.; Phillip, M.; Gat-Yablonski, G. MicroRNAs in the growth plate are responsive to nutritional cues: Association between miR-140 and SIRT1. J. Nutr. Biochem. 2012, 23, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [PubMed]
- Baumeister, F.A.; Engelsberger, I.; Schulze, A. Pancreatic agenesis as cause for neonatal diabetes mellitus. Klin. Padiatr. 2005, 217, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I. Lilly lecture: Molecular mechanisms of insulin resistance. Lessons from patients with mutations in the insulin-receptor gene. Diabetes 1992, 41, 1473–1490. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, M.J.; Wit, J.M. Single gene mutations causing SGA. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Krook, A.; O’Rahilly, S. Mutant insulin receptors in syndromes of insulin resistance. Baillieres Clin. Endocrinol. Metab. 1996, 10, 97–122. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, A.T.; Beards, F.; Ballantyne, E.; Appleton, M.; Harvey, R.; Ellard, S. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat. Genet. 1998, 19, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.; Cohen, P. Disorders of growth hormone/insulin like growth factor secretion and action in pediatric endocrinology. In Pediatric Endocrinology; Sperling, M., Ed.; Saunders: Philadelphia, PA, USA, 2002; pp. 211–288. [Google Scholar]
- Cosmi, E.; Fanelli, T.; Visentin, S.; Trevisanuto, D.; Zanardo, V. Consequences in infants that were intrauterine growth restricted. J. Pregnancy 2011, 2011, 364381. [Google Scholar] [CrossRef] [PubMed]
- Berends, L.M.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Cripps, R.L.; Ozanne, S.E. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int. J. Obes. 2013, 37, 1051–1057. [Google Scholar] [CrossRef]
- Deng, H.Z.; Deng, H.; Su, Z.; Li, Y.H.; Ma, H.M.; Chen, H.S.; Du, M.L. Insulin resistance and adiponectin levels are associated with height catch-up growth in pre-pubertal Chinese individuals born small for gestational age. Nutr. Metab. 2012, 9, 107. [Google Scholar] [CrossRef]
- Jou, M.Y.; Lonnerdal, B.; Griffin, I.J. Effects of early postnatal growth restriction and subsequent catch-up growth on body composition, insulin sensitivity, and behavior in neonatal rats. Pediatr. Res. 2013, 73, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Wagner, J.; Zapf, J. Differential effects of insulin-like growth factor I and growth hormone on developmental stages of rat growth plate chondrocytes in vivo. J. Clin. Investig. 1994, 93, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, J.; Grossman, D.I.; Peng, R.K.; Famula, T.R.; Oberbauer, A.M. Spatial distribution of growth hormone receptor, insulin-like growth factor-I receptor and apoptotic chondrocytes during growth plate development. J. Endocrinol. 2005, 184, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [PubMed]
- Woods, K.A.; Camacho-Hubner, C.; Savage, M.O.; Clark, A.J. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N. Engl. J. Med. 1996, 335, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Abuzzahab, M.J.; Schneider, A.; Goddard, A.; Grigorescu, F.; Lautier, C.; Keller, E.; Kiess, W.; Klammt, J.; Kratzsch, J.; Osgood, D.; et al. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N. Engl. J. Med. 2003, 349, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Raile, K.; Klammt, J.; Schneider, A.; Keller, A.; Laue, S.; Smith, R.; Pfaffle, R.; Kratzsch, J.; Keller, E.; Kiess, W. Clinical and functional characteristics of the human Arg59Ter insulin-like growth factor I receptor (IGF1R) mutation: Implications for a gene dosage effect of the human IGF1R. J. Clin. Endocrinol. Metab. 2006, 91, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Tiulpakov, A.; Rubtsov, P.; Sverdlova, P.; Peterkova, V.; Yakar, S.; Terekhov, S.; LeRoith, D. A familial insulin-like growth factor-I receptor mutant leads to short stature: Clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 2007, 92, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Peoples, R.; Milatovich, A.; Francke, U. Hemizygosity at the insulin-like growth factor I receptor (IGF1R) locus and growth failure in the ring chromosome 15 syndrome. Cytogenet. Cell Genet. 1995, 70, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, M.J.; de Muinck Keizer-Schrama, S.M.; de Mos, M.; Kalf, M.E.; van Duyvenvoorde, H.A.; Boot, A.M.; Kant, S.G.; White, S.J.; Losekoot, M.; Den Dunnen, J.T.; et al. Successful long-term growth hormone therapy in a girl with haploinsufficiency of the insulin-like growth factor-I receptor due to a terminal 15q26.2->qter deletion detected by multiplex ligation probe amplification. J. Clin. Endocrinol. Metab. 2008, 93, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Van Duyvenvoorde, H.A.; van Doorn, J.; Koenig, J.; Gauguin, L.; Oostdijk, W.; Wade, J.D.; Karperien, M.; Ruivenkamp, C.A.; Losekoot, M.; van Setten, P.A.; et al. The severe short stature in two siblings with a heterozygous IGF1 mutation is not caused by a dominant negative effect of the putative truncated protein. Growth Horm. IGF Res. 2011, 21, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, J.S.; Derr, M.; Rosenfeld, R.G.; Hwa, V. Identification of a novel heterozygous IGF1 splicing mutation in a large kindred with familial short stature. Horm. Res. Paediatr. 2012, 78, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mosier, H.D., Jr.; Jansons, R.A. Growth hormone during catch-up growth and failure of catch-up growth in rats. Endocrinology 1976, 98, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hermanussen, M.; Rol de Lama, M.A.; Romero, A.P.; Ruiz, C.A.; Burmeister, J.; Tresguerres, J.A. Differential catch-up in body weight and bone growth after short-term starvation in rats. Growth Regul. 1996, 6, 230–237. [Google Scholar] [PubMed]
- Gat-Yablonski, G.; Shtaif, B.; Abraham, E.; Phillip, M. Nutrition-induced catch-up growth at the growth plate. J. Pediatr. Endocrinol. Metab. 2008, 21, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Pando, R.; Shtaif, B.; Phillip, M.; Gat-Yablonski, G. A serum component mediates food restriction-induced growth attenuation. Endocrinology 2014, 155, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L., Jr.; Adamo, M.; Werner, H.; Roberts, C.T., Jr.; LeRoith, D. Regulation by fasting of rat insulin-like growth factor I and its receptor. Effects on gene expression and binding. J. Clin. Investig. 1989, 84, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Ketelslegers, J.M.; Underwood, L.E. Low circulating somatomedin-C/insulin-like growth factor I in insulin-dependent diabetes and malnutrition: Growth hormone receptor and post-receptor defects. Acta Endocrinol. Suppl. 1986, 279, 86–92. [Google Scholar]
- Lee, P.D.; Suwanichkul, A.; DePaolis, L.A.; Snuggs, M.B.; Morris, S.L.; Powell, D.R. Insulin-like growth factor (IGF) suppression of IGFBP-1 production: Evidence for mediation by the type I IGF receptor. Regul. Pept. 1993, 48, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, N.; Seurin, D.; le Bouc, Y.; Binoux, M.; Berdal, A.; Menuelle, P.; Babajko, S. Insulin-like growth factor binding protein (IGFBP-1) involvement in intrauterine growth retardation: Study on IGFBP-1 overexpressing transgenic mice. Endocrinology 2006, 147, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, A.M.; Holly, J.M.; Wass, J.A. The regulation of insulin-like growth factor binding protein (IGFBP)-1 during prolonged fasting. Clin. Endocrinol. 1993, 39, 357–362. [Google Scholar] [CrossRef]
- Woodall, S.M.; Breier, B.H.; Johnston, B.M.; Gluckman, P.D. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: Effects on the somatotrophic axis and postnatal growth. J. Endocrinol. 1996, 150, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.S.; Burke, E.J.; Marten, N.W. Induction of insulin-like growth factor binding protein-1 gene expression in liver of protein-restricted rats and in rat hepatoma cells limited for a single amino acid. Endocrinology 1993, 132, 1090–1100. [Google Scholar] [PubMed]
- Takenaka, A.; Komori, K.; Morishita, T.; Takahashi, S.I.; Hidaka, T.; Noguchi, T. Amino acid regulation of gene transcription of rat insulin-like growth factor-binding protein-1. J. Endocrinol. 2000, 164, R11–R16. [Google Scholar] [CrossRef] [PubMed]
- Henning, P.C.; Scofield, D.E.; Rarick, K.R.; Pierce, J.R.; Staab, J.S.; Lieberman, H.R.; Nindl, B.C. Effects of acute caloric restriction compared to caloric balance on the temporal response of the IGF-I system. Metabolism 2013, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Abu Shehab, M.; Iosef, C.; Wildgruber, R.; Sardana, G.; Gupta, M.B. Phosphorylation of IGFBP-1 at discrete sites elicits variable effects on IGF-I receptor autophosphorylation. Endocrinology 2013, 154, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Wilson, E.M.; Rosenfeld, R.G.; Oh, Y. Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J. Biol. Chem. 1997, 272, 30729–30734. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Forcinito, P.; Chang, M.; Chen, W.; Barnes, K.M.; Baron, J. Coordinated postnatal down-regulation of multiple growth-promoting genes: Evidence for a genetic program limiting organ growth. FASEB J. 2010, 24, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Campfield, L.A. Metabolic and hormonal controls of food intake: Highlights of the last 25 years—1972–1997. Appetite 1997, 29, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, N.; Mercer, J.G.; Rayner, D.V.; Moar, K.; Trayhurn, P.; Williams, L.M. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem. Biophys. Res. Commun. 1997, 232, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M., Jr. Role of leptin in regulating appetite, neuroendocrine function, and bone remodeling. Am. J. Med. Genet. 2006, 140, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; Ben-Ari, T.; Shtaif, B.; Potievsky, O.; Moran, O.; Eshet, R.; Maor, G.; Segev, Y.; Phillip, M. Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology 2004, 145, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliezer, M.; Phillip, M.; Gat-Yablonski, G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine 2007, 32, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. Leptin controls bone formation through a hypothalamic relay. Recent Prog. Horm. Res. 2001, 56, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Kalra, S.P.; Wong, C.P.; Philbrick, K.A.; Lindenmaier, L.B.; Boghossian, S.; Iwaniec, U.T. Peripheral leptin regulates bone formation. J. Bone Min. Res. 2013, 28, 22–34. [Google Scholar] [CrossRef]
- Grisaru-Granovsky, S.; Samueloff, A.; Elstein, D. The role of leptin in fetal growth: A short review from conception to delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Apter, D. The role of leptin in female adolescence. Ann. N. Y. Acad. Sci. 2003, 997, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, A.R.; Trueman, J.A.; Whatmore, A.J.; Westwood, M.; Price, D.A.; Hall, C.M.; Clayton, P.E. The relationship between nocturnal urinary leptin and gonadotrophins as children progress towards puberty. Horm. Res. 2007, 68, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Crawford, D.T.; Chidsey-Frink, K.L.; Ke, H.; Swick, A.G. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul. Pept. 2000, 92, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, U.T.; Boghossian, S.; Lapke, P.D.; Turner, R.T.; Kalra, S.P. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides 2007, 28, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Senaris, R.; Considine, R.V.; Casanueva, F.F.; Dieguez, C. Regulation of in vivo growth hormone secretion by leptin. Endocrinology 1997, 138, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Burguera, B.G.; Couce, M.E.; Scheithauer, B.W.; Lamsan, J.; Eberhardt, N.L.; Kulig, E.; Lloyd, R.V. Leptin and leptin receptor expression in normal and neoplastic human pituitary: Evidence of a regulatory role for leptin on pituitary cell proliferation. J. Clin. Endocrinol. Metab. 1999, 84, 2903–2911. [Google Scholar] [PubMed]
- Goldstone, A.P.; Howard, J.K.; Lord, G.M.; Ghatei, M.A.; Gardiner, J.V.; Wang, Z.L.; Wang, R.M.; Girgis, S.I.; Bailey, C.J.; Bloom, S.R. Leptin prevents the fall in plasma osteocalcin during starvation in male mice. Biochem. Biophys. Res. Commun. 2002, 295, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, P.A.; Munno, A.; Gamberoni, M.; Viggiani, R.; de Ambrogi, M.; Tamanini, C.; Seren, E. Role of leptin on growth hormone and prolactin secretion by bovine pituitary explants. J. Dairy Sci. 2007, 90, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Maor, G.; Rochwerger, M.; Segev, Y.; Phillip, M. Leptin acts as a growth factor on the chondrocytes of skeletal growth centers. J. Bone Min. Res. 2002, 17, 1034–1043. [Google Scholar] [CrossRef]
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.M.; Karsenty, G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000, 100, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; Ding, K.H.; Ponnala, S.; Ferrari, S.L.; Isales, C.M. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: Implications for the regulation of bone mass by body weight. J. Bone Min. Res. 2008, 23, 870–878. [Google Scholar] [CrossRef]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Wangensteen, T.; Collins, S.; Kimber, W.; Matarese, G.; Keogh, J.M.; Lank, E.; Bottomley, B.; Lopez-Fernandez, J.; Ferraz-Amaro, I.; et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 2007, 356, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; Shtaif, B.; Phillip, M. Leptin stimulates parathyroid hormone related peptide expression in the endochondral growth plate. J. Pediatr. Endocrinol. Metab. 2007, 20, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; David, V.; Malaval, L.; Lafage-Proust, M.H.; Vico, L.; Thomas, T. Opposite effects of leptin on bone metabolism: A dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology 2007, 148, 3419–3425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devlin, M.J.; Cloutier, A.M.; Thomas, N.A.; Panus, D.A.; Lotinun, S.; Pinz, I.; Baron, R.; Rosen, C.J.; Bouxsein, M.L. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Min. Res. 2010, 25, 2078–2088. [Google Scholar] [CrossRef]
- Bar-El Dadon, S.; Shahar, R.; Katalan, V.; Monsonego-Ornan, E.; Reifen, R. Leptin administration affects growth and skeletal development in a rat intrauterine growth restriction model: Preliminary study. Nutrition 2011, 27, 973–977. [Google Scholar] [CrossRef] [PubMed]
- LaPaglia, N.; Steiner, J.; Kirsteins, L.; Emanuele, M.; Emanuele, N. Leptin alters the response of the growth hormone releasing factor- growth hormone—Insulin-like growth factor-I axis to fasting. J. Endocrinol. 1998, 159, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Underwood, L.E.; Clemmons, D.R.; Maes, M.; D’Ercole, A.J.; Ketelslegers, J.M. Regulation of somatomedin-C/insulin-like growth factor I by nutrients. Horm. Res. 1986, 24, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Kume, K.; Satomura, K.; Nishisho, S.; Kitaoka, E.; Yamanouchi, K.; Tobiume, S.; Nagayama, M. Potential role of leptin in endochondral ossification. J. Histochem. Cytochem. 2002, 50, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Inada, H.; Koike, T.; Yamano, T. Effects of leptin to cultured growth plate chondrocytes. Horm. Res. 2003, 60, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Steendijk, R. Remarkable catch-up growth in a boy with steroid-responsive nephrotic syndrome. Acta Endocrinol. Suppl. 1986, 279, 8–12. [Google Scholar]
- Codoner-Franch, P.; Bernard, O.; Alvarez, F. Long-term follow-up of growth in height after successful liver transplantation. J. Pediatr. 1994, 124, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Lucidarme, N.; Prieur, A.M.; Ruiz, J.C.; Czernichow, P. Linear growth in children suffering from juvenile idiopathic arthritis requiring steroid therapy: Natural history and effects of growth hormone treatment on linear growth. J. Pediatr. Endocrinol. Metab. 2001, 14, 1483–1486. [Google Scholar] [PubMed]
- Pass, C.; MacRae, V.E.; Ahmed, S.F.; Farquharson, C. Inflammatory cytokines and the GH/IGF-I axis: Novel actions on bone growth. Cell Biochem. Funct. 2009, 27, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Gospodarowicz, D. Stimulation by glucocorticoid of the synthesis of cartilage-matrix proteoglycans produced by rabbit costal chondrocytes in vitro. J. Biol. Chem. 1985, 260, 2364–2373. [Google Scholar] [PubMed]
- Schrier, L.; Ferns, S.P.; Barnes, K.M.; Emons, J.A.; Newman, E.I.; Nilsson, O.; Baron, J. Depletion of resting zone chondrocytes during growth plate senescence. J. Endocrinol. 2006, 189, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chagin, A.S.; Karimian, E.; Sundstrom, K.; Eriksson, E.; Savendahl, L. Catch-up growth after dexamethasone withdrawal occurs in cultured postnatal rat metatarsal bones. J. Endocrinol. 2010, 204, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, T.; Farquharson, C.; Seawright, E.; Ahmed, S.F. Glucocorticoid effects on chondrogenesis, differentiation and apoptosis in the murine ATDC5 chondrocyte cell line. J. Endocrinol. 2002, 175, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Jux, C.; Leiber, K.; Hugel, U.; Blum, W.; Ohlsson, C.; Klaus, G.; Mehls, O. Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-I production and expression of GH- and IGF-I-receptor in cultured rat chondrocytes. Endocrinology 1998, 139, 3296–3305. [Google Scholar] [PubMed]
- Robson, H.; Phillip, M.; Wit, J.M. The Second European Growth Plate Working Group Symposium 25th September 2002, Madrid, Spain. J. Pediatr. Endocrinol. Metab. 2003, 16, 461–466. [Google Scholar] [PubMed]
- Luo, J.; Reid, R.E.; Murphy, L.J. Dexamethasone increases hepatic insulin-like growth factor binding protein-1 (IGFBP-1) mRNA and serum IGFBP-1 concentrations in the rat. Endocrinology 1990, 127, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Robson, H.; Siebler, T.; Stevens, D.A.; Shalet, S.M.; Williams, G.R. Thyroid hormone acts directly on growth plate chondrocytes to promote hypertrophic differentiation and inhibit clonal expansion and cell proliferation. Endocrinology 2000, 141, 3887–3897. [Google Scholar] [PubMed]
- Kaneshige, M.; Suzuki, H.; Kaneshige, K.; Cheng, J.; Wimbrow, H.; Barlow, C.; Willingham, M.C.; Cheng, S. A targeted dominant negative mutation of the thyroid hormone α 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 15095–15100. [Google Scholar] [CrossRef] [PubMed]
- Maglich, J.M.; Watson, J.; McMillen, P.J.; Goodwin, B.; Willson, T.M.; Moore, J.T. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J. Biol. Chem. 2004, 279, 19832–19838. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, J.T.; Stacy, C.; Bertrand, H.A. Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech. Ageing Dev. 1990, 53, 9–16. [Google Scholar] [CrossRef] [PubMed]
- DeLany, J.P.; Hansen, B.C.; Bodkin, N.L.; Hannah, J.; Bray, G.A. Long-term calorie restriction reduces energy expenditure in aging monkeys. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, 5–13. [Google Scholar] [CrossRef]
- Roth, G.S.; Handy, A.M.; Mattison, J.A.; Tilmont, E.M.; Ingram, D.K.; Lane, M.A. Effects of dietary caloric restriction and aging on thyroid hormones of rhesus monkeys. Horm. Metab. Res. 2002, 34, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.H.; Swinhoe, R.; Chassande, O.; Samarut, J.; Williams, G.R. Thyroid hormone regulates heparan sulfate proteoglycan expression in the growth plate. Endocrinology 2006, 147, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.C.; Kharitonenkov, A. Hormone-like fibroblast growth factors and metabolic regulation. Biochim. Biophys. Acta 2011, 1812, 791–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dong, J.; Liu, H.; Li, L.; Yang, G. Effects of short-term continuous subcutaneous insulin infusion on fasting plasma fibroblast growth factor-21 levels in patients with newly diagnosed type 2 diabetes mellitus. PLoS One 2011, 6, e26359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Woelfle, J.; Wunsch, R.; Roth, C.L. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Feldstein, A.E.; Santoro, N.; Kim, G.; Kursawe, R.; Pierpont, B.; Caprio, S. Circulating levels of FGF-21 in obese youth: Associations with liver fat content and markers of liver damage. J. Clin. Endocrinol. Metab. 2013, 98, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Lundasen, T.; Andersson, E.M.; Snaith, M.; Lindmark, H.; Lundberg, J.; Ostlund-Lindqvist, A.M.; Angelin, B.; Rudling, M. Inhibition of intestinal bile acid transporter Slc10a2 improves triglyceride metabolism and normalizes elevated plasma glucose levels in mice. PLoS One 2012, 7, e37787. [Google Scholar] [CrossRef] [PubMed]
- Ables, G.P.; Perrone, C.E.; Orentreich, D.; Orentreich, N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One 2012, 7, e51357. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, E.B.; Moller, C.L.; Kievit, P.; Grove, K.L.; Andersen, B. Increased fibroblast growth factor 21 expression in high-fat diet-sensitive non-human primates (Macaca mulatta). Int. J. Obes. 2014, 38, 183–191. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Kubicky, R.A.; Wu, S.; Kharitonenkov, A.; de Luca, F. Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology 2012, 153, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Galman, C.; Lundasen, T.; Kharitonenkov, A.; Bina, H.A.; Eriksson, M.; Hafstrom, I.; Dahlin, M.; Amark, P.; Angelin, B.; Rudling, M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 2008, 8, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; Schwarz, F.; Bobbert, T.; Andres, J.; Assmann, A.; Pfeiffer, A.F.; Spranger, J. Relation between fibroblast growth factor-21, adiposity, metabolism, and weight reduction. Metabolism 2011, 60, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Levenson, A.; Kharitonenkov, A.; de Luca, F. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J. Biol. Chem. 2012, 287, 26060–26067. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Grunwald, T.; Kharitonenkov, A.; Dam, J.; Jockers, R.; de Luca, F. Increased expression of fibroblast growth factor 21 (FGF21) during chronic undernutrition causes growth hormone insensitivity in chondrocytes by inducing leptin receptor overlapping transcript (LEPROT) and leptin receptor overlapping transcript-like 1 (LEPROTL1) expression. J. Biol. Chem. 2013, 288, 27375–27383. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Rosell, M.; Gonzalez, F.J.; Giralt, M.; Iglesias, R.; Villarroya, F. Hepatic FGF21 expression is induced at birth via response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.M.; Aenlle, K.K.; Roos, B.A.; Howard, G.A. 24R,25-dihydroxyvitamin D3 promotes the osteoblastic differentiation of human mesenchymal stem cells. Mol. Endocrinol. 2014, 28, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Sylvia, V.L.; Luna, M.H.; deVeau, P.; Whetstone, R.; Dean, D.D.; Boyan, B.D. The effect of 24R,25-(OH)2D3 on protein kinase C activity in chondrocytes is mediated by phospholipase D whereas the effect of 1α,25-(OH)2D3 is mediated by phospholipase C. Steroids 2001, 66, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Jennings, E.G.; Wang, L.; Schwartz, Z. Mechanisms regulating differential activation of membrane-mediated signaling by 1α,25(OH)2D3 and 24R,25(OH)2D3. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Schwartz, Z. 1,25-Dihydroxy vitamin D3 is an autocrine regulator of extracellular matrix turnover and growth factor release via ERp60-activated matrix vesicle matrix metalloproteinases. Cells Tissues Organs 2009, 189, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Van der Meijden, K.; Lips, P.; van Driel, M.; Heijboer, A.C.; Schulten, E.A.; den Heijer, M.; Bravenboer, N. Primary human osteoblasts in response to 25-hydroxyvitamin D3, 1,25-dihydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3. PLoS One 2014, 9, e110283. [Google Scholar] [CrossRef] [PubMed]
- D’Alesio, A.; Garabedian, M.; Sabatier, J.P.; Guaydier-Souquieres, G.; Marcelli, C.; Lemacon, A.; Walrant-Debray, O.; Jehan, F. Two single-nucleotide polymorphisms in the human vitamin D receptor promoter change protein-DNA complex formation and are associated with height and vitamin D status in adolescent girls. Hum. Mol. Genet. 2005, 14, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Dempfle, A.; Wudy, S.A.; Saar, K.; Hagemann, S.; Friedel, S.; Scherag, A.; Berthold, L.D.; Alzen, G.; Gortner, L.; Blum, W.F.; et al. Evidence for involvement of the vitamin D receptor gene in idiopathic short stature via a genome-wide linkage study and subsequent association studies. Hum. Mol. Genet. 2006, 15, 2772–2783. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Svartberg, J.; Joakimsen, R.M.; Grimnes, G. Associations between polymorphisms related to calcium metabolism and human height: The Tromso Study. Ann. Hum. Genet. 2012, 76, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; Demay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997, 94, 9831–9835. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.M.; Demay, M.B. Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology 2002, 143, 3691–3694. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.K.; Griffiths-Jones, S.; Enright, A.J. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. USA 2007, 104, 17719–17724. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Rajewsky, N. The evolution of gene regulation by transcription factors and microRNAs. Nat. Rev. Genet. 2007, 8, 93–103. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef] [PubMed]
- Lovis, P.; Gattesco, S.; Regazzi, R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol. Chem. 2008, 389, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Krutzfeldt, J.; Stoffel, M. MicroRNAs: A new class of regulatory genes affecting metabolism. Cell Metab. 2006, 4, 9–12. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.T. MicroRNAs and cancer. Semin. Cancer Biol. 2003, 13, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Byun, D.S.; Nasser, S.; Murray, L.; Ayyanar, K.; Arango, D.; Figueroa, M.; Melnick, A.; Kao, G.D.; Augenlicht, L.H.; Mariadason, J.M. HDAC4 Promotes Growth of Colon Cancer Cells via Repression of p21. Mol. Biol. Cell 2008, 19, 4062–4075. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Wang, W.X.; Rajeev, B.W. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain. Pathol. 2008, 18, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Kim, S.Y.; Carmell, M.A.; Murchison, E.P.; Alcorn, H.; Li, M.Z.; Mills, A.A.; Elledge, S.J.; Anderson, K.V.; Hannon, G.J. Dicer is essential for mouse development. Nat. Genet. 2003, 35, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Lu, J.; Cobb, B.S.; Rodda, S.J.; McMahon, A.P.; Schipani, E.; Merkenschlager, M.; Kronenberg, H.M. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Harfe, B.D.; McManus, M.T.; Mansfield, J.H.; Hornstein, E.; Tabin, C.J. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc. Natl. Acad. Sci. USA 2005, 102, 10898–10903. [Google Scholar] [CrossRef] [PubMed]
- Wienholds, E.; Kloosterman, W.P.; Miska, E.; Alvarez-Saavedra, E.; Berezikov, E.; de Bruijn, E.; Horvitz, H.R.; Kauppinen, S.; Plasterk, R.H. MicroRNA expression in zebrafish embryonic development. Science 2005, 309, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.J.; Yang, X.; Wei, L.; Chen, Q. MiR-365: A mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 2011, 25, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.A.; Kong, L.; Bai, X.H.; Luan, Y.; Liu, C.J. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J. Biol. Chem. 2009, 284, 11326–11335. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Inloes, J.B.; Nakamura, Y.; Paltrinieri, E.; Kobayashi, T. let-7 and miR-140 microRNAs coordinately regulate skeletal development. Proc. Natl. Acad. Sci. USA 2013, 110, E3291–E3300. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, J.K.; He, X.; Swartz, M.E.; Yan, Y.L.; Song, H.; Boling, T.C.; Kunerth, A.K.; Walker, M.B.; Kimmel, C.B.; Postlethwait, J.H. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat. Genet. 2008, 40, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.E.; Pais, H.; Schwach, F.; Lindow, M.; Kauppinen, S.; Moulton, V.; Dalmay, T. mRNA expression profiling reveals conserved and non-conserved miR-140 targets. RNA Biol. 2011, 8, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, S.; Sato, T.; Inoue, A.; Otsuki, S.; Ito, Y.; Yokoyama, S.; Kato, Y.; Takemoto, F.; Nakasa, T.; Yamashita, S.; et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010, 24, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Yang, T.; Liu, Y.; Li, A.; Fu, S.; Wu, M.; Pan, Z.; Zhou, W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br. J. Cancer 2010, 103, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.P.; Chen, J.; Seok, H.Y.; Zhang, Z.; Kataoka, M.; Hu, X.; Wang, D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 2013, 112, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Takeshita, F.; Hino, Y.; Fukunaga, S.; Kudo, Y.; Tamaki, A.; Matsunaga, J.; Takahashi, R.U.; Takata, T.; Shimamoto, A.; et al. miR-22 represses cancer progression by inducing cellular senescence. J. Cell Biol. 2011, 193, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Izumiya, M.; Ogata-Kawata, H.; Okamoto, K.; Fujiwara, Y.; Nakai, M.; Okabe, A.; Schetter, A.J.; Bowman, E.D.; Midorikawa, Y.; et al. Tumor suppressor miR-22 determines p53-dependent cellular fate through post-transcriptional regulation of p21. Cancer Res. 2011, 71, 4628–4639. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J. Key roles of microRNA-22 family in complex organisms inferred from its evolution. Microrna 2014, 3, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.; Matsuda, K.; Oh, J.; Barbosa, A.C.; Yang, X.; Meadows, E.; McAnally, J.; Pomajzl, C.; Shelton, J.M.; Richardson, J.A.; et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 2004, 119, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Cellular and molecular interactions regulating skeletogenesis. J. Cell Biochem. 2005, 95, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.E.; Pais, H.; Schwach, F.; Lindow, M.; Kauppinen, S.; Moulton, V.; Dalmay, T. Experimental identification of microRNA-140 targets by silencing and overexpressing miR-140. RNA 2008, 14, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, S.F.; Alarcon, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massague, J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Krichevsky, A.M.; Kosik, K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005, 65, 6029–6033. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Hickey, M. Microarray evaluation of dietary restriction. J. Nutr. 2005, 135, 1343–1346. [Google Scholar] [PubMed]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 2001, 15, 2865–2876. [Google Scholar] [PubMed]
- Schipani, E. Hypoxia and HIF-1α in chondrogenesis. Semin. Cell Dev. Biol. 2005, 16, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E. Posttranslational modifications of collagens as targets of hypoxia and Hif-1α in endochondral bone development. Ann. N. Y. Acad. Sci. 2010, 1192, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Che, X.; Jeong, J.H.; Choi, J.Y.; Lee, Y.J.; Lee, Y.H.; Bae, S.C.; Lee, Y.M. Runx2 protein stabilizes hypoxia-inducible factor-1α through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J. Biol. Chem. 2012, 287, 14760–14771. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J. The correct way to monitor autophagy in higher eukaryotes. Autophagy 2005, 1, 65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Shapiro, I.M. Chondrocytes embedded in the epiphyseal growth plates of long bones undergo autophagy prior to the induction of osteogenesis. Autophagy 2006, 2, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Bohensky, J.; Shapiro, I.M. Autophagy: A new phase in the maturation of growth plate chondrocytes is regulated by HIF, mTOR and AMP kinase. Cells Tissues Organs 2009, 189, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Fullgrabe, J.; Klionsky, D.J.; Joseph, B. The return of the nucleus: Transcriptional and epigenetic control of autophagy. Nat. Rev. Mol. Cell Biol. 2014, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Si, M.L.; Zhu, S.; Wu, H.; Lu, Z.; Wu, F.; Mo, Y.Y. miR-21-mediated tumor growth. Oncogene 2007, 26, 2799–2803. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, C.; Yoshino, K.; Yonezawa, K. mTOR integrates amino acid- and energy-sensing pathways. Biochem. Biophys. Res. Commun. 2004, 313, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Frias, M.A.; Thoreen, C.C.; Jaffe, J.D.; Schroder, W.; Sculley, T.; Carr, S.A.; Sabatini, D.M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006, 16, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Pullen, N.; Thomas, G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997, 410, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Pullen, N.; Brennan, P.; Cantrell, D.; Dennis, P.B.; Thomas, G. Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J. Biol. Chem. 2002, 277, 20104–20112. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.M. The regulation and function of Class III PI3Ks: Novel roles for Vps34. Biochem. J. 2008, 410, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Phornphutkul, C.; Wu, K.Y.; Auyeung, V.; Chen, Q.; Gruppuso, P.A. mTOR signaling contributes to chondrocyte differentiation. Dev. Dyn. 2008, 237, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Savabi, F. Interaction of creatine kinase and adenylate kinase systems in muscle cells. Mol. Cell. Biochem. 1994, 133–134, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bohensky, J.; Leshinsky, S.; Srinivas, V.; Shapiro, I.M. Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mTOR suppression. Pediatr. Nephrol. 2010, 25, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouyssegur, J.; Mazure, N.M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009, 29, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Sterner, D.E.; Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Yokosuka, O.; Fukai, K.; Kojima, H.; Tada, M.; Arai, M.; Imazeki, F.; Saisho, H. Cell growth inhibition and gene expression induced by the histone deacetylase inhibitor, trichostatin A, on human hepatoma cells. Oncology 2004, 66, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Bolden, J.E.; Peart, M.J.; Johnstone, R.W. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006, 5, 769–784. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, A.J.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cueto, M.A.; Asselbergs, F.; Atadja, P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 2002, 277, 25748–25755. [Google Scholar] [CrossRef] [PubMed]
- Funato, H.; Oda, S.; Yokofujita, J.; Igarashi, H.; Kuroda, M. Fasting and high-fat diet alter histone deacetylase expression in the medial hypothalamus. PLoS One 2011, 6, e18950. [Google Scholar] [CrossRef] [PubMed]
- Furumatsu, T.; Tsuda, M.; Yoshida, K.; Taniguchi, N.; Ito, T.; Hashimoto, M.; Ito, T.; Asahara, H. Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J. Biol. Chem. 2005, 280, 35203–35208. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Derfoul, A.; Pereira-Mouries, L.; Hall, D.J. A novel domain in histone deacetylase 1 and 2 mediates repression of cartilage-specific genes in human chondrocytes. FASEB J. 2009, 23, 3539–3552. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.W.; Carpio, L.R.; Westendorf, J.J. Histone deacetylase 3 suppression increases PH domain and leucine-rich repeat phosphatase (Phlpp)1 expression in chondrocytes to suppress Akt signaling and matrix secretion. J. Biol. Chem. 2013, 288, 9572–9582. [Google Scholar] [CrossRef]
- Kanfi, Y.; Peshti, V.; Gozlan, Y.M.; Rathaus, M.; Gil, R.; Cohen, H.Y. Regulation of SIRT1 protein levels by nutrient availability. FEBS Lett. 2008, 582, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Kanfi, Y.; Shalman, R.; Peshti, V.; Pilosof, S.N.; Gozlan, Y.M.; Pearson, K.J.; Lerrer, B.; Moazed, D.; Marine, J.C.; de Cabo, R.; et al. Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett. 2008, 582, 543–548. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W.; Yang, X.; Jardine, K.; Hixon, M.; Boekelheide, K.; Webb, J.R.; Lansdorp, P.M.; Lemieux, M. The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 2003, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. SIRT1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 2004, 306, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Jing, E.; Gesta, S.; Kahn, C.R. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007, 6, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; McBurney, M.; Robbins, P.D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 2010, 5, e9199. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase SIRT1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Lee, Y.M.; Chun, Y.S.; Chen, J.; Kim, J.E.; Park, J.W. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Monteserin-Garcia, J.; Al-Massadi, O.; Seoane, L.M.; Alvarez, C.V.; Shan, B.; Stalla, J.; Paez-Pereda, M.; Casanueva, F.F.; Stalla, G.K.; Theodoropoulou, M. Sirt1 inhibits the transcription factor CREB to regulate pituitary growth hormone synthesis. FASEB J. 2013, 27, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Iguchi, G.; Fukuoka, H.; Suda, K.; Bando, H.; Takahashi, M.; Nishizawa, H.; Seino, S.; Takahashi, Y. SIRT1 regulates adaptive response of the growth hormone—Insulin-like growth factor-I axis under fasting conditions in liver. Proc. Natl. Acad. Sci. USA 2013, 110, 14948–14953. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Han, Y.; Bastianetto, S.; Dumont, Y.; Unterman, T.G.; Quirion, R. FoxO-dependent and -independent mechanisms mediate SIRT1 effects on IGFBP-1 gene expression. Biochem. Biophys. Res. Commun. 2005, 337, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liang, M.L.; Zhu, Y.; Gong, Y.Y.; Wang, Y.; Heng, D.; Lin, L. Resveratrol inhibits collagen I synthesis by suppressing IGF-1R activation in intestinal fibroblasts. World J. Gastroenterol. 2014, 20, 4648–4661. [Google Scholar] [CrossRef] [PubMed]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Schwer, B.; Schumacher, B.; Lombard, D.B.; Xiao, C.; Kurtev, M.V.; Gao, J.; Schneider, J.I.; Chai, H.; Bronson, R.T.; Tsai, L.H.; et al. Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 21790–21794. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Tsuji, K.; Ochi, H.; Iwata, M.; Koga, D.; Okawa, A.; Morita, S.; Takeda, S.; Asou, Y. Sirt6 regulates postnatal growth plate differentiation and proliferation via Ihh signaling. Sci. Rep. 2013, 3, 3022. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Takasaka, N.; Araya, J.; Hara, H.; Ito, S.; Kobayashi, K.; Kurita, Y.; Wakui, H.; Yoshii, Y.; Yumino, Y.; Fujii, S.; et al. Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. J. Immunol. 2014, 192, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; D’Urso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.D.; Nir, T.; et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell 2010, 140, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Panera, N.; Agostoni, C. Low birth weight and catch-up-growth associated with metabolic syndrome: A ten year systematic review. Pediatr. Endocrinol. Rev. 2008, 6, 241–247. [Google Scholar] [PubMed]
- Pando, R.; Masarwi, M.; Shtaif, B.; Idelevich, A.; Monsonego-Ornan, E.; Shahar, R.; Phillip, M.; Gat-Yablonski, G. Bone quality is affected by food restriction and by nutrition-induced catch-up growth. J. Endocrinol. 2014, 223, 227–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gat-Yablonski, G.; Phillip, M. Nutritionally-Induced Catch-Up Growth. Nutrients 2015, 7, 517-551. https://doi.org/10.3390/nu7010517
Gat-Yablonski G, Phillip M. Nutritionally-Induced Catch-Up Growth. Nutrients. 2015; 7(1):517-551. https://doi.org/10.3390/nu7010517
Chicago/Turabian StyleGat-Yablonski, Galia, and Moshe Phillip. 2015. "Nutritionally-Induced Catch-Up Growth" Nutrients 7, no. 1: 517-551. https://doi.org/10.3390/nu7010517
APA StyleGat-Yablonski, G., & Phillip, M. (2015). Nutritionally-Induced Catch-Up Growth. Nutrients, 7(1), 517-551. https://doi.org/10.3390/nu7010517




