Selected Activities of Citrus Maxima Merr. Fruits on Human Endothelial Cells: Enhancing Cell Migration and Delaying Cellular Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
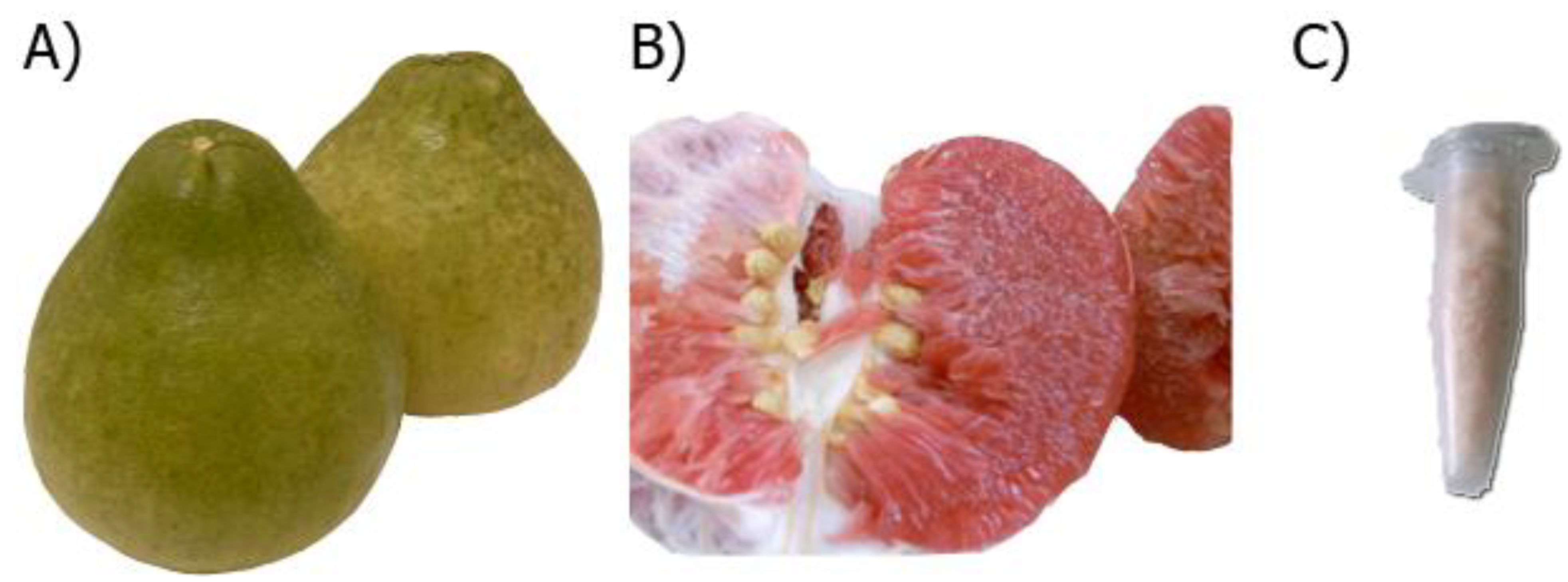
2.2. Preparation of C. maxima (CM) Fruit Extract
2.3. Determination of Antioxidant Capacity and Total Phenolic Compounds
2.4. Determination of Ascorbic Ccid, Gallic Ccid and Certain Citrus Flavonoids by HPLC
2.5. Endothelial Scratch Wound and Cell Migration Assays
| Agent | Mobile Phase | Flow Rate (mL/min) | Detection λ (nm) | Retention Time (min) | Reference |
|---|---|---|---|---|---|
| Ascorbic acid | 100 mM phosphate buffer (pH 2.5) 95%: methanol 5% | 0.4 | 243 | 6.1 | [13] |
| Hesperidin and Naringin | 12 mmol Heptafluorobutyric Acid in 0.05% Formic acid 80%: acetronitrile 20% | 1.2 | 283 | 6.8 and 7.3, respectively | [14] |
| Gallic acid | 0.02% dihydrogen phosphate 95%: acetronitrile 5% | 1.0 | 252 | 4.1 | [16] |
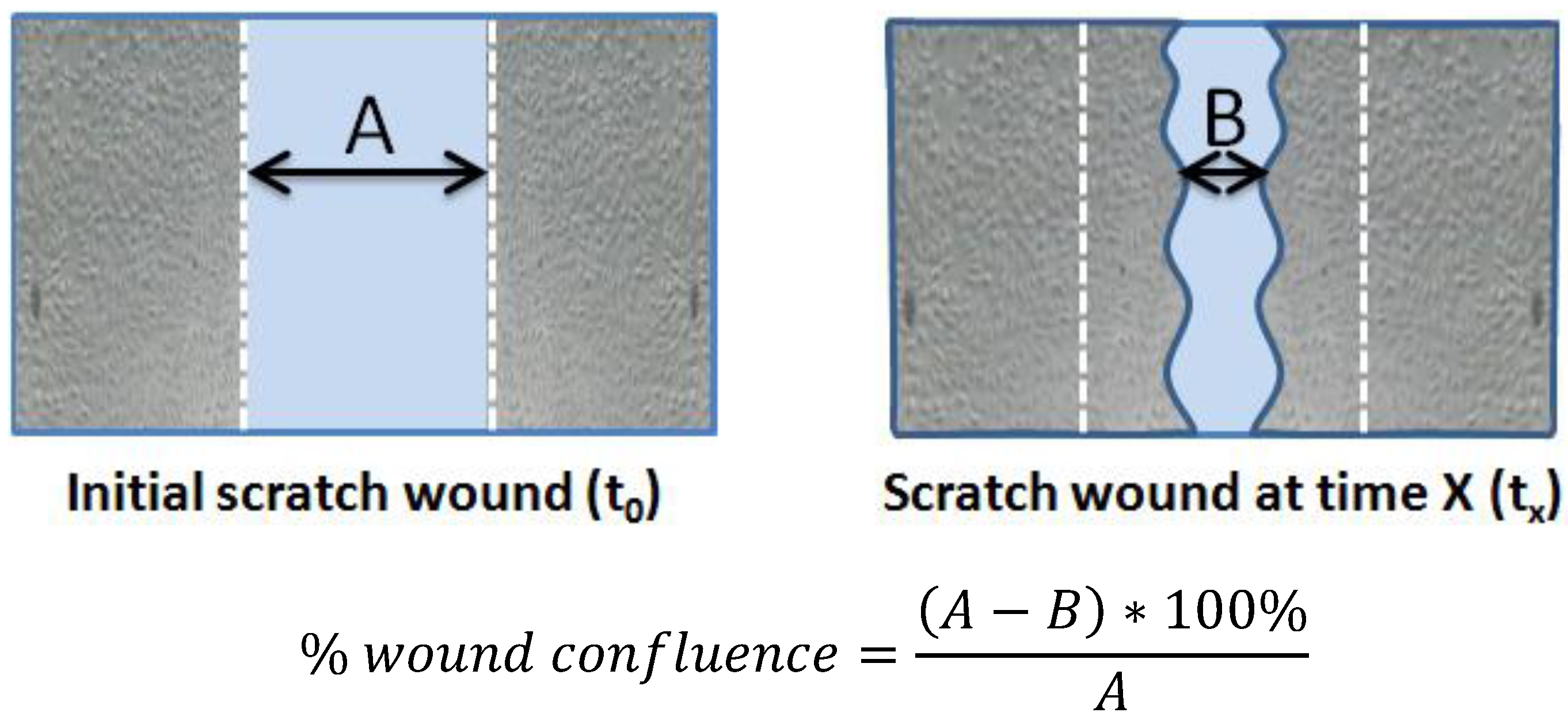
2.6. Cell Culture and Determination of Population Doubling Level (PDL)
2.7. Determination of Intracellular ROS
2.8. Senescence-Associated β-Galactosidase (SA-β-gal) Staining
2.9. Analyses of eNOS mRNA and Protein Expression
2.10. Statistical Analysis
3. Results
3.1. Antioxidant Capacity and Certain Antioxidant Compositions in CM Fruit
| Content | FRAP Value (μmol Fe2+/L) | GAE (mg/L) | Content in Dry Powder %(w/w) | Content in Fruit Juice (mg/L) |
|---|---|---|---|---|
| Total antioxidant power | 6609 | |||
| Total phenolics | 690 | |||
| Ascorbic acid | 0.476 | 423.5 | ||
| Gallic acid | 0.064 | 57.0 | ||
| Hesperidin | 0.100 | 89.1 | ||
| Naringin | 0.542 | 482.3 |
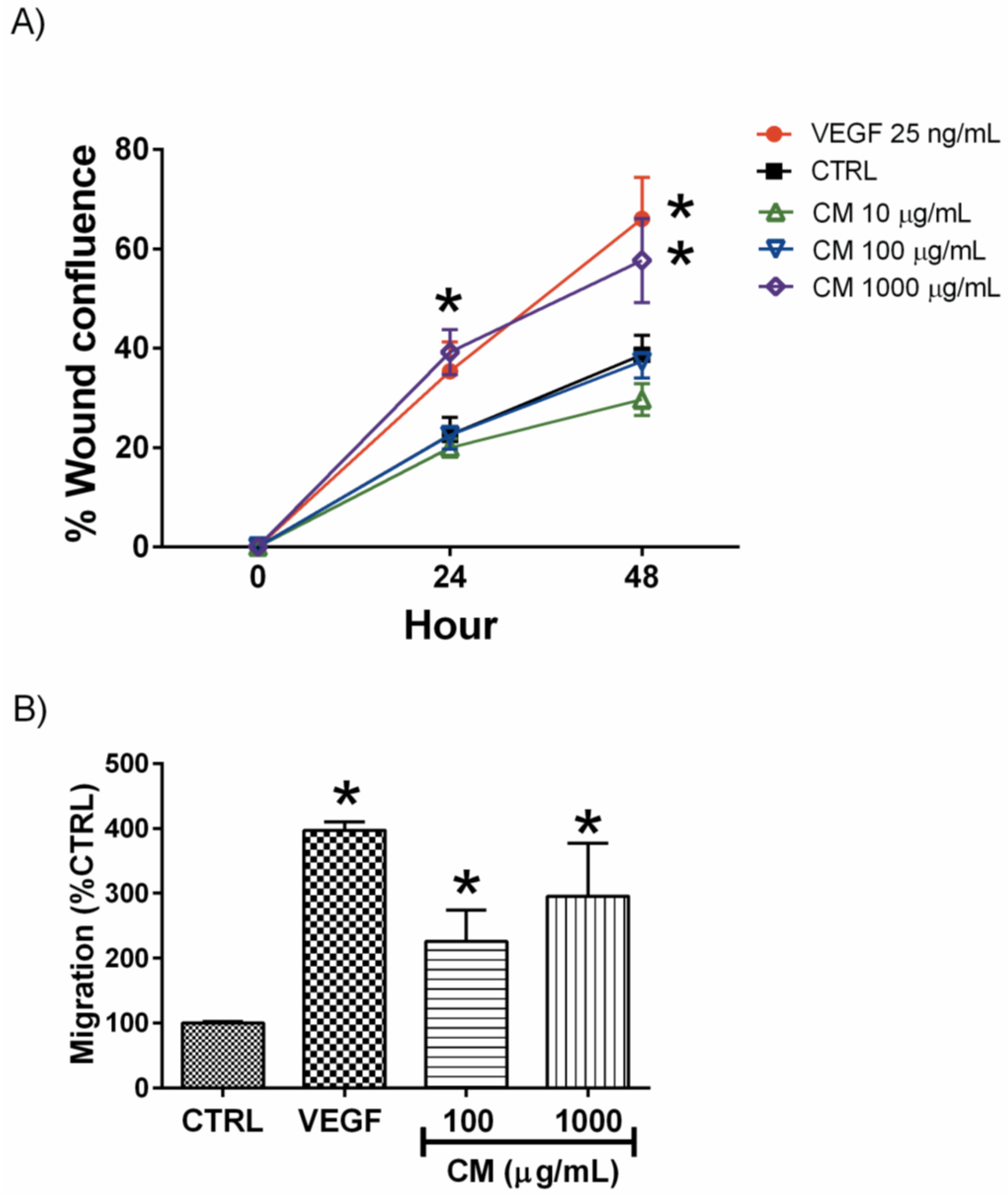
3.2. CM Enhanced Endothelial Cell Migration
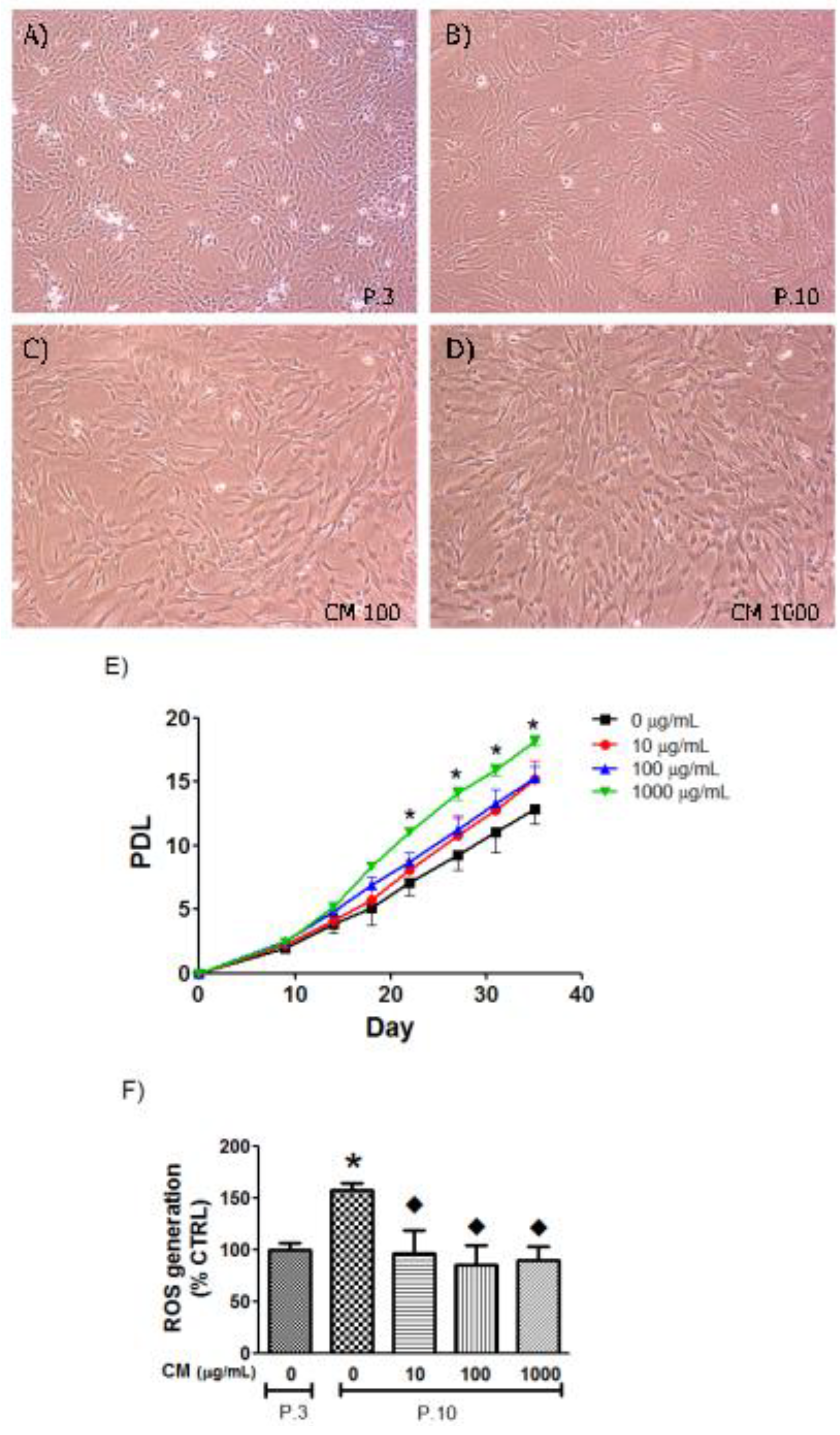
3.3. CM Modified HUVEC Population Doubling Level (PDL)
3.4. CM Decreased Intracellular ROS in Late Passage Cells
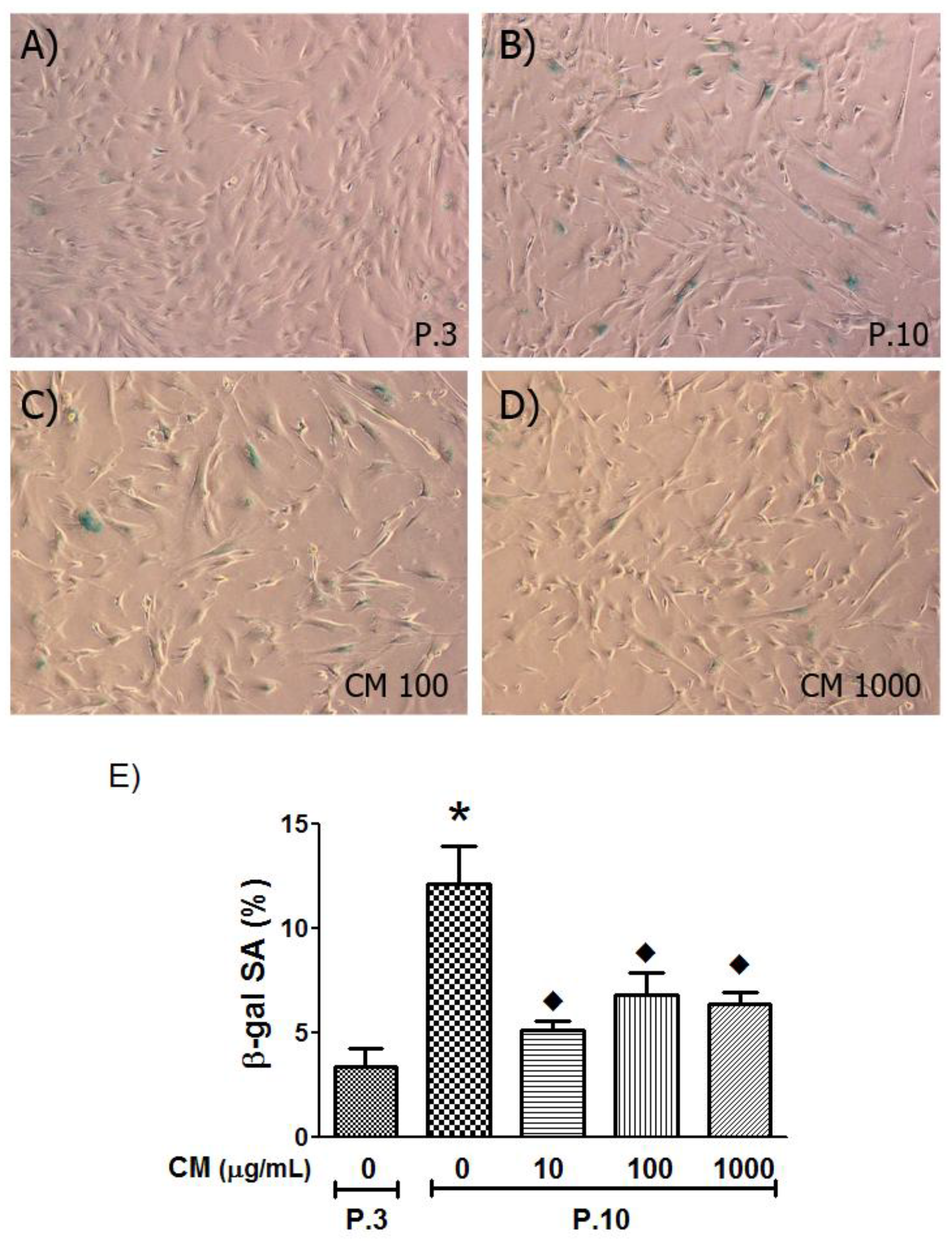
3.5. SA-β-Gal Activity Decreased with CM Treatment
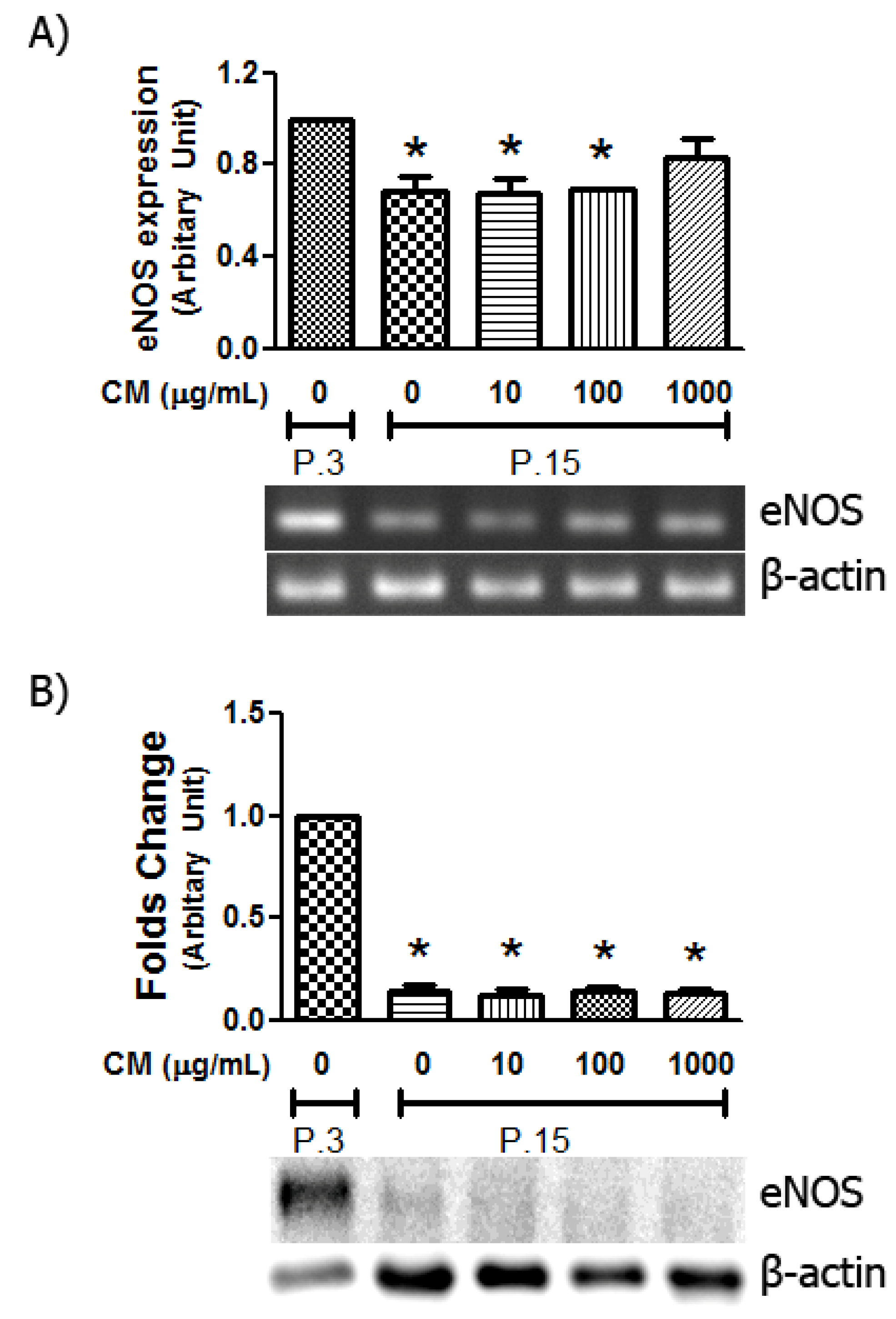
3.6. Alteration in eNOS Expression
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mulero, J.; Bernabe, J.; Cerda, B.; García-Viguera, C.; Moreno, D.A.; Albaladejo, M.D.; Avilés, F.; Parra, S.; Abellán, J.; Zafrilla, P. Variations on cardiovascular risk factors in metabolic syndrome after consume of a citrus-based juice. Clin. Nutr. 2012, 31, 372–377. [Google Scholar] [CrossRef]
- Cassidy, A.; Rimm, E.B.; O’Reilly, E.J.; Logroscino, G.; Kay, C.; Chiuve, S.E.; Rexrode, K.M. Dietary flavonoids and risk of stroke in women. Stroke 2012, 43, 946–951. [Google Scholar] [CrossRef]
- Herrera, M.D.; Mingorance, C.; Rodriguez-Rodriguez, R.; Alvarez de Sotomayor, M. Endothelial dysfunction and aging: An update. Ageing Res. Rev. 2009, 9, 142–152. [Google Scholar]
- Collins, C.; Tzima, E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp. Gerontol. 2011, 46, 185–188. [Google Scholar] [CrossRef]
- Papaharalambus, C.A.; Griendling, K.K. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc. Med. 2007, 17, 48–54. [Google Scholar] [CrossRef]
- Tsai, H.L.; Chang, S.K.; Chang, S.J. Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J. Agric. Food Chem. 2007, 55, 2867–2872. [Google Scholar] [CrossRef]
- Mokbel, M.S.; Hashinaga, F. Evaluation of the antioxidant activity of extracts from buntan (Citrus grandis Osbeck) fruit tissues. Food Chem. 2006, 94, 529–534. [Google Scholar] [CrossRef]
- Jang, H.-D.; Chang, K.-S.; Chang, T.-C.; Hsu, C.-L. Antioxidant potentials of buntan pumelo (Citrus grandis Osbeck) and its ethanolic and acetified fermentation products. Food Chem. 2010, 118, 554–558. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef]
- Benzie, I.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Lester, P., Ed.; Academic Press, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Fernandez-Robredo, P.; Moya, D.; Rodriguez, J.A.; Garcia-Layana, A. Vitamins C and E reduce retinal oxidative stress and nitric oxide metabolites and prevent ultrastructural alterations in porcine hypercholesterolemia. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1140–1146. [Google Scholar] [CrossRef]
- Ding, L.; Luo, X.; Tang, F.; Yuan, J.; Liu, Q.; Yao, S. Simultaneous determination of flavonoid and alkaloid compounds in Citrus herbs by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry. J. Chromatogr. B 2007, 857, 202–209. [Google Scholar] [CrossRef]
- Chularojmontri, L.; Suwatronnakorn, M.; Wattanapitayakul, S.K. Phyllanthus emblica L. enhances human umbilical vein endothelial wound healing and sprouting. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Nitric oxide radical scavenging active components from Phyllanthus emblica L. Plant Foods Hum. Nutr. 2006, 61, 1–5. [Google Scholar] [CrossRef]
- Jendrach, M.; Mai, S.; Pohl, S.; Voth, M.; Bereiter-Hahn, J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion 2008, 8, 293–304. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Bachschmid, M.M.; Schildknecht, S.; Matsui, R.; Zee, R.; Haeussler, D.; Cohen, R.A.; Pimental, D.; van der Loo, B. Vascular aging: Chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann. Med. 2013, 45, 17–36. [Google Scholar] [CrossRef]
- Yamada, T.; Hayasaka, S.; Shibata, Y.; Ojima, T.; Saegusa, T.; Gotoh, T.; Ishikawa, S.; Nakamura, Y.; Kayaba, K.; Jichi Medical School Cohort Study Group. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: The Jichi Medical School cohort study. J. Epidemiol. 2011, 21, 169–175. [Google Scholar] [CrossRef]
- Ettenson, D.S.; Gotlieb, A.I. Endothelial wounds with disruption in cell migration repair primarily by cell proliferation. Microvasc. Res. 1994, 48, 328–337. [Google Scholar] [CrossRef]
- Pintucci, G.; Moscatelli, D.; Saponara, F.; Biernacki, P.R.; Baumann, F.G.; Bizekis, C.; Galloway, A.C.; Basilico, C.; Mignatti, P. Lack of ERK activation and cell migration in FGF-2-deficient endothelial cells. FASEB J. 2002, 16, 598–600. [Google Scholar]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Myung, S.K.; Ju, W.; Cho, B.; Oh, S.-W.; Park, S.M.; Koo, B.-K.; Park, B.-J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 346, f10. [Google Scholar] [CrossRef]
- Oude Griep, L.M.; Verschuren, W.M.; Kromhout, D.; Ocke, M.C.; Geleijnse, J.M. Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in the Netherlands. Eur. J. Clin. Nutr. 2011, 65, 791–799. [Google Scholar] [CrossRef]
- Griep, L.M.; Verschuren, W.M.; Kromhout, D.; Ocke, M.C.; Geleijnse, J.M. Variety in fruit and vegetable consumption and 10-year incidence of CHD and stroke. Public Health Nutr. 2012, 15, 2280–2286. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary Fiber Intake and risk of first stroke: A systematic review and meta-analysis. Stroke 2013, 44, 1360–1368. [Google Scholar]
- Kurz, D.J.; Decary, S.; Hong, Y.; Trivier, E.; Akhmedov, A.; Erusalimsky, J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004, 117, 2417–2426. [Google Scholar] [CrossRef]
- Burger, D.; Kwart, D.G.; Montezano, A.C.; Read, N.C.; Kennedy, C.R.J.; Thompson, C.S.; Touyz, R.M. Microparticles induce cell cycle arrest through redox-sensitive processes in endothelial cells: Implications in vascular senescence. J. Am. Heart Assoc. 2012, 1, e001842. [Google Scholar]
- Ravelojaona, V.; Robert, A.M.; Robert, L. Expression of senescence-associated beta-galactosidase (SA-β-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch. Gerontol. Geriatr. 2009, 48, 151–154. [Google Scholar] [CrossRef]
- Kruse, P.F.; Patterson, M.K. Tissue Culture. Methods and Applications; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Zarzuelo, M.J.; Lopez-Sepulveda, R.; Sanchez, M.; Romero, M.; Gómez-Guzmán, M.; Ungvary, Z.; Pérez-Vizcaíno, F.; Jiménez, R.; Duarte, J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: Implications for vascular aging. Biochem. Pharmacol. 2013, 85, 1288–1296. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.Z.; Wan, Y.Z.; Zhang, Q.J.; Wei, Y.S.; Huang, S.; Liu, J.J.; Lu, Y.B.; Zhang, Z.Q.; Yang, R.F.; et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ. Res. 2011, 109, 639–648. [Google Scholar] [CrossRef]
- Kao, C.L.; Chen, L.K.; Chang, Y.L.; Yung, M.C.; Hsu, C.C.; Chen, Y.C.; Lo, W.L.; Chen, S.J.; Ku, H.H.; Hwang, S.J. Resveratrol protects human endothelium from H2O2-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler. Thromb. 2010, 17, 970–979. [Google Scholar]
- Peterson, J.J.; Beecher, G.R.; Bhagwat, S.A.; Dwyer, J.T.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in grapfruit, lemons, and limes: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S74–S80. [Google Scholar]
- Nizamutdinova, I.T.; Jeong, J.J.; Xu, G.H.; Lee, S.H.; Kang, S.S.; Kim, Y.S.; Chang, K.C.; Kim, H.J. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-α-stimulated human umbilical vein endothelial cells. Int. Immunopharmacol. 2008, 8, 670–678. [Google Scholar] [CrossRef]
- Choi, I.Y.; Kim, S.J.; Jeong, H.J.; Park, S.H.; Song, Y.S.; Lee, J.H.; Kang, T.H.; Park, J.H.; Hwang, G.S.; Lee, E.J.; et al. Hesperidin inhibits expression of hypoxia inducible factor-1α and inflammatory cytokine production from mast cells. Mol. Cell. Biochem. 2007, 305, 153–161. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metabolism. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Bérard, A.M. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J. Nutr. Biochem. 2012, 23, 469–477. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, H.J.; Lee, J.S.; Lee, M.K.; Kim, H.O.; Park, E.J.; Kim, H.K.; Jeong, T.S.; Choi, M.S. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003, 22, 561–568. [Google Scholar]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care 2013, 36, 4104–4108. [Google Scholar] [CrossRef]
- Mortensen, A.; Lykkesfeldt, J. Does vitamin C enhance nitric oxide bioavailability in a tetrahydrobiopterin-dependent manner? In vitro, in vivo and clinical studies. Nitric Oxide 2014, 36, 51–57. [Google Scholar] [CrossRef]
- Pichaiyongvongdee, S.; Haruenkit, R. Investigation of limonoids, flavanones, total polyphenol content and antioxidant activity in seven thai pummelo cultivars. Kasetsart J. 2009, 43, 458–466. [Google Scholar]
- Van der Loo, B.; Schildknecht, S.; Zee, R.; Bachschmid, M.M. Signalling processes in endothelial ageing in relation to chronic oxidative stress and their potential therapeutic implications in humans. Exp. Physiol. 2009, 94, 305–310. [Google Scholar]
- Diaz, M.N.; Frei, B.; Vita, J.A.; Keaney, J.F., Jr. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 1997, 337, 408–416. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Buachan, P.; Chularojmontri, L.; Wattanapitayakul, S.K. Selected Activities of Citrus Maxima Merr. Fruits on Human Endothelial Cells: Enhancing Cell Migration and Delaying Cellular Aging. Nutrients 2014, 6, 1618-1634. https://doi.org/10.3390/nu6041618
Buachan P, Chularojmontri L, Wattanapitayakul SK. Selected Activities of Citrus Maxima Merr. Fruits on Human Endothelial Cells: Enhancing Cell Migration and Delaying Cellular Aging. Nutrients. 2014; 6(4):1618-1634. https://doi.org/10.3390/nu6041618
Chicago/Turabian StyleBuachan, Paiwan, Linda Chularojmontri, and Suvara K. Wattanapitayakul. 2014. "Selected Activities of Citrus Maxima Merr. Fruits on Human Endothelial Cells: Enhancing Cell Migration and Delaying Cellular Aging" Nutrients 6, no. 4: 1618-1634. https://doi.org/10.3390/nu6041618
APA StyleBuachan, P., Chularojmontri, L., & Wattanapitayakul, S. K. (2014). Selected Activities of Citrus Maxima Merr. Fruits on Human Endothelial Cells: Enhancing Cell Migration and Delaying Cellular Aging. Nutrients, 6(4), 1618-1634. https://doi.org/10.3390/nu6041618







