Comparison of Two Doses of Elemental Iron in the Treatment of Latent Iron Deficiency: Efficacy, Side Effects and Blinding Capabilities
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
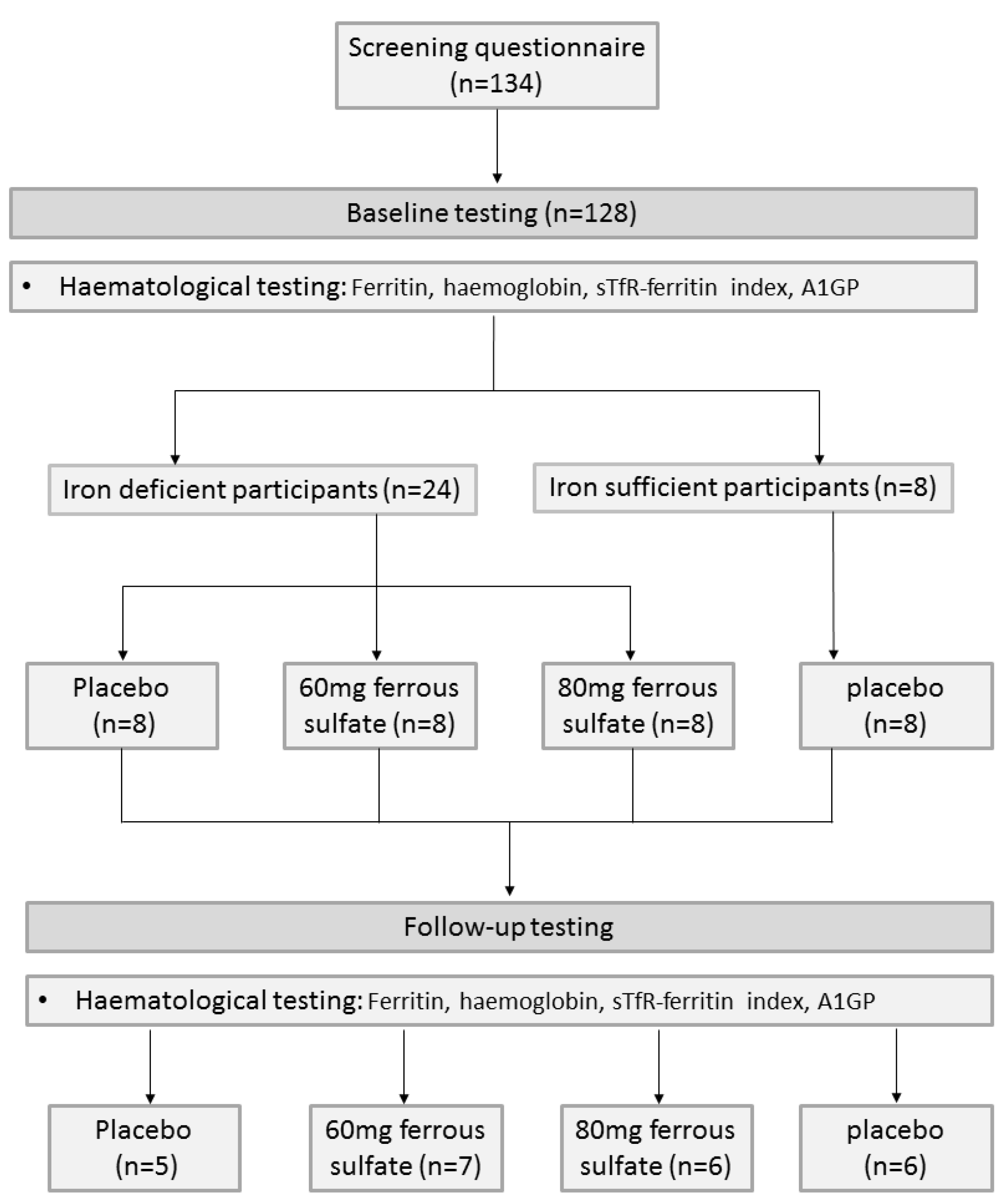
2.2. Haematological Testing
2.3. Pilot Testing of Supplementation
2.4. Capsules and Randomisation
2.5. Statistical Analysis
3. Results
3.1. Participants
| 60 mg iron | 80 mg iron | Placebo | Control | |
|---|---|---|---|---|
| Age (years) | 27.9 ± 5.1 | 24.5 ± 3.4 | 24.8 ± 3.8 | 24.7 ± 3.7 |
| BMI (kg/m2) | 20.8 ± 1.7 | 21.7 ± 1.2 | 21.9 ± 2.4 | 20.6 ± 2.0 |
| Origin | ||||
| Australia | 4 | 3 | 5 | 2 |
| Asia | 1 | 0 | 0 | 1 |
| Canada | 1 | 0 | 0 | 0 |
| United Kingdom | 0 | 1 | 0 | 0 |
| OCP use (total) | 3 | 3 | 4 | 2 |
3.2. Iron Status
| Iron marker | 60 mg iron | 80 mg iron | Placebo | Control |
|---|---|---|---|---|
| Ferritin (µg/L) | ||||
| Baseline | 11.1 ± 1.9 | 10.5 ± 1.7 | 13.5 ± 2.1 | 30.4 ± 2.9 |
| Follow-up | 34.4 ± 10.2 | 30.7 ± 7.0 | 15.1 ± 1.8 | 31.9 ± 5.0 |
| Change | 23.3 ± 10.6 | 20.3 ± 5.6 | 1.6 ± 2.0 | 1.5 ± 4.8 |
| Haemoglobin (g/L) | ||||
| Baseline | 125.8 ± 3.7 | 133.7 ± 2.0 | 132.2 ± 3.3 | 126.8 ± 1.9 |
| Follow-up | 130.1 ± 2.3 | 136.3 ± 4.2 | 131.6 ± 3.9 | 129.0 ± 4.6 |
| Change | 4.3 ± 4.0 | 2.7 ± 3.3 | −0.6 ± 2.3 | 2.2 ± 3.6 |
| sTfR-index | ||||
| Baseline | 1.4 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.7 ± 0.6 |
| Follow-up | 0.8 ± 0.8 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.6 ± 0.9 |
| Change | −0.3 ± 0.2 | −0.6 ± 0.2 | −0.18 ± 0.1 | −0.0 ± 0.1 |
3.2.1. Baseline
| Comparisons (p value) | Ferritin | Haemoglobin | sTfR-Index |
|---|---|---|---|
| Baseline | |||
| Controls vs. Iron deficient | <0.01 | 0.30 | <0.01 |
| Follow-up | |||
| Placebo vs. Controls, 60 mg, 80 mg | <0.01 | 1.0 | 0.11 |
| Change score | |||
| Iron treatment vs. Placebo | <0.01 | 0.45 | 0.07 |
3.2.2. Follow-Up
3.2.3. Change Scores
| Participant group | Outcome | Compliance (%) * | Side effects | Treatment guess | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | ID | DNF | Nil | Nausea | Dark stools | Constipation | Diarrhoea | Iron | Placebo | Unsure | ||
| 60 mg iron (n = 7) | 6 | 1 | 1 | 85.7 ± 17.7 | 5 | 1 | 1 | 0 | 0 | 6 | 1 | 0 |
| 80 mg iron (n = 6) | 4 | 2 | 2 | 93.3 ± 10.6 | 2 | 1 | 4 | 1 | 2 | 3 | 2 | 1 |
| ID placebo (n = 5) | 1 | 4 | 3 | 92.3 ± 5.3 | 4 | 1 | 0 | 0 | 2 | 2 | 1 | 2 |
| IS controls (n = 6) | 5 | 1 | 2 | 88.7 ± 9.1 | 5 | 1 | 1 | 0 | 0 | 1 | 5 | 0 |
3.3. Side Effects and Compliance
3.4. Participants’ Treatment Guesses
4. Discussion
4.1. Change in Iron Status
4.2. Compliance
4.3. Side Effects and Treatment Guess
4.4. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Iron deficiency anaemia: Assessment, prevention, and control. A guide for programme managers. Available online: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf (accessed on 10 August 2013).
- Fayet, F.; Flood, V.; Petocz, P.; Samman, S. Relative and biomarker-based validity of a food frequency questionnaire that measures the intakes of vitamin B(12), folate, iron, and zinc in young women. Nutr. Res. 2010, 31, 14–20. [Google Scholar]
- Patterson, A.J.; Brown, W.J.; Powers, J.R.; Roberts, D.C. Iron deficiency, general health and fatigue: Results from the Australian Longitudinal Study on Women’s Health. Qual. Life Res. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Patterson, A.J.; Brown, W.J.; Roberts, D.C. Dietary and supplement treatment of iron deficiency results in improvements in general health and fatigue in Australian women of childbearing age. J. Am. Coll. Nutr. 2001, 20, 337–342. [Google Scholar] [CrossRef]
- Mora, J.O. Iron supplementation: Overcoming technical and practical barriers. J. Nutr. 2002, 132, S853–S855. [Google Scholar]
- International Nutritional Anemia Consultative Group (INACG). Guidelines for the Use of Iron Supplements to Prevent and Treat. Iron Deficiency Anaemia; Stoltzfus, R.J., Dreyfuss, M.L., Eds.; International Life Sciences Institute: Washington, DC, USA, 1998. [Google Scholar]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B.; British Soc, G. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef]
- Australian Medicines Handbook Pty Ltd. Australian Medicines Handbook; Australian Medicines Handbook Pty Ltd.: Adelaide, Australia, 2010. [Google Scholar]
- Therapeutic Guidelines Ltd. Therapeutic Guidelines: Gastrointestinal; eTG: Melbourne, Australia, 2006. [Google Scholar]
- National Prescribing Service. Available online: http://www.nps.org.au/publications/health-professional/nps-news/2010/iron-anaemia (accessed on 10 September 2013).
- Fernandez-Gaxiola, A.C.; De-Regil, L.M. Intermittent iron supplementation for reducing anaemia and its associated impairments in menstruating women. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Hallberg, L.; Ryttinge, L.; Solvell, L. Side-effects of oral iron therapy—A double blind study of different iron compounds in tablet form. Acta Med. Scand. 1966, 180, 3–21. [Google Scholar] [CrossRef]
- Zhu, A.; Kaneshiro, M.; Kaunitz, J.D. Evaluation and Treatment of Iron Deficiency Anemia: A Gastroenterological Perspective. Dig. Dis. Sci. 2010, 55, 548–559. [Google Scholar] [CrossRef]
- Macdougall, I.C. Strategies for iron supplementation: Oral vs. intravenous. Kidney Int. 1999, 55, S61–S66. [Google Scholar] [CrossRef]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef]
- Makrides, M.; Crowther, C.A.; Gibson, R.A.; Gibson, R.S.; Skeaff, C.M. Efficacy and tolerability of low-dose iron supplements during pregnancy: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 145–153. [Google Scholar]
- Mozaffari-Khosravi, H.; Noori-Shadkam, M.; Fatehi, F.; Naghiaee, Y. Once Weekly Low-dose Iron Supplementation Effectively Improved Iron Status in Adolescent Girls. Biol. Trace Elem. Res. 2010, 135, 22–30. [Google Scholar] [CrossRef]
- Rockey, D. Treatment of iron deficiency. Gastroenterology 2006, 130, 1367–1368. [Google Scholar] [CrossRef]
- Leonard, A.; Hutchesson, M.; Patterson, A.; Chalmers, K.; Collins, C. Recruitment and retention of young women into nutrition research studies: Practical considerations. Trials 2014, 15, 23. [Google Scholar] [CrossRef]
- Ahmed, F.; Coyne, T.; Dobson, A.; McClintock, C. Iron status among Australian adults: Findings of a population based study in Queensland, Australia. Asia Pac. J. Clin. Nut. 2008, 17, 40–47. [Google Scholar]
- Suominen, P.; Punnonen, K.; Rajamaki, A.; Majuri, R.; Hanninen, V.; Irjala, K. Automated immunoturbidimetric method for measuring serum transferrin receptor. Clin. Chem. 1999, 45, 1302–1305. [Google Scholar]
- Leonard, A.J.; Patterson, A.J.; Collins, C.E.; Chalmers, K.A. Is soluble transferrin receptor a useful marker in early stage iron deficiency? e-SPEN J. 2013, 8, e210–e212. [Google Scholar] [CrossRef]
- Larsson, A.P.M.; Hansson, L.-O.; Basu, S.; Axelsson, O. Reference values for a1-acid glycoprotein, a1-antitrypsin, albumin, haptoglobin, C-reactive protein, IgA, IgG and IgM during pregnancy. Acta Obstet. Gynecol. 2008, 87, 1084–1088. [Google Scholar] [CrossRef]
- Koulaouzidis, A.; Said, E.; Cottier, R.; Saeed, A.A. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J. Gastrointest. Liver Dis. 2009, 18, 345–352. [Google Scholar]
- Olivares, M.; Walter, T.; Cook, J.D.; Hertrampf, E.; Pizarro, F. Usefulness of serum transferrin receptor and serum ferritin in diagnosis of iron deficiency in infancy. Am. J. Clin. Nutr. 2000, 72, 1191–1195. [Google Scholar]
- Saghaei, M. Random Allocation Software. 2004. Available online: http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html#Random Allocation Software (accessed on 20 September 2013).
- Bruner, A.B.; Joffe, A.; Duggan, A.K.; Casella, J.F.; Brandt, J. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet 1996, 348, 992–996. [Google Scholar] [CrossRef]
- Galloway, R.; McGuire, J. Determinants of compliance with iron supplementation: Supplies, side effects, or psychology? Soc. Sci. Med. 1994, 39, 381–390. [Google Scholar] [CrossRef]
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, S594–S597. [Google Scholar]
- Borel, M.J.; Smith, S.M.; Derr, J.; Beard, J.L. Day-to-day variation in iron-status indexes in healthy-men and women. Am. J. Clin. Nutr. 1991, 54, 729–735. [Google Scholar]
- Kim, I.; Yetley, E.A.; Calvo, M.S. Variations in iron-status measures during the menstrual cycle. Am. J. Clin. Nutr. 1993, 58, 705–709. [Google Scholar]
- Maes, M.; Bosmans, E.; Scharpe, S.; Hendriks, D.; Cooremans, W.; Neels, H.; DeMeyer, F.; Dhondt, P.; Peeters, D. Components of biological variation in serum soluble transferrin receptor: Relationships to serum iron, transferrin and ferritin concentrations, and immune and haematological variables. Scand. J. Clin. Lab. Investig. 1997, 57, 31–41. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leonard, A.J.; Chalmers, K.A.; Collins, C.E.; Patterson, A.J. Comparison of Two Doses of Elemental Iron in the Treatment of Latent Iron Deficiency: Efficacy, Side Effects and Blinding Capabilities. Nutrients 2014, 6, 1394-1405. https://doi.org/10.3390/nu6041394
Leonard AJ, Chalmers KA, Collins CE, Patterson AJ. Comparison of Two Doses of Elemental Iron in the Treatment of Latent Iron Deficiency: Efficacy, Side Effects and Blinding Capabilities. Nutrients. 2014; 6(4):1394-1405. https://doi.org/10.3390/nu6041394
Chicago/Turabian StyleLeonard, Alecia J., Kerry A. Chalmers, Clare E. Collins, and Amanda J. Patterson. 2014. "Comparison of Two Doses of Elemental Iron in the Treatment of Latent Iron Deficiency: Efficacy, Side Effects and Blinding Capabilities" Nutrients 6, no. 4: 1394-1405. https://doi.org/10.3390/nu6041394
APA StyleLeonard, A. J., Chalmers, K. A., Collins, C. E., & Patterson, A. J. (2014). Comparison of Two Doses of Elemental Iron in the Treatment of Latent Iron Deficiency: Efficacy, Side Effects and Blinding Capabilities. Nutrients, 6(4), 1394-1405. https://doi.org/10.3390/nu6041394






