Nutrients Utilization in Obese Individuals with and without Hypertriglyceridemia
Abstract
:1. Introduction
2. Method
2.1. Nutritional Intake and Anthropometric Measurements
2.2. Blood Pressure Measurement
2.3. RQ and RMR Measurement
2.4. Biochemical Evaluation
2.5. Statistical Analysis
3. Results
| Variables | Controls | Cases | p |
|---|---|---|---|
| Age (years) | 49.7 ± 9 | 49.3 ± 8 | 0.901 |
| RMR (Joule) | 6970.18 ± 1105 | 7223.06 ± 1419 | 0.534 |
| RQ | 0.84 ± 0.06 | 0.89 ± 0.07 | 0.020 |
| BMI | 36.3 ± 5 | 35.7 ± 5 | 0.782 |
| Weight (kg) | 97.8 ± 19 | 95.6 ± 23 | 0.749 |
| WC (cm) | 111.3 ± 13 | 116.1 ± 19 | 0.406 |
| HC (cm) | 115.1 ± 11 | 114.7 ± 15 | 0.931 |
| SBP (mmHg) | 126.5 ± 16 | 127.6 ± 16 | 0.831 |
| DBP (mmHg) | 78.4 ± 7 | 80.0 ± 10 | 0.586 |
| Glucose (mmol/L) | 91 ± 10 | 93.7 ± 9 | 0.398 |
| Creatinin (µmol/L) | 0.82 ± 0.2 | 0.77 ± 0.2 | 0.407 |
| Tot Cholesterol (mmol/L) | 205.6 ± 43 | 220.6 ± 34 | 0.235 |
| LDL-Cholesterol (mmol/L) | 135.1 ± 40 | 125.4 ± 36 | 0.467 |
| HDL-Cholesterol (mmol/L) | 49.15 ± 12 | 37.61 ± 9 | 0.002 |
| Triglyceride (mmol/L) | 109.1 ± 33 | 341.5 ± 125 | <0.001 |
| Uric Acid (mmol/L) | 5.1 ± 1.0 | 6.1 ± 1 | 0.070 |
| Apo B100 (mmol/L) | 1.15 ± 0.3 | 1.20 ± 0.2 | 0.691 |
| Insulin (pmol/L) | 148.8 ± 71 | 161.8 ± 28 | 0.712 |
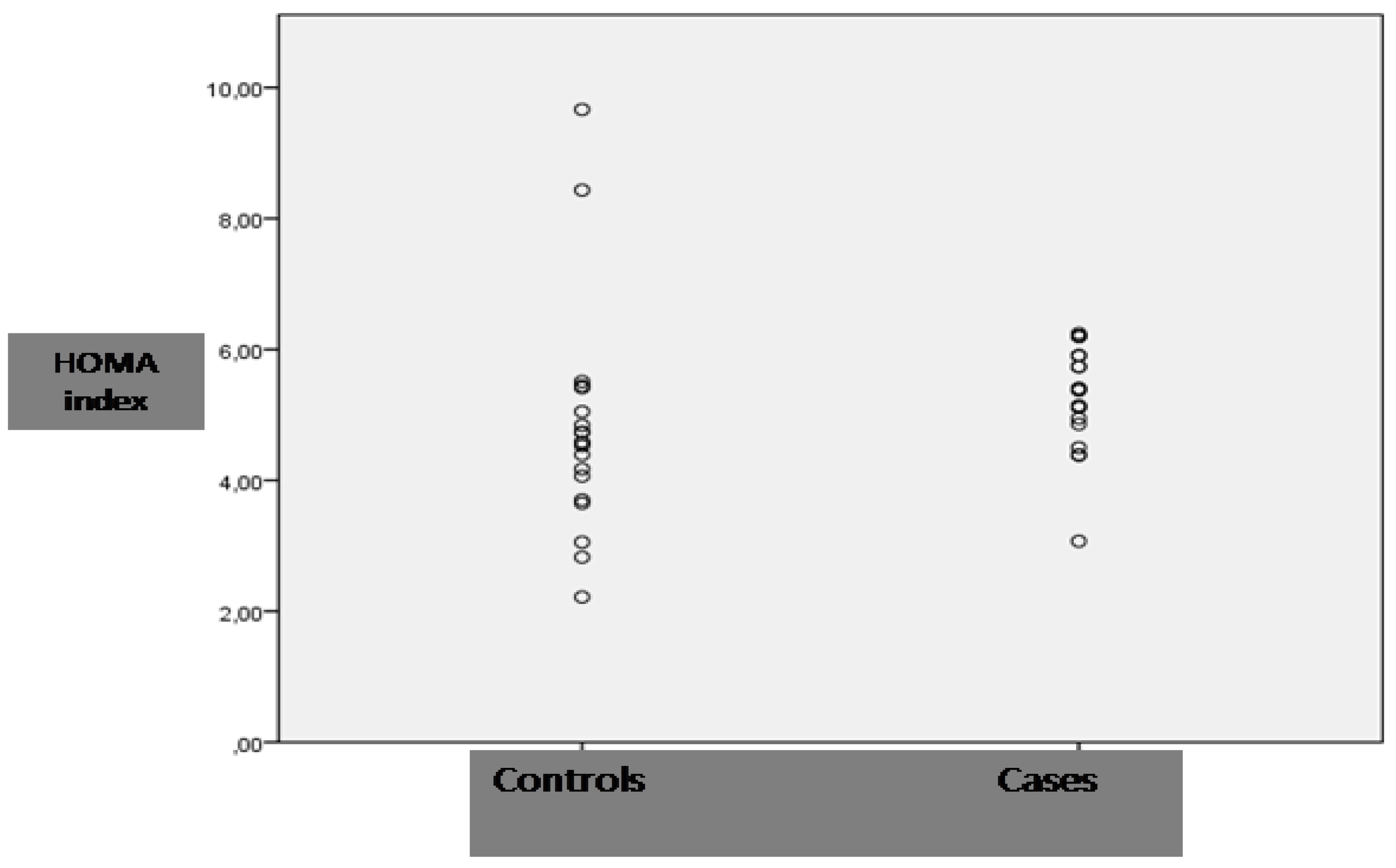
| HOMA index | 4.7 ± 1.7 | 5.2 ± 0.8 | 0.233 |
| Independent Variables | B | SE | Beta | t | p |
|---|---|---|---|---|---|
| Hypertriglyceridemia | 0.052 | 0.024 | 0.343 | 2.192 | 0.035 |
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Bjorkman, O. Fuel metabolism during exercise in normal and diabetic man. Diabetes Metab. Rev. 1986, 1, 319–357. [Google Scholar] [CrossRef]
- Wisneski, J.A.; Gertz, E.W.; Neese, R.A.; Mayr, M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J. Clin. Investig. 1987, 79, 359–366. [Google Scholar] [CrossRef]
- Gold, M.; Spitzer, J.J. Metabolism of free fatty acids by myocardium and kidney. Am. J. Physiol. 1964, 206, 27–31. [Google Scholar]
- Andres, R.; Cader, G.; Zierler, K.L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state: Measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J. Clin. Investig. 1956, 35, 671–682. [Google Scholar] [CrossRef]
- Ferrannini, E.; Barrett, E.J.; Bevilacqua, S.; DeFronzo, R.A. Effect of fatty acids on glucose production and utilization in man. J. Clin. Investig. 1983, 72, 1737–1747. [Google Scholar] [CrossRef]
- Mcneill, G.; Bruce, A.C.; Ralph, A.; James, W.P.T. Interindividual differences in fasting nutrient oxidation and the influence of diet composition. Int. J. Obes. 1988, 12, 445–463. [Google Scholar]
- Zurlo, F.; Lillioja, S.; Esposito-Del Puente, A.; Nyomba, B.L.; Raz, I.; Saad, M.F.; Swinburn, B.A.; Knowler, W.C.; Bogardus, C.; Ravussin, E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. Am. J. Physiol. Endocrinol. Metab. 1990, 259, E650–E657. [Google Scholar]
- Montalcini, T.; Gazzaruso, C.; Ferro, Y.; Migliaccio, V.; Rotundo, S.; Castagna, A.; Montalcini, T.; Gazzaruso, C.; Ferro, Y.; Migliaccio, V.; et al. Metabolic fuel utilization and subclinical atherosclerosis in overweight/obese subjects. Endocrine 2012, 44, 380–385. [Google Scholar]
- Ferro, Y.; Gazzaruso, C.; Coppola, A.; Romeo, S.; Migliaccio, V.; Giustina, A.; Ferro, Y.; Gazzaruso, C.; Coppola, A.; Romeo, S.; et al. Fat utilization and arterial hypertension in overweight/obese subjects. J. Transl. Med. 2013, 11, 159. [Google Scholar] [CrossRef]
- Kissebah, A.H.; Alfarsi, S.; Adams, P.W.; Seed, M.; Folkyard, J.; Wynn, V. Transport kinetics of plasma free fatty acid, very low density lipoprotein triglycerides and apoprotein in patients with endogenous hypertriglyceridaemia. Atherosclerosis 1976, 24, 199–218. [Google Scholar]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef]
- Hokanson, J.E.; Austin, M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef]
- Carey, V.J.; Bishop, L.; Laranjo, N.; Harshfield, B.J.; Kwiat, C.; Sacks, F.M. Contribution of high plasma triglycerides and low high-density lipoprotein cholesterol to residual risk of coronary heart disease after establishment of low-density lipoprotein cholesterol control. Am. J. Cardiol. 2010, 106, 757–763. [Google Scholar] [CrossRef]
- Stein, E.A.; Lane, M.; Laskarzewski, P. Comparison of statins in hypertriglyceridemia. Am. J. Cardiol. 1998, 81, 66B–69B. [Google Scholar] [CrossRef]
- Llurba, E.; Casals, E.; Domínguez, C.; Delgado, J.; Mercadé, I.; Crispi, F.; Llurba, E.; Casals, E.; Domínguez, C.; Delgado, J.; et al. Atherogenic lipoprotein subfraction profile in preeclamptic women with and without high triglycerides: Different pathophysiologic subsets in preeclampsia. Metabolism 2005, 54, 1504–1509. [Google Scholar] [CrossRef]
- Damci, T.; Tatliagac, S.; Osar, Z.; Ilkova, H. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur. J. Intern. Med. 2003, 14, 357–360. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group Report on hypertension in the elderly. Hypertension 1994, 23, 275–285. [Google Scholar]
- Psaty, B.M.; Furberg, C.D.; Kuller, L.H.; Bild, D.E.; Rautaharju, P.M.; Polak, J.F.; Psaty, B.M.; Furberg, C.D.; Kuller, L.H.; Bild, D.E.; et al. Traditional risk factors and subclinical disease measures as predictors of first myocardial infarction in older adults: The Cardiovascular Health Study. Arch. Intern. Med. 1999, 59, 1339–1347. [Google Scholar]
- Montalcini, T.; Gorgone, G.; Garzaniti, A.; Gazzaruso, C.; Pujia, A. Artery remodeling and abdominal adiposity in nonobese postmenopausal women. Eur. J. Clin. Nutr. 2010, 64, 1022–1024. [Google Scholar] [CrossRef]
- Montalcini, T.; Gorgone, G.; Fava, A.; Romeo, S.; Gazzaruso, C.; Pujia, A. Carotid and brachial arterial enlargement in postmenopausal women with hypertension. Menopause 2012, 19, 145–149. [Google Scholar] [CrossRef]
- Zemel, M.B.; Bruckbauer, A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients 2012, 4, 529–541. [Google Scholar] [CrossRef]
- Byrne, C.D.; Wareham, N.J.; Brown, D.C.; Clark, P.M.; Cox, L.J.; Day, N.E.; Byrne, C.D.; Wareham, N.J.; Brown, D.C.; Clark, P.M.; et al. Hypertriglyceridaemia in subjects with normal and abnormal glucose tolerance: Relative contributions of insulin secretion, insulin resistance and suppression of plasma non-esterified fatty acids. Diabetologia 1994, 37, 889–896. [Google Scholar] [CrossRef]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; et al. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef]
- Gorostiaga, E.M.; Maurer, C.A.; Eclache, J.P. Decrease in respiratory quotient during exercise following l-carnitine supplementation. Int. J. Sports Med. 1989, 10, 169–174. [Google Scholar] [CrossRef]
- Somesh, B.P.; Verma, M.K.; Sadasivuni, M.K.; Mammen-Oommen, A.; Biswas, S.; Shilpa, P.C.; Reddy, A.K.; Yateesh, A.N.; Pallavi, P.M.; Nethra, S. Chronic glucolipotoxic conditions in pancreatic islets impair insulin secretion due to dysregulated calcium dynamics, glucose responsiveness and mitochondrial activity. BMC Cell Biol. 2013, 14. [Google Scholar] [CrossRef]
- Boden, G.; Jadali, F.; White, J.; Liang, Y.; Mozzoli, M.; Chen, X.; Boden, G.; Jadali, F.; White, J.; Liang, Y.; et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J. Clin. Investig. 1991, 88, 960–966. [Google Scholar] [CrossRef]
- Åberg, V.; Thörne, A.; Alvestrand, A.; Nordenström, J. Combined hypertriglyceridemic and insulin-glucose clamps for the characterization of substrate oxidation and plasma elimination of a long-chain triglyceride emulsion in healthy men. Metabolism 2012, 61, 221–228. [Google Scholar] [CrossRef]
- Bonen, A.; Parolin, M.L.; Steinberg, G.R.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F.; Bonen, A.; Parolin, M.L.; Steinberg, G.R.; Calles-Escandon, J.; et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004, 18, 1144–1146. [Google Scholar]
- Steensberg, A.; Keller, C.; Starkie, R.L.; Osada, T.; Febbraio, M.A.; Pedersen, B.K. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1272–E1278. [Google Scholar]
- Chabowski, A.; Zmijewska, M.; Gorski, J.; Bonen, A.; Kaminski, K.; Kozuch, M.; Chabowski, A.; Zmijewska, M.; Gorski, J.; Bonen, A.; et al. IL-6 deficiency increases fatty acid transporters and intramuscular lipid contentin red but not white skeletal muscle. J. Physiol. Pharmacol. 2008, 7, 105–117. [Google Scholar]
- Nyman, L.R.; Tian, L.; Hamm, D.A.; Schoeb, T.R.; Gower, B.A.; Nagy, T.R.; Nyman, L.R.; Wood, P.A. Long term effects of high fat or high carbohydrate diets on glucose tolerance in mice with heterozygous carnitine palmitoyltransferase-1a (CPT-1a) deficiency: Diet influences on CPT1a deficient mice. Nutr. Diabetes 2011, 1, e14. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Montalcini, T.; Lamprinoudi, T.; Morrone, A.; Mazza, E.; Gazzaruso, C.; Romeo, S.; Pujia, A. Nutrients Utilization in Obese Individuals with and without Hypertriglyceridemia. Nutrients 2014, 6, 790-798. https://doi.org/10.3390/nu6020790
Montalcini T, Lamprinoudi T, Morrone A, Mazza E, Gazzaruso C, Romeo S, Pujia A. Nutrients Utilization in Obese Individuals with and without Hypertriglyceridemia. Nutrients. 2014; 6(2):790-798. https://doi.org/10.3390/nu6020790
Chicago/Turabian StyleMontalcini, Tiziana, Theodora Lamprinoudi, Attilio Morrone, Elisa Mazza, Carmine Gazzaruso, Stefano Romeo, and Arturo Pujia. 2014. "Nutrients Utilization in Obese Individuals with and without Hypertriglyceridemia" Nutrients 6, no. 2: 790-798. https://doi.org/10.3390/nu6020790
APA StyleMontalcini, T., Lamprinoudi, T., Morrone, A., Mazza, E., Gazzaruso, C., Romeo, S., & Pujia, A. (2014). Nutrients Utilization in Obese Individuals with and without Hypertriglyceridemia. Nutrients, 6(2), 790-798. https://doi.org/10.3390/nu6020790







