A Novel Role of Eruca sativa Mill. (Rocket) Extract: Antiplatelet (NF-κB Inhibition) and Antithrombotic Activities
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Antibodies
2.2. Processing Material
2.3. Preparation of Extract
2.4. Preparation of Human Platelet Suspensions
2.5. Flow Cytometry Analysis for P-Selectin
2.6. Measurement of Platelet Aggregation
2.7. Measurement of Thromboxane B2, CCL5, TGF-1β and IL-1β Levels
2.8. Measurement of cAMP Levels in Human Platelets
2.9. Western Blotting
2.10. Murine Model of Thrombosis
2.11. Bleeding Assay
2.12. Statistical Analysis
3. Results
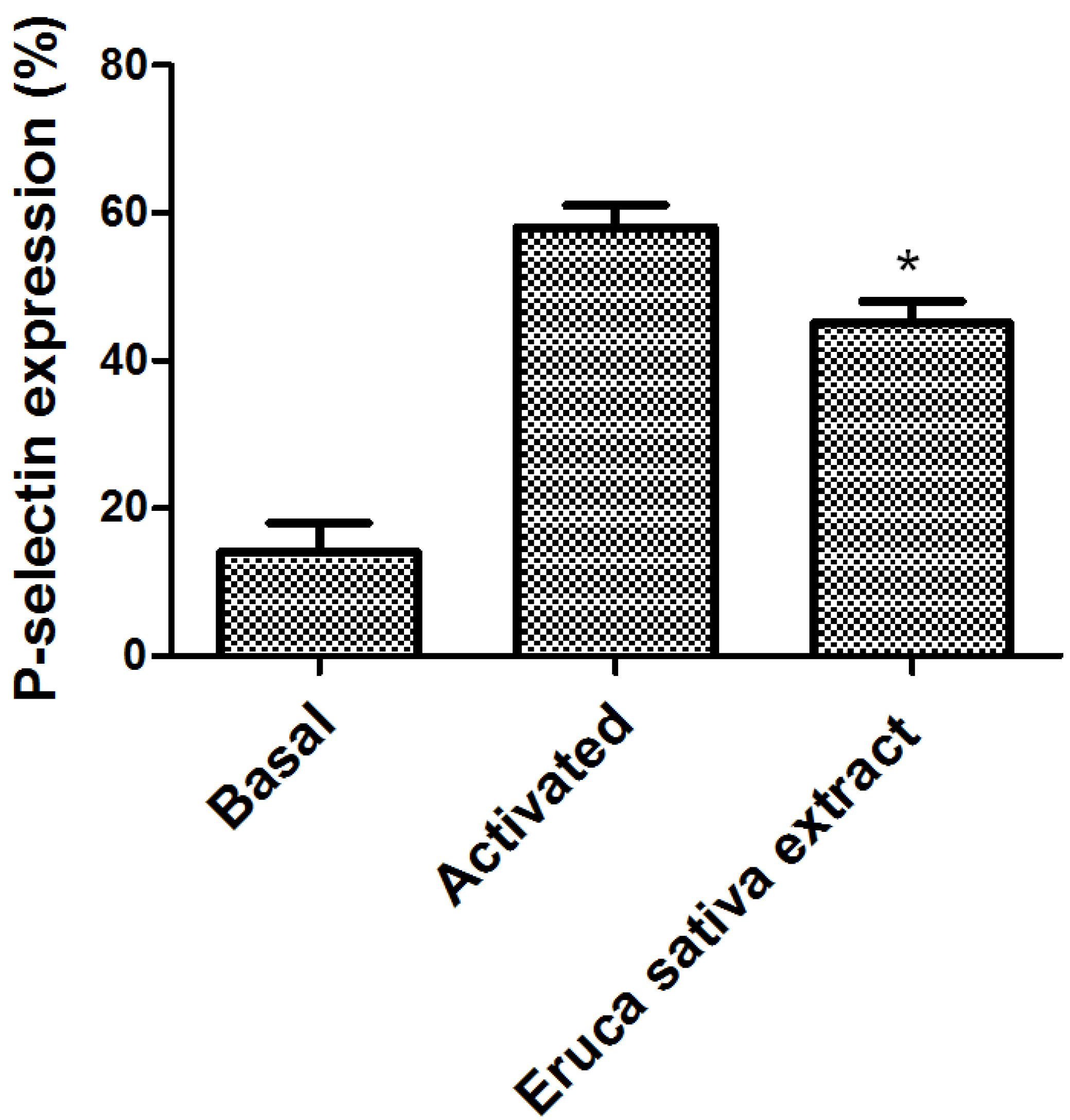
3.1. Effect of Eruca sativa Mill. Extract on Platelet Activation
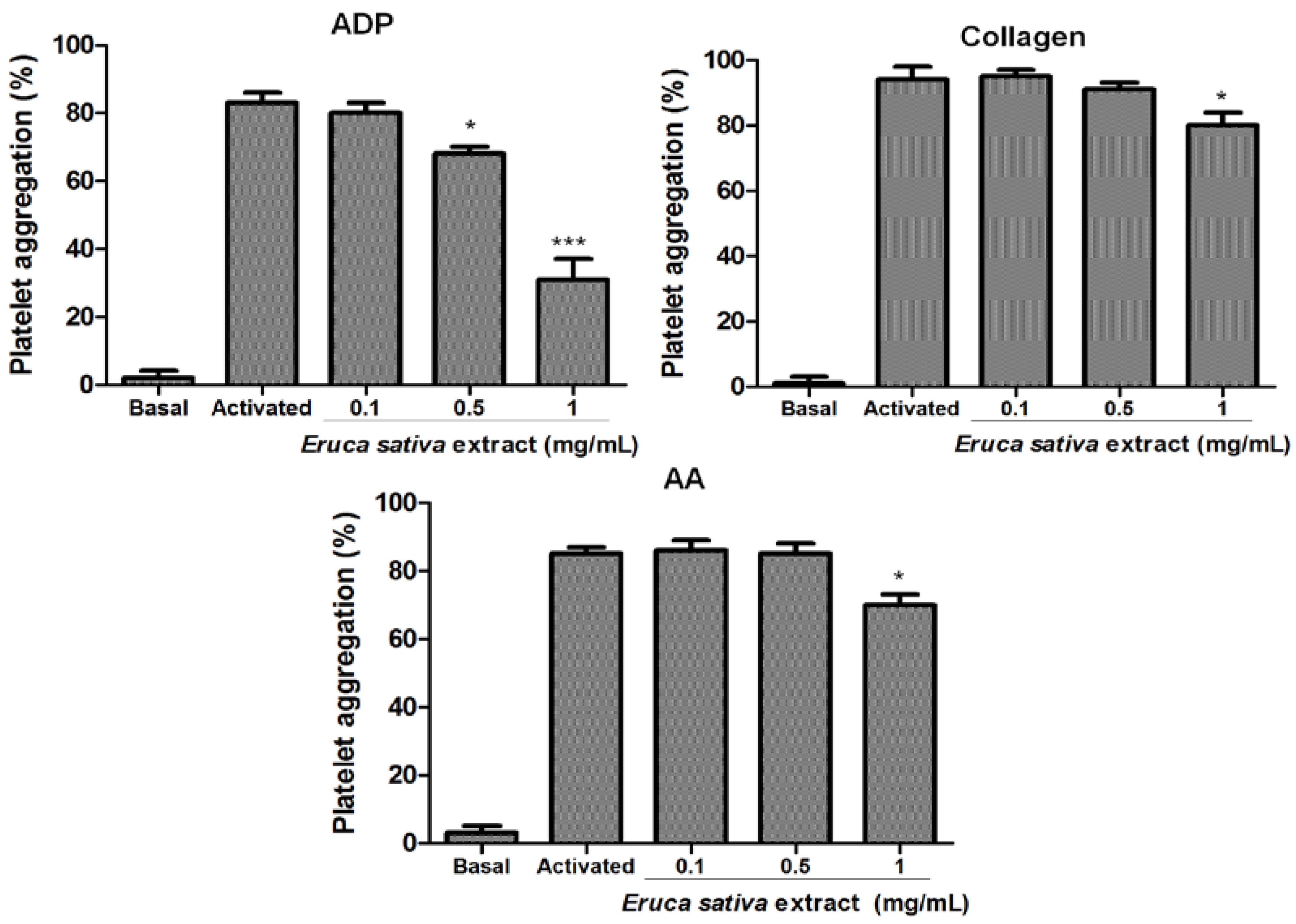
3.2. Effect of Eruca sativa Mill. Extract on Platelet Aggregation
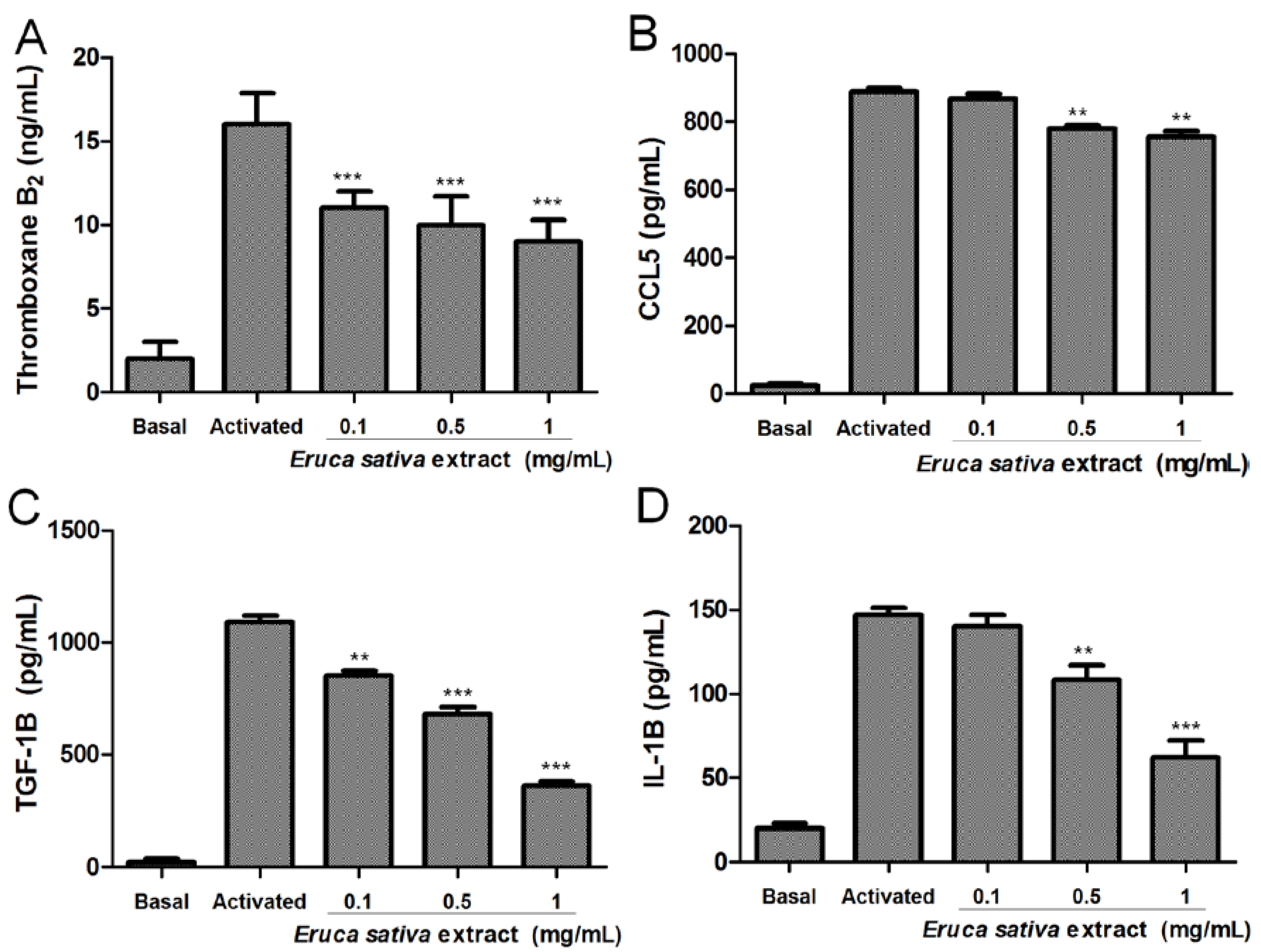
3.3. Effects of Eruca sativa Mill. Extract on Thromboxane B2, CCL5, TGF-1β and IL-1β Levels
3.4. Eruca sativa Mill. Extract and Intraplatelet Levels of cAMP
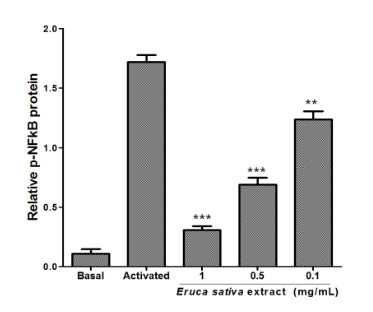
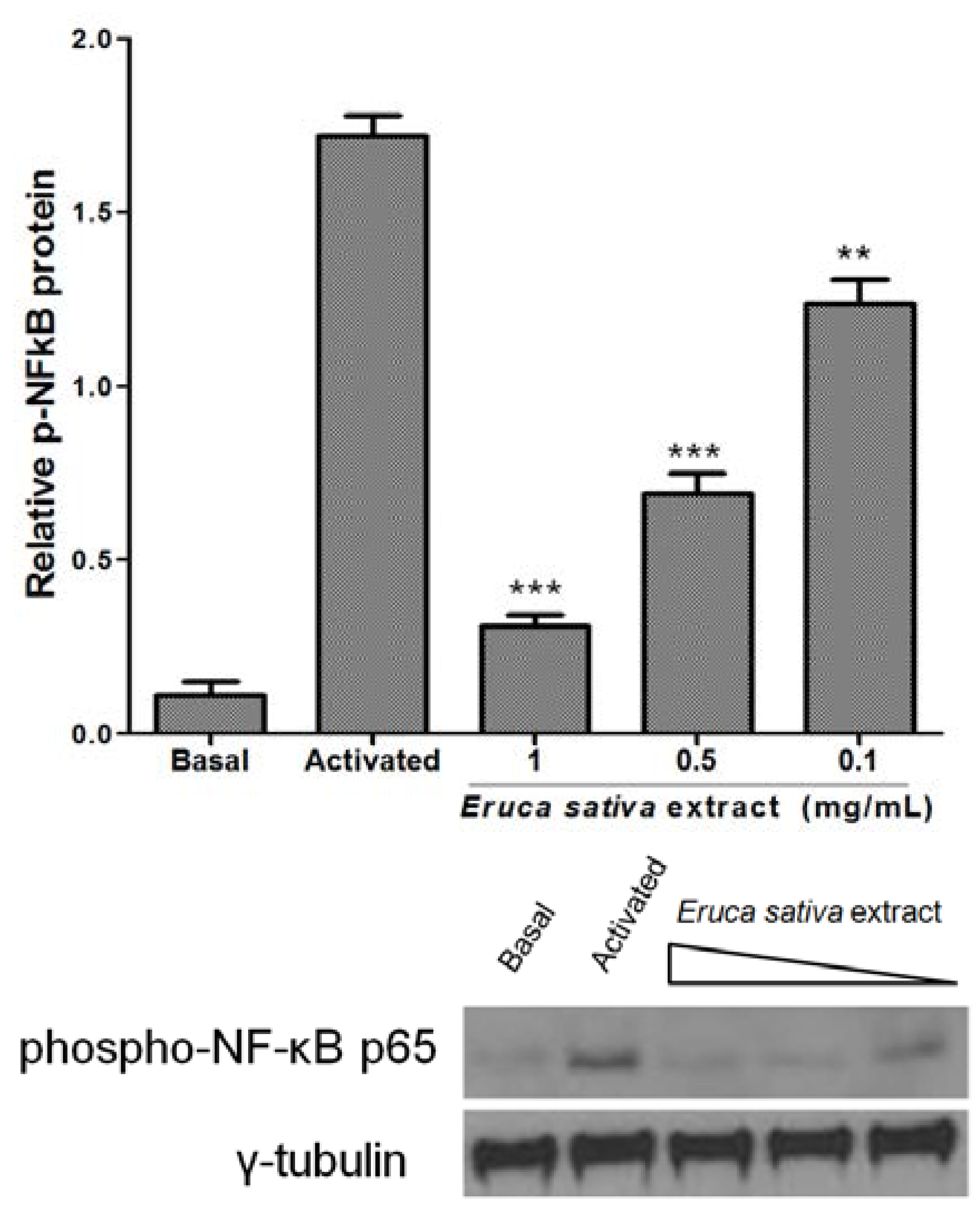
3.5. Effects of Eruca sativa Mill. Extract on PKA and NF-κB
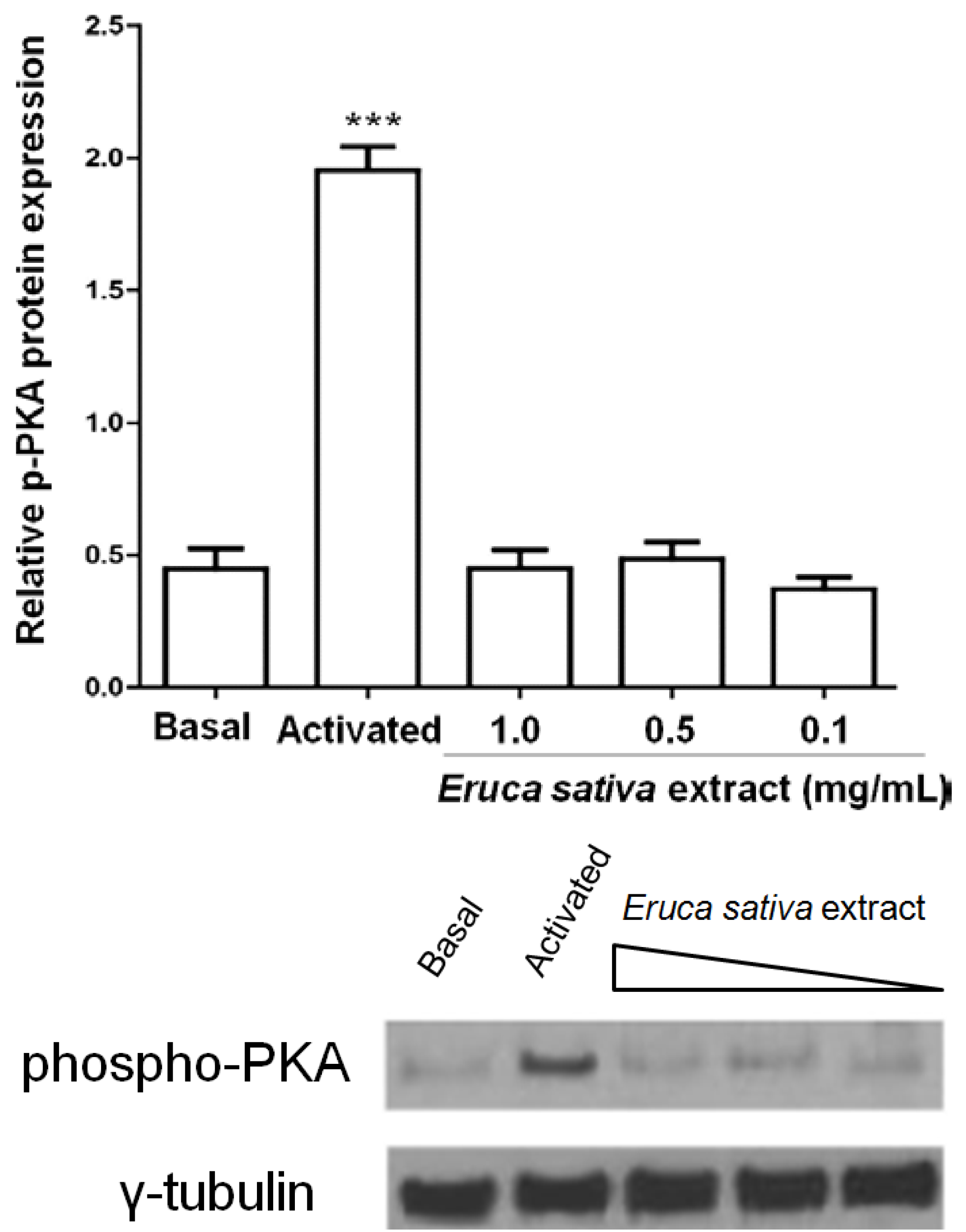
3.6. Effect of Eruca sativa Mill. Extract on Arterial Thrombus Formation and Bleeding Time
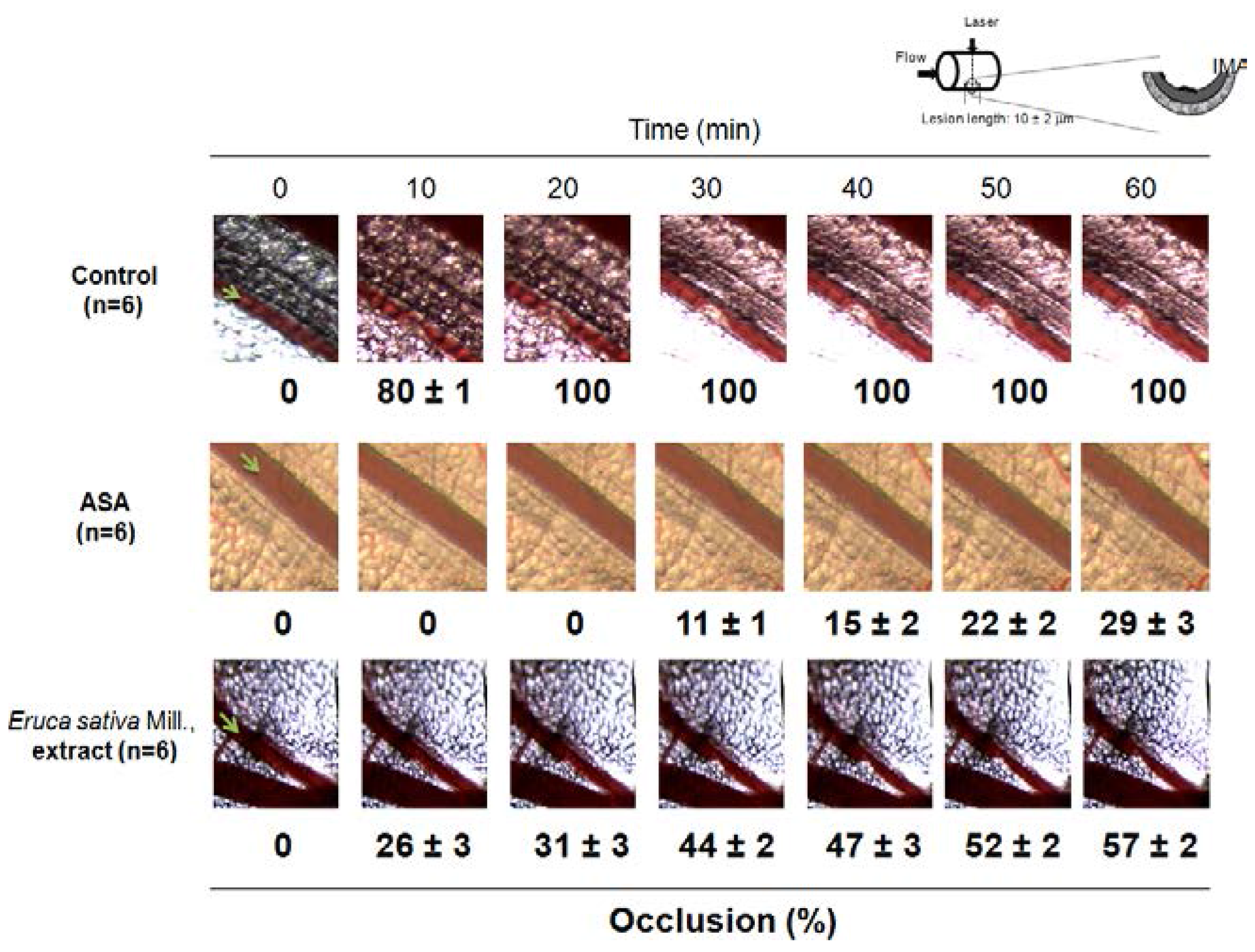
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflict of Interest
References
- Bennett, R.N.; Rosa, E.A.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D.; et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001, 134, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Koroleva, O.A.; Gibson, T.; Swanston, J.; Magan, J.; Zhang, Y.; Rowland, I.R.; Wagstaff, C. Analysis of phytochemical composition and chemoprotective capacity of rocket (Eruca sativa and Diplotaxis tenuifolia) leafy salad following cultivation in different environments. J. Agric. Food Chem. 2009, 57, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Jakse, M.; Hacin, J.; Kacjan, N. Production of rocket (Eruca sativa Mill.) on plug trays and on a floating system in relation to reduced nitrate content. Acta Agric. Slov. 2013, 101, 59–68. [Google Scholar]
- Villatoro-Pulido, M.; Font, R.; Saha, S.; Obregon-Cano, S.; Anter, J.; Munoz-Serrano, A.; De Haro-Bailon, A.; Alonso-Moraga, A.; Del Rio-Celestino, M. In vivo biological activity of rocket extracts (Eruca vesicaria subsp. sativa (Miller) Thell) and sulforaphane. Food Chem. Toxicol. 2012, 50, 1384–1392. [Google Scholar] [CrossRef]
- Alqasoumi, S.; Al-Sohaibani, M.; Al-Howiriny, T.; Al-Yahya, M.; Rafatullah, S. Rocket “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J. Gastroenterol. 2009, 15, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Khan, M.A. Antiulcer Effect of Extract/Fractions of Eruca sativa : Attenuation of Urease Activity. J. Evid. Based Complement. Altern. Med. 2014, 19, 176–180. [Google Scholar] [CrossRef]
- Nieswandt, B.; Pleines, I.; Bender, M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 92–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, G.; Le Breton, G.C.; Gao, X.; Malik, A.B.; Du, X. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J. Biol. Chem. 2003, 278, 30725–30731. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Penz, S.; Reininger, A.J.; Brandl, R.; Goyal, P.; Rabie, T.; Bernlochner, I.; Rother, E.; Goetz, C.; Engelmann, B.; Smethurst, P.A.; et al. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. FASEB J. 2005, 19, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, G. Semilleros en sistema flotante. In Tratado de Cultivos sin Suelo; Urresterazu, M., Ed.; Ediciones Mundi Prensa: Madrid, España, 2004; p. 914. [Google Scholar]
- Carrasco, G.; Izquierdo, J. Almaciguera flotante para la producción de almácigos hortícolas. In Manual Técnico; Universidad de Talca y FAO Oficina Regional para América Latina y El Caribe, Universidad de Talca: Talca, Chile, 2005. [Google Scholar]
- Fuentes, E.; Castro, R.; Astudillo, L.; Carrasco, G.; Alarcón, M.; Gutierrez, M.; Palomo, I. Bioassay-guided isolation and HPLC determination of bioactive compound that relate to the anti-platelet activity (adhesion, secretion and aggregation) from Solanum lycopersicum. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Frojmovic, M.; Wong, T.; van de Ven, T. Dynamic measurements of the platelet membrane glycoprotein IIb-IIIa receptor for fibrinogen by flow cytometry. I. Methodology, theory and results for two distinct activators. Biophys. J. 1991, 59, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V.; Cross, M.J. The Aggregation of Blood Platelets. J. Physiol. 1963, 168, 178–195. [Google Scholar] [PubMed]
- Przyklenk, K.; Whittaker, P. Adaptation of a photochemical method to initiate recurrent platelet-mediated thrombosis in small animals. Lasers Med. Sci. 2007, 22, 42–45. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, F.; Goossens, J.; Reneman, R. Effects of anti-inflammatory, anticoagulant and vasoactive compounds on tail bleeding time, whole blood coagulation time and platelet retention by glass beads in rats. Thromb. Res. 1976, 8, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Morris, S.; Epps, J.; Carroll, R. Demonstration of an activation regulated NF-kappaB/I-kappaBalpha complex in human platelets. Thromb. Res. 2002, 106, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Michelson, A.D. Advances in antiplatelet therapy. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 62–69. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Schroder, J.; Paulus, S.; Brenk, P.; Stahl, T.; Mersch-Sundermann, V. Antigenotoxic properties of Eruca sativa (rocket plant), erucin and erysolin in human hepatoma (HepG2) cells towards benzo(a)pyrene and their mode of action. Food Chem. Toxicol. 2008, 46, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Verardo, V.; Cerretani, L.; Caboni, M.F.; D’Antuono, L.F. Rocket salad (Diplotaxis and Eruca spp.) sensory analysis and relation with glucosinolate and phenolic content. J. Sci. Food Agric. 2011, 91, 2858–2864. [Google Scholar] [PubMed]
- Weckerle, B.; Michel, K.; Balazs, B.; Schreier, P.; Toth, G. Quercetin 3,3′,4′-tri-O-beta-d-glucopyranosides from leaves of Eruca sativa (Mill.). Phytochemistry 2001, 57, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.H.; Ko, W.C.; Ko, F.N.; Teng, C.M. Inhibition of platelet aggregation by some flavonoids. Thromb. Res. 1991, 64, 91–100. [Google Scholar] [CrossRef]
- Sempinska, E.; Kostka, B.; Krolikowska, M.; Kalisiak, E. Effect of flavonoids on the platelet adhesiveness in repeatedly bred rats. Pol. J. Pharmacol. Pharm. 1977, 29, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Mosawy, S.; Jackson, D.E.; Woodman, O.L.; Linden, M.D. Treatment with quercetin and 3′,4′-dihydroxyflavonol inhibits platelet function and reduces thrombus formation in vivo. J. Thromb. Thromb. 2013, 36, 50–57. [Google Scholar] [CrossRef]
- Ferroni, P.; Basili, S.; Davi, G. Platelet activation, inflammatory mediators and hypercholesterolemia. Curr. Vasc. Pharmacol. 2003, 1, 157–169. [Google Scholar]
- Malaver, E.; Romaniuk, M.A.; D’Atri, L.P.; Pozner, R.G.; Negrotto, S.; Benzadon, R.; Schattner, M. NF-kappaB inhibitors impair platelet activation responses. J. Thromb. Haemost. 2009, 7, 1333–1343. [Google Scholar] [PubMed]
- Fukuoka, T.; Hattori, K.; Maruyama, H.; Hirayama, M.; Tanahashi, N. Laser-induced thrombus formation in mouse brain microvasculature: effect of clopidogrel. J. Thromb. Thrombolysis 2012, 34, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.L.; Malinin, A.I.; Eisert, R.M.; Sane, D.C. Risk of bleeding complications with antiplatelet agents: Meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials. Am. J. Hematol. 2004, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, E.; Alarcón, M.; Fuentes, M.; Carrasco, G.; Palomo, I. A Novel Role of Eruca sativa Mill. (Rocket) Extract: Antiplatelet (NF-κB Inhibition) and Antithrombotic Activities. Nutrients 2014, 6, 5839-5852. https://doi.org/10.3390/nu6125839
Fuentes E, Alarcón M, Fuentes M, Carrasco G, Palomo I. A Novel Role of Eruca sativa Mill. (Rocket) Extract: Antiplatelet (NF-κB Inhibition) and Antithrombotic Activities. Nutrients. 2014; 6(12):5839-5852. https://doi.org/10.3390/nu6125839
Chicago/Turabian StyleFuentes, Eduardo, Marcelo Alarcón, Manuel Fuentes, Gilda Carrasco, and Iván Palomo. 2014. "A Novel Role of Eruca sativa Mill. (Rocket) Extract: Antiplatelet (NF-κB Inhibition) and Antithrombotic Activities" Nutrients 6, no. 12: 5839-5852. https://doi.org/10.3390/nu6125839
APA StyleFuentes, E., Alarcón, M., Fuentes, M., Carrasco, G., & Palomo, I. (2014). A Novel Role of Eruca sativa Mill. (Rocket) Extract: Antiplatelet (NF-κB Inhibition) and Antithrombotic Activities. Nutrients, 6(12), 5839-5852. https://doi.org/10.3390/nu6125839