Fatty Acid Metabolism in Carriers of Apolipoprotein E Epsilon 4 Allele: Is It Contributing to Higher Risk of Cognitive Decline and Coronary Heart Disease?
Abstract
:1. Introduction
2. Fatty Acids Composition of the Human Brain and Heart
3. LC Omega-3, Cognition and APOE4
4. Prospective Studies on APOE4 and CHD
5. LC Omega-3, CHD and APOE4
6. Clinical Trials with Dietary Interventions
6.1. Dietary Interventions with LC Omega-3 and Cognition
6.2. Dietary Interventions with LC Omega-3 and CHD
7. Fatty Acid Metabolism in APOE4 Carriers
8. Animal Studies
9. Overlap between Cognitive Decline, Load and CHD
10. Does Fatty Acid Metabolism Disruption Contributes to Higher Risk of Cognitive Decline and CHD in APOE4 Carriers?
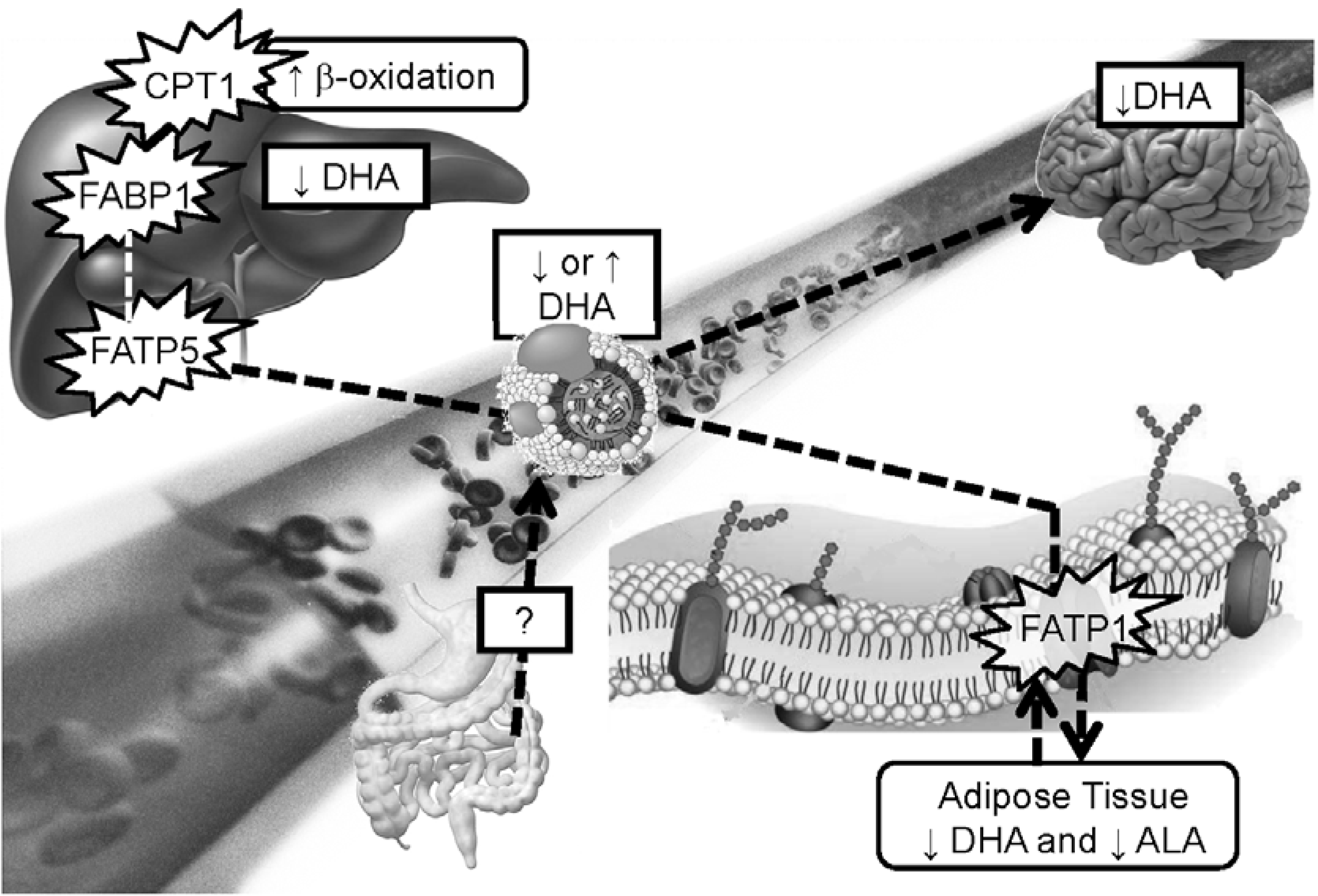
11. Conclusions
- Shift in fatty acid selection for β-oxidation where DHA becomes highly β-oxidized in APOE4 carriers whereas in the non-carriers, DHA is highly conserved.
- In APOE4 carriers, brain uptake of DHA seems lower resulting in lower brain membrane DHA over time. This could play a role in neurotransmission and expression of genes and proteins involved in brain health but this needs further investigation.
- APOE4 carriers respond differently than non-carriers to dietary interventions involving lipids such that modulating lipoprotein levels may include managing fatty acid circulating in the blood. Providing higher doses of LC omega-3 to this population could be necessary to obtain a similar response compared to the non-carriers supplemented with lower doses of LC omega-3.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weisgraber, K.H.; Rall, S.C., Jr.; Mahley, R.W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the Apo-E isoforms. J. Biol. Chem. 1981, 256, 9077–9083. [Google Scholar] [PubMed]
- Garenc, C.; Aubert, S.; Laroche, J.; Girouard, J.; Vohl, M.C.; Bergeron, J.; Rousseau, F.; Julien, P. Population prevalence of APOE, APOC3 and PPAR-alpha mutations associated to hypertriglyceridemia in French Canadians. J. Hum. Genet. 2004, 49, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Bullido, M.J.; Artiga, M.J.; Recuero, M.; Sastre, I.; Garcia, M.A.; Aldudo, J.; Lendon, C.; Han, S.W.; Morris, J.C.; Frank, A.; et al. A polymorphism in the regulatory region of APOE associated with risk for Alzheimer’s dementia. Nat. Genet. 1998, 18, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Rall, S.C., Jr.; Weisgraber, K.H.; Mahley, R.W. Human apolipoprotein E. The complete amino acid sequence. J. Biol. Chem. 1982, 257, 4171–4178. [Google Scholar] [PubMed]
- Mahley, R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Xu, Y.F.; Wu, J.Y.; Chan, L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J. Clin. Investig. 1986, 78, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Elshourbagy, N.A.; Liao, W.S.; Mahley, R.W.; Taylor, J.M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc. Natl. Acad. Sci. USA 1985, 82, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kuhel, D.G.; Shen, L.; Hui, D.Y.; Woods, S.C. Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R903–R908. [Google Scholar] [CrossRef] [PubMed]
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Foss, D.; Mahley, R.W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta 1987, 917, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Farrer, L.A.P.; Cupples, L.A.P.; Haines, J.L.P.; Hyman, B.M.D.P.; Kukull, W.A.P.; Mayeux, R.M.D.; Myers, R.H.P.; Pericak-Vance, M.A.P.; Risch, N.P.; van Duijn, C.M.P. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Coon, K.D.; Myers, A.J.; Craig, D.W.; Webster, J.A.; Pearson, J.V.; Lince, D.H.; Zismann, V.L.; Beach, T.G.; Leung, D.; Bryden, L.; et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J. Clin. Psychiatry 2007, 68, 613–618. [Google Scholar] [CrossRef]
- Kok, E.; Haikonen, S.; Luoto, T.; Huhtala, H.; Goebeler, S.; Haapasalo, H.; Karhunen, P.J. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann. Neurol. 2009, 65, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Filippini, N.; Zarei, M.; Beckmann, C.F.; Galluzzi, S.; Borsci, G.; Testa, C.; Bonetti, M.; Beltramello, A.; Ghidoni, R.; Benussi, L.; et al. Regional atrophy of transcallosal prefrontal connections in cognitively normal APOE epsilon4 carriers. J. Magn. Reson. Imaging 2009, 29, 1021–1026. [Google Scholar] [CrossRef]
- Jak, A.J.; Houston, W.S.; Nagel, B.J.; Corey-Bloom, J.; Bondi, M.W. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement. Geriatr. Cogn. Disord. 2007, 23, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Reiman, E.M.; Alexander, G.E.; Caselli, R.J.; Gerkin, R.; Bandy, D.; Domb, A.; Osborne, D.; Fox, N.; Crum, W.R.; et al. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am. J. Psychiatry 2007, 164, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, M.H.; Ngandu, T.; Rovio, S.; Helkala, E.L.; Uusitalo, U.; Viitanen, M.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Fat intake at midlife and risk of dementia and Alzheimer’s disease: A population-based study. Dement. Geriatr. Cogn. Disord. 2006, 22, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.X.; Shea, S.; Mayeux, R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002, 59, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.W.; Salmon, D.P.; Galasko, D.; Thomas, R.G.; Thal, L.J. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol. Aging 1999, 14, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.M.; Lambert, C.; Sunderland, T.; Parasuraman, R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the national institute of mental health’s biocard study. Neuropsychology 2005, 19, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Baxter, L.C.; Caselli, R.J.; Johnson, S.C.; Reiman, E.; Osborne, D. Apolipoprotein E epsilon 4 affects new learning in cognitively normal individuals at risk for Alzheimer’s disease. Neurobiol. Aging 2003, 24, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Habeck, C.G.; Hilton, J.; Anderson, K.E.; Flynn, J.; Park, A.; Stern, Y. APOE related alterations in cerebral activation even at college age. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.M.; Sunderland, T.; Putnam, K.; Levy, J.; Parasuraman, R. Scaling of visuospatial attention undergoes differential longitudinal change as a function of APOE genotype prior to old age: Results from the NIMH BIOCARD study. Neuropsychology 2005, 19, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Stampfer, M.J.; Liu, S. Meta-analysis: Apolipoprotein E genotypes and risk for coronary heart disease. Ann. Intern. Med. 2004, 141, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.; Mitrou, P.N.; Bowman, R.; Luben, R.; Wareham, N.J.; Khaw, K.T.; Bingham, S. APOE genotype, lipids, and coronary heart disease risk: A prospective population study. Arch. Intern. Med. 2009, 169, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, J.M.; Guesnet, P.; Vancassel, S.; Astorg, P.; Denis, I.; Langelier, B.; Aid, S.; Poumes-Ballihaut, C.; Champeil-Potokar, G.; Lavialle, M. Polyunsaturated fatty acids in the central nervous system: Evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 2004, 44, 509–538. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Lim, G.P.; Yang, F.; Morihara, T.; Teter, B.; Ubeda, O.; Rostaing, P.; Triller, A.; Salem, N., Jr.; Ashe, K.H.; et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron 2004, 43, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Botolin, D.; Wang, Y.; Xu, J.; Christian, B.; Demeure, O. Fatty acid regulation of hepatic gene transcription. J. Nutr. 2005, 135, 2503–2506. [Google Scholar] [PubMed]
- Bouwens, M.; van de Rest, O.; Dellschaft, N.; Bromhaar, M.G.; de Groot, L.C.; Geleijnse, J.M.; Muller, M.; Afman, L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, S1467–S1476. [Google Scholar]
- Vidgren, H.M.; Agren, J.J.; Schwab, U.; Rissanen, T.; Hanninen, O.; Uusitupa, M.I. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 1997, 32, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as suppements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Bernoud, N.; Fenart, L.; Benistant, C.; Pageaux, J.F.; Dehouck, M.P.; Moliere, P.; Lagarde, M.; Cecchelli, R.; Lecerf, J. Astrocytes are mainly responsible for the polyunsaturated fatty acid enrichment in blood-brain barrier endothelial cells in vitro. J. Lipid Res. 1998, 39, 1816–1824. [Google Scholar] [PubMed]
- Boudrault, C.; Bazinet, R.P.; Ma, D.W. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Ma, Q.L.; Frautschy, S.A. Dietary fatty acids and the aging brain. Nutr. Rev. 2010, 68, S102–S111. [Google Scholar] [CrossRef] [PubMed]
- Rocquelin, G.; Guenot, L.; Astorg, P.O.; David, M. Phospholipid content and fatty acid composition of human heart. Lipids 1989, 24, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [PubMed]
- Rocquelin, G.; Guenot, L.; Justrabo, E.; Grynberg, A.; David, M. Fatty acid composition of human heart phospholipids: Data from 53 biopsy specimens. J. Mol. Cell. Cardiol. 1985, 17, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Letenneur, L.; Deschamps, V.; Peres, K.; Dartigues, J.F.; Renaud, S. Fish, meat, and risk of dementia: Cohort study. BMJ 2002, 325, 932–933. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Alperovitch, A. Dietary patterns and risk of dementia: The three-city cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Kaufman, J.S.; Sloane, P.D.; Heiss, G.; Ibrahim, J. n-3 Fatty acids, hypertension and risk of cognitive decline among older adults in the atherosclerosis risk in communities (ARIC) study. Public Health Nutr. 2008, 11, 17–29. [Google Scholar] [CrossRef]
- Eskelinen, M.H.; Ngandu, T.; Helkala, E.L.; Tuomilehto, J.; Nissinen, A.; Soininen, H.; Kivipelto, M. Fat intake at midlife and cognitive impairment later in life: A population-based CAIDE study. Int. J. Geriatr. Psychiatry 2008, 23, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Zandi, P.P.; Tucker, K.L.; Fitzpatrick, A.L.; Kuller, L.H.; Fried, L.P.; Burke, G.L.; Carlson, M.C. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005, 65, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Feskens, E.J.; Launer, L.J.; Kromhout, D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997, 145, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997, 42, 776–782. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Fish consumption and cognitive decline with age in a large community study. Arch. Neurol. 2005, 62, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, B.M.; Tijhuis, M.; Kalmijn, S.; Kromhout, D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen elderly study. Am. J. Clin. Nutr. 2007, 85, 1142–1147. [Google Scholar]
- Vercambre, M.N.; Boutron-Ruault, M.C.; Ritchie, K.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Whalley, L.J.; Fox, H.C.; Wahle, K.W.; Starr, J.M.; Deary, I.J. Cognitive aging, childhood intelligence, and the use of food supplements: Possible involvement of n-3 fatty acids. Am. J. Clin. Nutr. 2004, 80, 1650–1657. [Google Scholar] [PubMed]
- Beydoun, M.A.; Kaufman, J.S.; Satia, J.A.; Rosamond, W.; Folsom, A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: The atherosclerosis risk in communities study. Am. J. Clin. Nutr. 2007, 85, 1103–1111. [Google Scholar] [PubMed]
- Dullemeijer, C.; Durga, J.; Brouwer, I.A.; van de Rest, O.; Kok, F.J.; Brummer, R.J.; van Boxtel, M.P.; Verhoef, P. n-3 Fatty acid proportions in plasma and cognitive performance in older adults. Am. J. Clin. Nutr. 2007, 86, 1479–1485. [Google Scholar] [PubMed]
- Heude, B.; Ducimetiere, P.; Berr, C. Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA study. Am. J. Clin. Nutr. 2003, 77, 803–808. [Google Scholar] [PubMed]
- Samieri, C.; Feart, C.; Letenneur, L.; Dartigues, J.F.; Peres, K.; Auriacombe, S.; Peuchant, E.; Delcourt, C.; Barberger-Gateau, P. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am. J. Clin. Nutr. 2008, 88, 714–721. [Google Scholar] [PubMed]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Gao, Q.; Niti, M.; Feng, L.; Yap, K.B. Omega-3 polyunsaturated fatty acid supplements and cognitive decline: Singapore Longitudinal Aging Studies. J. Nutr. Health Aging 2011, 15, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Kritz-Silverstein, D.; Lopez, L.B.; Barrett Connor, E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: The Rancho Bernardo study. J. Nutr. Health Aging 2011, 15, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Whalley, L.J.; Deary, I.J.; Starr, J.M.; Wahle, K.W.; Rance, K.A.; Bourne, V.J.; Fox, H.C. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: An observational follow-up study in late adulthood. Am. J. Clin. Nutr. 2008, 87, 449–454. [Google Scholar] [PubMed]
- Stuyt, P.M.; Brenninkmeijer, B.J.; Demacker, P.N.; Hendriks, J.C.; van Elteren, P.; Stalenhoef, A.F.; van’t Laar, A. Apolipoprotein E phenotypes, serum lipoproteins and apolipoproteins in angiographically assessed coronary heart disease. Scand. J. Clin. Lab. Investig. 1991, 51, 425–435. [Google Scholar] [CrossRef]
- Salazar, L.A.; Hirata, M.H.; Giannini, S.D.; Forti, N.; Diament, J.; Lima, T.M.; Hirata, R.D. Seven DNA polymorphisms at the candidate genes of atherosclerosis in Brazilian women with angiographically documented coronary artery disease. Clin. Chim. Acta 2000, 300, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Haan, M.N.; Mayeda, E.R. Apolipoprotein E genotype and cardiovascular diseases in the elderly. Curr. Cardiovasc. Risk Rep. 2010, 4, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Shah, T.; Prieto, D.; Zhang, W.; Price, J.; Fowkes, G.R.; Cooper, J.; Talmud, P.J.; Humphries, S.E.; Sundstrom, J.; et al. Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: Systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int. J. Epidemiol. 2013, 42, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Akinkuolie, A.O.; Wu, J.H.; Ding, E.L.; Gaziano, J.M. Fish consumption, omega-3 fatty acids and risk of heart failure: A meta-analysis. Clin. Nutr. 2012, 31, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Raghunathan, T.E.; King, I.; Weinmann, S.; Wicklund, K.G.; Albright, J.; Bovbjerg, V.; Arbogast, P.; Smith, H.; Kushi, L.H.; et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 1995, 274, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; Pelucchi, C.; Negri, E.; Bertuzzi, M.; la Vecchia, C. n-3 Polyunsaturated fatty acids, fish, and nonfatal acute myocardial infarction. Circulation 2001, 104, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Hennekens, C.H.; O’Donnell, C.J.; Ajani, U.A.; Carey, V.J.; Willett, W.C.; Ruskin, J.N.; Manson, J.E. Fish consumption and risk of sudden cardiac death. JAMA 1998, 279, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, M.; Plourde, M.; Chouinard-Watkins, R.; Castellano, C.A.; Barberger-Gateau, P.; Cunnane, S.C. Ageing and APOE change DHA homeostasis: Relevance to age-related cognitive decline. Proc. Nutr. Soc. 2014, 73, 80–86. [Google Scholar] [PubMed]
- Fortier, M.; Tremblay-Mercier, J.; Plourde, M.; Chouinard-Watkins, R.; Vandal, M.; Pifferi, F.; Freemantle, E.; Cunnane, S.C. Higher plasma n-3 fatty acid status in the moderately healthy elderly in southern Quebec: Higher fish intake or aging-related change in n-3 fatty acid metabolism? Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 277–280. [Google Scholar]
- Carvalho-Wells, A.L.; Jackson, K.G.; Gill, R.; Olano-Martin, E.; Lovegrove, J.A.; Williams, C.M.; Minihane, A.M. Interactions between age and APOE genotype on fasting and postprandial triglycerides levels. Atherosclerosis 2010, 212, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Steffen, L.M.; Steffen, B.T.; Guan, W.; Weir, N.L.; Rich, S.S.; Manichaikul, A.; Vargas, J.D.; Tsai, M.Y. APOE genotype modifies the association between plasma omega-3 fatty acids and plasma lipids in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2013, 228, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Su, K.P.; Cheng, T.C.; Liu, H.C.; Chang, C.J.; Dewey, M.E.; Stewart, R.; Huang, S.Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Eriksdotter-Jonhagen, M.; Cederholm, T.; Basun, H.; Faxen-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Kotani, S.; Sakaguchi, E.; Warashina, S.; Matsukawa, N.; Ishikura, Y.; Kiso, Y.; Sakakibara, M.; Yoshimoto, T.; Guo, J.; Yamashima, T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci. Res. 2006, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Morikawa, Y.; Takahashi, H. Effect of DHA oil supplementation on intelligence and visual acuity in the elderly. World Rev. Nutr. Diet. 2001, 88, 68–71. [Google Scholar] [PubMed]
- Terano, T.; Fujishiro, S.; Ban, T.; Yamamoto, K.; Tanaka, T.; Noguchi, Y.; Tamura, Y.; Yazawa, K.; Hirayama, T. Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases. Lipids 1999, 34, S345–S346. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Kamphuis, P.J.; Verhey, F.R.; Olde Rikkert, M.G.; Wurtman, R.J.; Wilkinson, D.; Twisk, J.W.; Kurz, A. Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement. 2010, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N., Jr.; Stedman, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Feart, C.; Proust-Lima, C.; Peuchant, E.; Dartigues, J.F.; Amieva, H.; Barberger-Gateau, P. Omega-3 fatty acids and cognitive decline: Modulation by apoeepsilon4 allele and depression. Neurobiol. Aging 2011, 32, e2313–e2322. [Google Scholar]
- Van de Rest, O.; Geleijnse, J.M.; Kok, F.J.; van Staveren, W.A.; Dullemeijer, C.; Olderikkert, M.G.; Beekman, A.T.; de Groot, C.P. Effect of fish oil on cognitive performance in older subjects: A randomized, controlled trial. Neurology 2008, 71, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Yaqoob, P. Marine omega-3 fatty acids and coronary heart disease. Curr. Opin. Cardiol. 2012, 27, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef]
- Oikawa, S.; Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: Sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2009, 206, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Gissi, H.F.I.; Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.; Soranna, D.; Catapano, A.L.; Corrao, G. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, placebo controlled trials [corrected]. Atheroscler. Suppl. 2013, 14, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M.; Alpha Omega Trial Group. n-3 Fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S.; SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef] [PubMed]
- Rauch, B.; Schiele, R.; Schneider, S.; Diller, F.; Victor, N.; Gohlke, H.; Gottwik, M.; Steinbeck, G.; del Castillo, U.; Sack, R.; et al. Omega, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010, 122, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Wells, A.L.; Jackson, K.G.; Lockyer, S.; Lovegrove, J.A.; Minihane, A.M. APOE genotype influences triglyceride and C-reactive protein responses to altered dietary fat intake in UK adults. Am. J. Clin. Nutr. 2012, 96, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Khan, S.; Leigh-Firbank, E.C.; Talmud, P.; Wright, J.W.; Murphy, M.C.; Griffin, B.A.; Williams, C.M. APOE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Anil, E.; Caslake, M.J.; Packard, C.J.; Bedford, D.; Stewart, G.; Peiris, D.; Williams, C.M.; Minihane, A.M. Contribution of apolipoprotein E genotype and docosahexaenoic acid to the LDL-cholesterol response to fish oil. Atherosclerosis 2010, 209, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Vohl, M.C.; Vandal, M.; Couture, P.; Lemieux, S.; Cunnane, S.C. Plasma n-3 fatty acid response to an n-3 fatty acid supplement is modulated by APOE epsilon4 but not by the common PPAR-alpha L162V polymorphism in men. Br. J. Nutr. 2009, 102, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Rioux-Perreault, C.; Fortier, M.; Tremblay-Mercier, J.; Zhang, Y.; Lawrence, P.; Vohl, M.C.; Perron, P.; Lorrain, D.; Brenna, J.T.; et al. Disturbance in uniformly 13C-labelled DHA metabolism in elderly human subjects carrying the APOE epsilon4 allele. Br. J. Nutr. 2013, 110, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Chouinard-Watkins, R.; Rioux-Perreault, C.; Fortier, M.; Dang, M.T.; Allard, M.J.; Tremblay-Mercier, J.; Zhang, Y.; Lawrence, P.; Vohl, M.C.; et al. Kinetics of 13C-DHA before and during fish-oil supplementation in healthy older individuals. Am. J. Clin. Nutr. 2014, 100, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Conway, V.; Allard, M.J.; Minihane, A.M.; Jackson, K.G.; Lovegrove, J.A.; Plourde, M. Postprandial enrichment of triacylglycerol-rich lipoproteins with omega-3 fatty acids: Lack of an interaction with apolipoprotein E genotype? Lipids Health Dis. 2014, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.M.; Conway, V.; Plourde, M. Disrupt Fatty Acid Distribution in HDL and LDL According to Apolipoprotein E Genotype. In Proceedings of the 7th Congress of the International Society of Nutrigenetics/Nutrigenomics (ISNN), Quebec City, QC, Canada, 6–8 October 2013; Volume 6, pp. 199–253.
- Gregg, R.E.; Zech, L.A.; Schaefer, E.J.; Stark, D.; Wilson, D.; Brewer, H.B., Jr. Abnormal in vivo metabolism of apolipoprotein E4 in humans. J. Clin. Investig. 1986, 78, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Weisgraber, K.H. Apolipoprotein E: Structure-function relationships. Adv. Protein Chem. 1994, 45, 249–302. [Google Scholar] [PubMed]
- Bour, A.; Grootendorst, J.; Vogel, E.; Kelche, C.; Dodart, J.C.; Bales, K.; Moreau, P.H.; Sullivan, P.M.; Mathis, C. Middle-aged human APOE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 2008, 193, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Haley, G.E.; Raber, J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol. Aging 2010, 33, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J. Nutr. Health Aging 2004, 8, 163–174. [Google Scholar] [PubMed]
- Klein, R.C.; Mace, B.E.; Moore, S.D.; Sullivan, P.M. Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2. Neuroscience 2010, 171, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, K.; Hosono, T.; Nakamura, T.; Bu, G.; Michikawa, M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 2011, 286, 17536–17542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Trotter, J.; Zhang, J.; Peters, M.M.; Cheng, H.; Bao, J.; Han, X.; Weeber, E.J.; Bu, G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 2010, 30, 17068–17078. [Google Scholar] [CrossRef] [PubMed]
- Bu, G. Apolipoprotein E and its receptors in Alzheimer’s disease: Pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009, 10, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E: Structure determines function, from atherosclerosis to Alzheimer’s disease to aids. J. Lipid Res. 2009, 50, S183–S188. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, C.; Rousselle, C.; Pollack, G.M.; Scherrmann, J.M. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein-deficient mice. J. Cereb. Blood Flow Metab. 2000, 20, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S.; LaFerla, F.; Bazinet, R.P.; Calon, F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Vandal, M.; Alata, W.; Tremblay, C.; Rioux-Perreault, C.; Salem, N., Jr.; Calon, F.; Plourde, M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J. Neurochem. 2014, 129, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Poirier, J. Apolipoprotein E represents a potent gene-based therapeutic target for the treatment of sporadic Alzheimer’s disease. Alzheimers Dement. 2008, 4, S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Conway, V.; Larouche, A.; Alata, W.; Vandal, M.; Calon, F.; Plourde, M. Apolipoprotein E isoforms disrupt long-chain fatty acid distribution in the plasma, the liver and the adipose tissue of mice. Prostaglandins Leukot. Essent. Fatty Acids 2014. in Press. [Google Scholar]
- Ravona-Springer, R.; Davidson, M.; Noy, S. Is the distinction between Alzheimer’s disease and vascular dementia possible and relevant? Dialogues Clin. Neurosci. 2003, 5, 7–15. [Google Scholar]
- Roher, A.E.; Debbins, J.P.; Malek-Ahmadi, M.; Chen, K.; Pipe, J.G.; Maze, S.; Belden, C.; Maarouf, C.L.; Thiyyagura, P.; Mo, H.; et al. Cerebral blood flow in Alzheimer’s disease. Vasc. Health Risk Manag. 2012, 8, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N.; Ballard, C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis. Assoc. Disord. 1999, 13, S115–S123. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Helkala, E.L.; Laakso, M.P.; Hanninen, T.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissinen, A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ 2001, 322, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Notkola, I.L.; Sulkava, R.; Pekkanen, J.; Erkinjuntti, T.; Ehnholm, C.; Kivinen, P.; Tuomilehto, J.; Nissinen, A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 1998, 17, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Pomara, N.; Sagare, A.P.; Mack, W.J.; Frangione, B.; Zlokovic, B.V. Relationship between cyclophilin A levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein E4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013, 70, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Vandal, M.; Freemantle, E.; Tremblay-Mercier, J.; Plourde, M.; Fortier, M.; Bruneau, J.; Gagnon, J.; Begin, M.; Cunnane, S.C. Plasma omega-3 fatty acid response to a fish oil supplement in the healthy elderly. Lipids 2008, 43, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Tremblay-Mercier, J.; Fortier, M.; Pifferi, F.; Cunnane, S.C. Eicosapentaenoic acid decreases postprandial beta-hydroxybutyrate and free fatty acid responses in healthy young and elderly. Nutrition 2009, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Sepe, S.; D’Amelio, M.; Bernardi, C.; Cristiano, L.; Cimini, A.; Cecconi, F.; Ceru, M.P.; Moreno, S. Age-dependent roles of peroxisomes in the hippocampus of a transgenic mouse model of Alzheimer’s disease. Mol. Neurodegener. 2013, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, Y.; Shi, Y.; Shi, S.; Jiang, L. Inhibition of peroxisomal β-oxidation by thioridazine increases the amount of VLCFAs and Aβ generation in the rat brain. Neurosci. Lett. 2012, 528, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Jung, K.M.; Berchtold, N.C.; Nguyen, V.Q.; Gillen, D.L.; Head, E.; Cotman, C.W.; Piomelli, D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS One 2010, 5, e12538. [Google Scholar] [CrossRef] [PubMed]
- Lizard, G.; Rouaud, O.; Demarquoy, J.; Cherkaoui-Malki, M.; Iuliano, L. Potential roles of peroxisomes in Alzheimer’s disease and in dementia of the Alzheimer’s type. J. Alzheimer’s Dis. 2012, 29, 241–254. [Google Scholar]
- Guzzardi, M.A.; Iozzo, P. Fatty heart, cardiac damage, and inflammation. Rev. Diabet. Stud. 2011, 8, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; Gomez, M.A.; Guillen, F.; Bornstein, B.; Campos, Y.; Rubio, J.C.; de la Calzada, C.S.; Arenas, J. Myocardial carnitine and carnitine palmitoyltransferase deficiencies in patients with severe heart failure. Biochim. Biophys. Acta 2000, 1502, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zlokovic, B.V. Blood-brain barrier: A dual life of Mfsd2a? Neuron 2014, 82, 728–730. [Google Scholar] [CrossRef]
- Betsholtz, C. Physiology: Double function at the blood-brain barrier. Nature 2014, 509, 432–433. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouinard-Watkins, R.; Plourde, M. Fatty Acid Metabolism in Carriers of Apolipoprotein E Epsilon 4 Allele: Is It Contributing to Higher Risk of Cognitive Decline and Coronary Heart Disease? Nutrients 2014, 6, 4452-4471. https://doi.org/10.3390/nu6104452
Chouinard-Watkins R, Plourde M. Fatty Acid Metabolism in Carriers of Apolipoprotein E Epsilon 4 Allele: Is It Contributing to Higher Risk of Cognitive Decline and Coronary Heart Disease? Nutrients. 2014; 6(10):4452-4471. https://doi.org/10.3390/nu6104452
Chicago/Turabian StyleChouinard-Watkins, Raphaël, and Mélanie Plourde. 2014. "Fatty Acid Metabolism in Carriers of Apolipoprotein E Epsilon 4 Allele: Is It Contributing to Higher Risk of Cognitive Decline and Coronary Heart Disease?" Nutrients 6, no. 10: 4452-4471. https://doi.org/10.3390/nu6104452
APA StyleChouinard-Watkins, R., & Plourde, M. (2014). Fatty Acid Metabolism in Carriers of Apolipoprotein E Epsilon 4 Allele: Is It Contributing to Higher Risk of Cognitive Decline and Coronary Heart Disease? Nutrients, 6(10), 4452-4471. https://doi.org/10.3390/nu6104452



