Anti-Osteoporotic Effects of Angelica sinensis (Oliv.) Diels Extract on Ovariectomized Rats and Its Oral Toxicity in Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Animal and Treatments
2.3. Rat Toxicity Studies
2.4. Bone Mineral Density Measurements
2.5. Serum Estradiol and Bone Marker Analysis
2.6. Statistical Analysis
3. Results
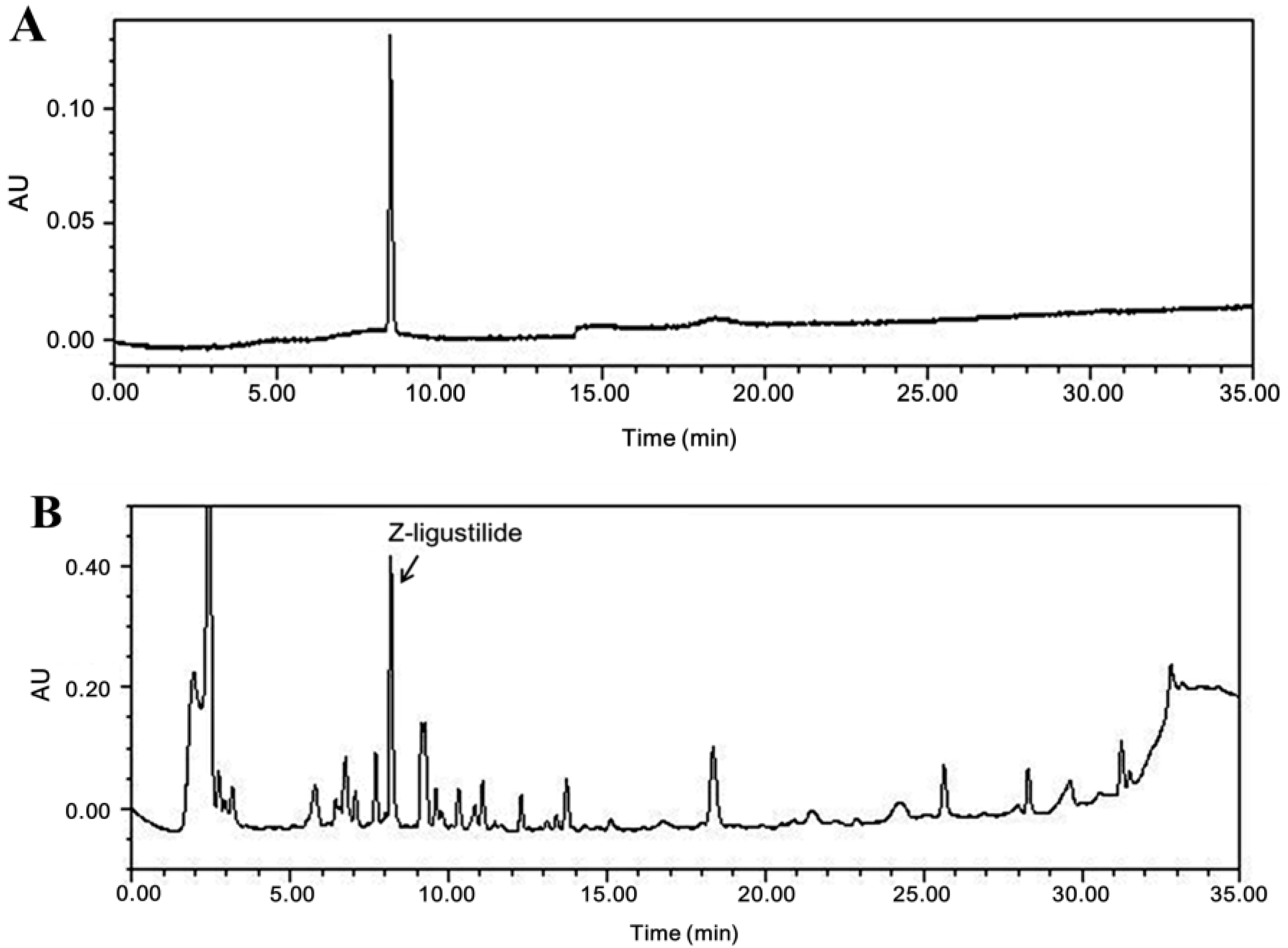
3.1. Compositional Analysis of Z-Ligustilide from A. sinensis Extract
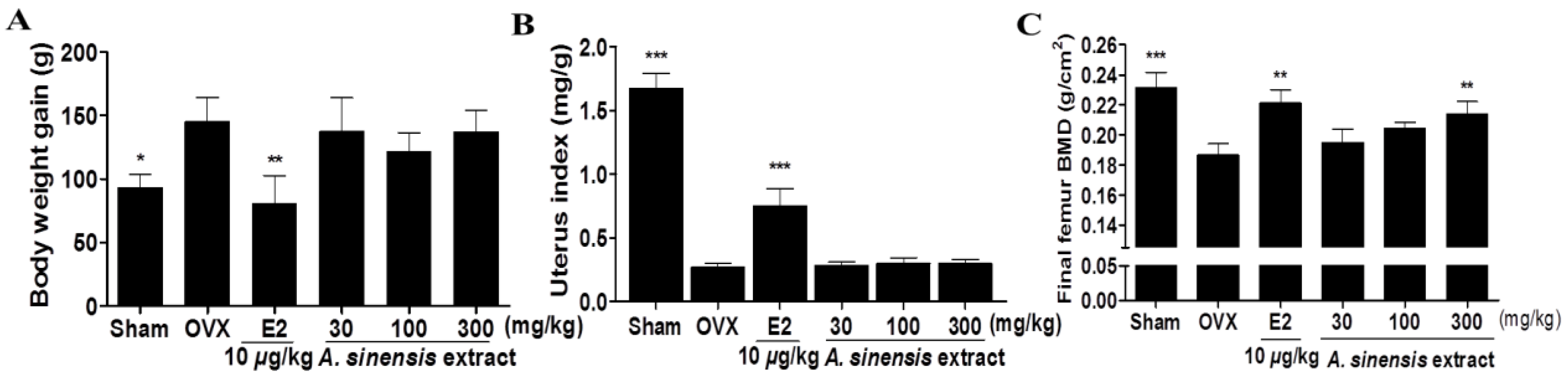
3.2. Body Weight Gain, Uterus Index, and Bone Mineral Density in Rats Treated with A. sinensis Extract
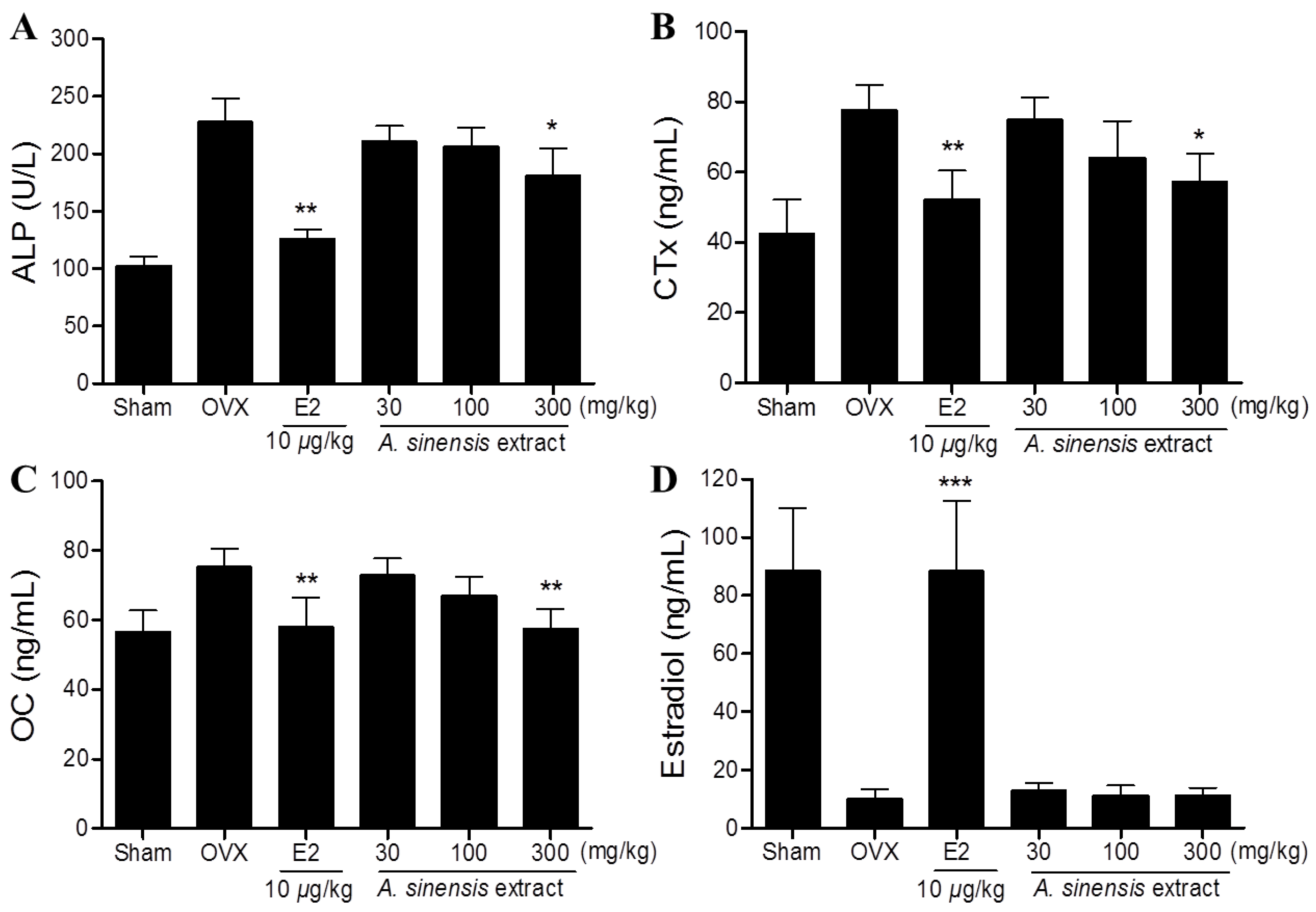
3.3. Serum Bone Marker in Rats Treated with A. sinensis Extract
3.4. Effects of A. sinensis Extract on Sub-Chronic Toxicity
| Week | Mean Body Weight (g ± SD) | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Normal | Extract (mg/kg) | Normal | Extract (mg/kg) | |||
| 1000 | 2000 | 1000 | 2000 | |||
| 0 | 234.3 ± 9.1 | 227.6 ± 10.3 | 230.0 ± 8.5 | 187.6 ± 3.8 | 185.7 ± 4.9 | 182.8 ± 5.0 |
| 1 | 281.7 ± 13.5 | 273.8 ± 12.9 | 279.5 ± 10.4 | 199.0 ± 10.4 | 210.8 ± 7.1 | 212.4 ± 10.8 |
| 2 | 321.3 ± 19.2 | 312.9 ± 16.4 | 318.5 ± 12.6 | 219.0 ± 12.2 | 229.0 ± 9.8 | 230.1 ± 7.8 |
| 3 | 343.1 ± 22.8 | 338.9 ± 16.6 | 341.2 ± 15.5 | 225.6 ± 8.5 | 230.9 ± 9.1 | 228.4 ± 10.0 |
| 4 | 370.1 ± 20.9 | 366.2 ± 19.3 | 368.5 ± 15.8 | 236.4 ± 8.6 | 243.7 ± 10.5 | 240.6 ± 11.4 |
| Organ | Mean Weight (g ± SD) | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Normal | Extract (mg/kg) | Normal | Extract (mg/kg) | |||
| 1000 | 2000 | 1000 | 2000 | |||
| Heart | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.1 |
| Liver | 10.1 ± 1.0 | 9.9 ± 1.5 | 9.9 ± 1.0 | 6.6 ± 0.5 | 6.5 ± 0.7 | 6.6 ± 0.0 |
| Spleen | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.0 |
| Kidney | 1.2 ± 0.1 | 1.2 ±0.1 | 1.2 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.1 | 0.7 ± 0.0 |
| Testis | 1.9 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.2 | - | - | - |
| Uterus | - | - | - | 0.8 ± 0.1 | 0.7 ± 0.0 | 0. 8 ± 0.0 |
| Serum Parameter | Mean Weight (g ± SD) | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Normal | Extract (mg/kg) | Normal | Extract (mg/kg) | |||
| 1000 | 2000 | 1000 | 2000 | |||
| AST (U/L) | 66.3 ± 12.2 | 76.0 ± 8.5 | 72.2 ± 10.5 | 64.3 ± 10.8 | 60.5 ± 6.4 | 62.8 ± 5.5 |
| ALT (U/L) | 29.8 ± 3.4 | 25.0 ± 1.41 | 26.0 ± 4.2 | 24.7 ± 4.7 | 26.0 ± 2.8 | 24.0 ± 2.5 |
| GGT (U/L) | 5.2 ± 3.7 | 4.0 ± 1.4 | 4.5 ± 2.4 | 4.2 ± 3.1 | 3.0 ± 0.0 | 3.8 ± 0.0 |
| GLU (mmol/L) | 6.3 ± 0.85 | 6.1.0 ± 0.50 | 5.9 ± 2.01 | 5.8 ± 1.08 | 6.0.0 ± 0.75 | 5.9 ± 0.67 |
| BUN (mg/dL) | 24.1 ± 2.5 | 25.7 ± 1.9 | 24.2 ± 3.4 | 19.6 ± 7.5 | 22.5 ± 3.7 | 22.4 ± 3.0 |
| ALP (U/L) | 582.0 ± 20.1 | 652.0 ± 9.9 | 630.0 ± 4.8 | 293.2 ± 52.5 | 312.5 ± 54.5 | 318.5 ± 50.2 |
| CRE (mg/dL) | 0.21 ± 0.03 | 0.15 ± 0.07 | 0.18 ± 0.01 | 0.21 ± 0.03 | 0.20 ± 0.00 | 0.20 ± 0.01 |
| TP (g/dL) | 6.0 ± 0.23 | 6.2 ± 0.64 | 6.2 ± 0.52 | 6.0 ± 0.25 | 5.9 ± 0.07 | 6.0 ± 0.05 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hook, I.L. Danggui to angelica sinensis root: Are potential benefits to european women lost in translation? A review. J. Ethnopharmacol. 2014, 152, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.N.; Liu, E.S.; Li, Y.; So, H.L.; Cho, C.C.; Sheng, H.P.; Lee, S.S.; Cho, C.H. Protective effect of polysaccharides-enriched fraction from angelica sinensis on hepatic injury. Life Sci. 2001, 69, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Lin, C.M.; Chiang, B.H. Protective effects of angelica sinensis extract on amyloid beta-peptide-induced neurotoxicity. Phytomed. Int. J. Phytother. Phytopharmacol. 2008, 15, 710–721. [Google Scholar] [CrossRef]
- Wu, S.J.; Ng, L.T.; Lin, C.C. Antioxidant activities of some common ingredients of traditional chinese medicine, angelica sinensis, lycium barbarum and poria cocos. Phytother. Res. PTR 2004, 18, 1008–1012. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Y.S.; Liu, J.; Li, J.; Tan, Y.; Li, X.J.; Magdalou, J.; Mei, Q.B.; Wang, H.; Chen, L.B. Effect of angelica sinensis polysaccharides on osteoarthritis in vivo and in vitro: A possible mechanism to promote proteoglycans synthesis. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Lai, J.N.; Wu, C.T.; Wang, J.D. Prescription pattern of chinese herbal products for breast cancer in taiwan: A population-based study. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Chen, X.P.; Li, W.; Xiao, X.F.; Zhang, L.L.; Liu, C.X. Phytochemical and pharmacological studies on radix angelica sinensis. Chin. J. Nat. Med. 2013, 11, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [PubMed]
- Evans, D.B.; Bunning, R.A.; Russell, R.G. The effects of recombinant human interleukin-1 beta on cellular proliferation and the production of prostaglandin e2, plasminogen activator, osteocalcin and alkaline phosphatase by osteoblast-like cells derived from human bone. Biochem. Biophys. Res. Commun. 1990, 166, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Dewhirst, F.E.; Peros, W.J.; Kent, R.L.; Ago, J.M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J. Immunol. 1987, 138, 1464–1468. [Google Scholar] [PubMed]
- McLean, R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Hamer, A.J.; Rogers, A.; Stockley, I.; Eastell, R. Bone mineral density and biochemical markers of bone turnover in aseptic loosening after total hip arthroplasty. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2003, 21, 691–696. [Google Scholar] [CrossRef]
- Ma, Z.; Bai, L. Anti-inflammatory effects of Z-ligustilide nanoemulsion. Inflammation 2013, 36, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Wu, Q.; Su, Z.Y.; Wang, H.; Yang, Y.; Xu, X.; Huang, Y.; Khor, T.O.; Kong, A.N. Effects of natural phytochemicals in angelica sinensis (danggui) on nrf2-mediated gene expression of phase ii drug metabolizing enzymes and anti-inflammation. Biopharm. Drug Dispos. 2013, 34, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kong, X.; Zhou, H.; Liu, C.; Zhao, X.; Zhou, X.; Su, Y.; Sharma, H.S.; Feng, S. An experimental novel study: Angelica sinensis prevents epidural fibrosis in laminectomy rats via downregulation of hydroxyproline, IL-6, and TGF-β1. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Thiede, M.A.; Smock, S.L.; Petersen, D.N.; Grasser, W.A.; Thompson, D.D.; Nishimoto, S.K. Presence of messenger ribonucleic acid encoding osteocalcin, a marker of bone turnover, in bone marrow megakaryocytes and peripheral blood platelets. Endocrinology 1994, 135, 929–937. [Google Scholar] [PubMed]
- Coleman, R.E. The clinical use of bone resorption markers in patients with malignant bone disease. Cancer 2002, 94, 2521–2533. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Andersen, P.; Tanko, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.M.; Delaisse, J.M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res. Ther. 2004, 6, R169–R180. [Google Scholar] [CrossRef] [PubMed]
- Jee, W.S.; Yao, W. Overview: Animal models of osteopenia and osteoporosis. J. Musculoskelet. Neuronal Interact. 2001, 1, 193–207. [Google Scholar] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- Nishizawa, Y.; Nakatsuka, K. Gguideline for adequate use of metabolic bone markers in osteoporosis. Nihon Rinsho. Jpn. J. Clin. Med. 2004, 62 (Suppl. 2), S325–S332. [Google Scholar]
- Yogesh, H.S.; Chandrashekhar, V.M.; Katti, H.R.; Ganapaty, S.; Raghavendra, H.L.; Gowda, G.K.; Goplakhrishna, B. Anti-osteoporotic activity of aqueous-methanol extract of berberis aristata in ovariectomized rats. J. Ethnopharmacol. 2011, 134, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Kim, Y.T. Dried root of rehmannia glutinosa prevents bone loss in ovariectomized rats. Molecules 2013, 18, 5804–5813. [Google Scholar] [CrossRef] [PubMed]
- Hertrampf, T.; Schleipen, B.; Offermanns, C.; Velders, M.; Laudenbach, U.; Diel, P. Comparison of the bone protective effects of an isoflavone-rich diet with dietary and subcutaneous administrations of genistein in ovariectomized rats. Toxicol. Lett. 2009, 184, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Devareddy, L.; Khalil, D.A.; Smith, B.J.; Lucas, E.A.; Soung, D.Y.; Marlow, D.D.; Arjmandi, B.H. Soy moderately improves microstructural properties without affecting bone mass in an ovariectomized rat model of osteoporosis. Bone 2006, 38, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.C.; van Bezooijen, R.L.; Karperien, M.; Papapoulos, S.E.; Lowik, C.W.G.M. Exposure of ks483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J. Bone Miner. Res. 2002, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Joyner, J.M.; Hutley, L.J.; Cameron, D.P. Estrogen receptors in human preadipocytes. Endocrine 2001, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Korach, K.S. Oestrogen receptor knockout mice: Roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction 2003, 125, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clini. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bai, L. The anti-inflammatory effect of Z-ligustilide in experimental ovariectomized osteopenic rats. Inflammation 2012, 35, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.W.; Kim, Y.T. Anti-Osteoporotic Effects of Angelica sinensis (Oliv.) Diels Extract on Ovariectomized Rats and Its Oral Toxicity in Rats. Nutrients 2014, 6, 4362-4372. https://doi.org/10.3390/nu6104362
Lim DW, Kim YT. Anti-Osteoporotic Effects of Angelica sinensis (Oliv.) Diels Extract on Ovariectomized Rats and Its Oral Toxicity in Rats. Nutrients. 2014; 6(10):4362-4372. https://doi.org/10.3390/nu6104362
Chicago/Turabian StyleLim, Dong Wook, and Yun Tai Kim. 2014. "Anti-Osteoporotic Effects of Angelica sinensis (Oliv.) Diels Extract on Ovariectomized Rats and Its Oral Toxicity in Rats" Nutrients 6, no. 10: 4362-4372. https://doi.org/10.3390/nu6104362
APA StyleLim, D. W., & Kim, Y. T. (2014). Anti-Osteoporotic Effects of Angelica sinensis (Oliv.) Diels Extract on Ovariectomized Rats and Its Oral Toxicity in Rats. Nutrients, 6(10), 4362-4372. https://doi.org/10.3390/nu6104362





