Abstract
Obesity, a chronic low-grade inflammatory condition is associated with the development of many comorbidities including dyslipidemia. This review examines interactions between single nucleotide polymorphisms (SNP) in the inflammatory genes tumor necrosis alpha (TNFA) and interleukin-6 (IL-6) and dietary fatty acids, and their relationship with obesity and serum lipid levels. In summary, dietary fatty acids, in particular saturated fatty acids and the omega-3 and omega-6 polyunsaturated fatty acids, impact the expression of the cytokine genes TNFA and IL-6, and alter TNFα and IL-6 production. In addition, sequence variants in these genes have also been shown to alter their gene expression and plasma levels, and are associated with obesity, measures of adiposity and serum lipid concentrations. When interactions between dietary fatty acids and TNFA and IL-6 SNPs on obesity and serum lipid were analyzed, both the quantity and quality of dietary fatty acids modulated the relationship between TNFA and IL-6 SNPs on obesity and serum lipid profiles, thereby impacting the association between phenotype and genotype. Researching these diet–gene interactions more extensively, and understanding the role of ethnicity as a confounder in these relationships, may contribute to a better understanding of the inter-individual variability in the obese phenotype.
1. Introduction
Obesity, a chronic low-grade inflammatory condition, is associated with the development of many comorbidities, including cardiovascular disease (CVD), type 2 diabetes, and a number of cancers [1]. As more is understood about obesity, the complexity of this chronic disorder becomes more apparent, exhibiting a multi-factorial etiology [2]. Lifestyle factors such as diet and exercise continue to be recognized to play an important role in the development and progression of obesity and its comorbidities. However, genetic variation is also known to contribute to the obese phenotype. Lifestyle factors, including dietary components, such as fatty acids, interact with genetic variants to regulate the development and progression of obesity and its comorbidities. These complex interactions may explain differences observed in the obese phenotype and its comorbidities that vary both within and across populations [3,4,5,6].
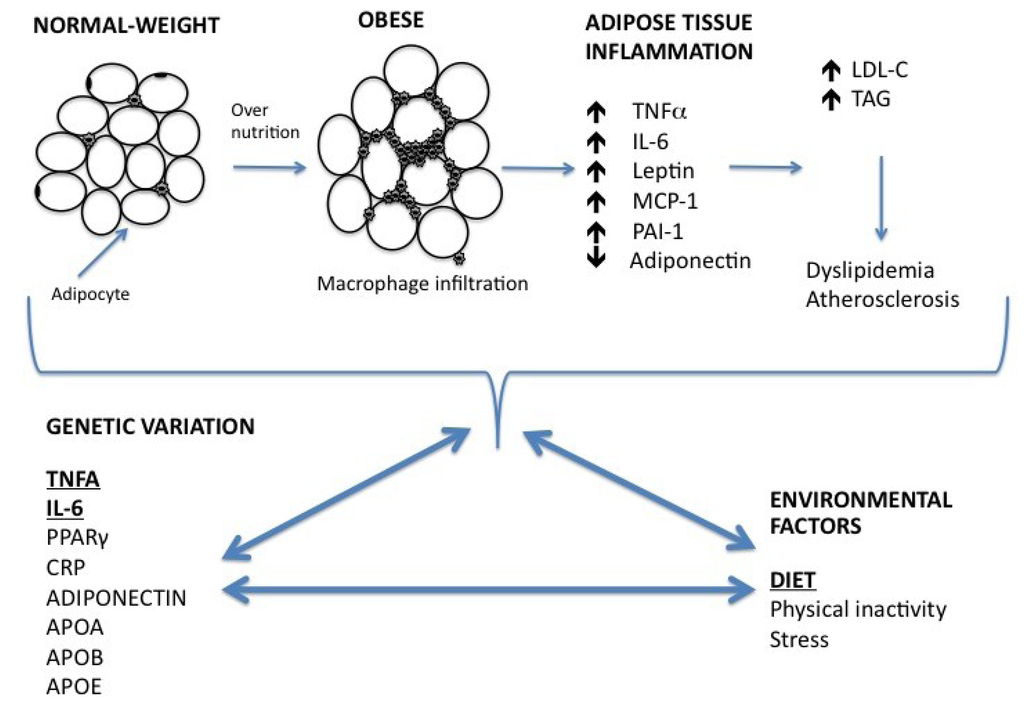
Figure 1.
A proposed schematic diagram for obesity-associated low-grade inflammation, and the relationship of diet–gene interactions on obesity and dyslipidemia. Adipocytes become hypertrophic through over-nutrition. Expansion of adipose tissue in obesity leads to a subsequent increase in the production of chemokines by the adipocytes, resulting in increasing macrophage infiltration and enhanced production of pro-inflammatory cytokines, such as TNFα and IL-6. Obesity-associated low-grade inflammation results in an increase in serum trigycerides, and LDL-C concentrations and is associated with dyslipidemia. Environmental factors and DNA sequence variations in inflammatory genes, interact to impact molecular processes of the inflammatory pathway, serum lipids and the obese phenotype. APOA, Apolipoprotein A; APOB, Apolipoprotein B; APOE, Apolipoprotein E; CRP, C-reactive protein; IL-6, interleukin-6; LDL-C, low-density lipoprotein cholesterol; MCP-1, monocyte chemotactic protein-1; PAI, plasminogen activated inhibitor; PPARγ, peroxisome proliferator-activated receptor gamma; TAG, triglycerides; TNFα, tumor necrosis factor.
The aim of this review is to illustrate the current state of knowledge regarding the interaction between dietary fatty acids and cytokines associated with obesity and serum lipids. Dietary fatty acids modulate the regulation and production of tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6), thereby influencing inflammatory status. Furthermore, emerging research suggests that diet–gene interactions play an important role in the regulation of these inflammatory cytokines, impacting the obese phenotype. This review focuses on interactions between single nucleotide polymorphisms (SNPs) in the inflammatory genes TNFA and IL-6, and dietary fatty acids, and their relationship with obesity and serum lipid levels as proof-of-principle examples. Figure 1 illustrates the development of obesity-associated low-grade inflammation and the impact of diet–gene interactions on obesity and dyslipidemia.
2. Relationship between Inflammation, Obesity and Lipid Metabolism
White adipose tissue constitutes the majority of adipose tissue (AT) [7] and can be divided into two fractions; the adipocyte fraction composed of mature adipocytes, and the stroma vascular fraction (SVF) composed of many other cell types, including preadipocytes, macrophages, endothelial cells and fibroblasts [8]. Although AT is considered a metabolically active endocrine organ, the macrophages present in the SVF are the primary source of obesity-induced inflammation [9,10,11]. The number of macrophages present in the SVF is directly correlated with the level of adiposity and adipocyte size [10,12]. Adipocyte hypertrophy, results in increased chemokine secretion e.g., transforming growth factor β1, soluble ICAM, and monocyte chemotactic protein-1 (MCP-1) [10]. There is a subsequent increase in the infiltration of macrophages, which in turn secrete cytokines such as IL-6 and TNFα. Since AT expansion is characterized by increased macrophage infiltration, these cells are responsible for almost all TNFα and significant amounts of IL-6 secreted by AT [10].
Low-grade chronic inflammation also mediates all stages of atherosclerosis from initiation through to the complications of thrombosis. So much so, that elevated inflammatory markers are used to define risk of atherosclerotic complications, independent of myocardial damage [13]. One of the proposed mechanisms, linking inflammatory processes to the development of atheroma, is the low-density lipoprotein (LDL) oxidation hypothesis [14]. LDL in the intima, the innermost layer of an artery or vein, undergoes oxidative modification, inducing expression of proinflammatory cytokines, chemokines and other mediators of inflammation. Very-low density lipoprotein (VLDL) and intermediate-density molecules are also believed to undergo oxidative modification and may themselves activate the inflammatory processes of the vascular endothelial cells [15] through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [16].
Genetic sequence variants—known as polymorphisms—within the promoter region of inflammatory genes influence gene transcription, altering protein production. Functional polymorphisms have been reported in the TNFA, IL-1, IL-6 and lymphotoxin-α (LTA) inflammatory genes, altering cytokine production [17,18,19,20]. These polymorphisms have been shown to interact with dietary fatty acids to regulate production and secretion of cytokines, predisposing an individual to inflammation and altering obesity and associated comorbidity risk [6,21].
In a recent review article by Phillips [22], it has been suggested that inconsistencies in previous studies on the influence of cytokine polymorphisms in the risk of obesity, diabetes and the metabolic syndrome (MetS), may be in part explained by the fact that cytokines act in a complex network, and that studying single genes may not provide full insight [22]. The recent LIPGENE-SU.VI.MAX MetS case control study reported by Phillips et al., examined the relationship between LTA, IL-6 and TNFA gene variants and MetS [23]. They reported that the G allele of the TNFA –308 G > A SNP and the minor A allele of the LTA rs915654 SNP were associated with a 20%–40% higher MetS risk [23], but the combined effect of carrying both risk genotypes further increased MetS risk. It was also shown that total plasma PUFA/SFA levels modified the observed additive genetic effects of IL-6, TNFA and LTA [23], highlighting the importance of studying nutrigenetic SNP-SNP or gene-gene interactions.
3. Dietary Fatty Acids and Inflammation
Dietary fatty acids have received considerable attention for their ability to regulate inflammatory gene expression and secretion. It has been proposed that dietary fatty acids affect inflammatory processes through the modulation of transcription factors such as NFκB and peroxisome proliferator-activated receptor gamma (PPARγ) [24,25]. PPARγ inhibits NFκB, and both transcription factors are sensitive to dietary fatty acids [26].
There is general agreement that increasing dietary SFA intake, especially in overweight or obese individuals, is associated with raised inflammatory markers [27], predominately by activating the toll-like receptor 4 (TLR4) pathway. The TLR4 pathway is expressed in both subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). SFAs serve as ligands for TLR4, inducing inflammatory changes in both adipocytes and macrophages through NFκB activation [28], increasing adipocytokine gene expression and production [29,30]. It has been shown that when adipocytes are exposed to the SFA, palmitic acid, IL-6 mRNA expression and protein production increased, most likely through activation of NFκB [31,32]. Similarly, monocytes were directly activated when exposed to SFA, especially lauric acid [30,33].
The n-6 PUFA, linoleic acid (LA), constitutes the majority of PUFA intake in the western diet. LA is the precursor of the n-6 PUFA arachidonic acid (AA). A high LA intake is considered proinflammatory, however the evidence to support this is contradictory and not conclusive [27,34,35,36]. AA intake in the diet is low relative to LA intake, its metabolic precursor. However, AA is the most prevalent n-6 PUFA in inflammatory cell membranes and is the substrate for the synthesis of the proinflammatory eicosanoids, including prostaglandin E2 (PGE2) and 4-series leukotrienes, associated with inflammatory processes [27]. Despite this, studies investigating the impact of AA on inflammatory markers are inconclusive, and few human intervention studies have reported on the effect of dietary intake of AA on low-grade inflammation [37,38,39].
The n-3 PUFA, alpha-linolenic acid (ALA) is an 18-carbon n-3 essential fatty acid common in canola, soybean oil and some nuts, but in greatest concentrations in flaxseed and flaxseed oil [40]. ALA is elongated and desaturated to eicosapentanoic acid (EPA) and further to decosahexanoic acid (DHA), however the efficiency of this conversion has been debated [41]. Association studies between dietary intake of ALA and inflammatory markers suggest a modest anti-inflammatory effect of ALA [27,34,36,42,43,44].
The long chain n-3 PUFAs, EPA and DHA are found in seafood, especially oily fish and in some algal oils. Is it proposed that n-3 PUFAs affect inflammation mainly through altered eicosanoid production, but potentially also impacting cell signaling and gene expression [27,42]. When EPA and DHA are incorporated into human inflammatory cells, this is partly at the expense of AA, providing less substrate for eicosanoid production [27]. Culture systems, animal models and human intervention studies have been generally consistent in recognizing the anti-inflammatory actions of n-3 PUFAs [24,25,42,45,46,47,48].
4. Tumor Necrosis Factor-α
TNFα was the first cytokine associated with inflammation [20]. TNFα is overexpressed in the AT of obese individuals, with greater expression in visceral fat than in subcutaneous fat [1,49]. TNFα acts in a paracrine manner, suggesting that circulating TNFα levels may not be indicative of actual TNFα levels [50]. Despite this caution, elevated circulating levels of TNFα have been observed in the obese phenotype, which decrease with weight loss [51]. It was originally proposed that adipocytes were the principle source of raised TNFα levels in obesity, however, it is now well recognized that TNFα is abundantly produced by macrophages in the SVF [1,10,49,50]. TNFα has numerous effects in AT including regulating adipogenesis, lipid metabolism and insulin signaling [6,11]. In addition to its ability to increase other pro-inflammatory cytokines [49,52], TNFα has also been associated with a reduction in anti-inflammatory adipokines such as adiponectin [53,54]. Conclusively, TNFα plays a powerful role in regulating inflammatory pathways, favoring an overall inflammatory state [6].
The influence of TNFα on lipid metabolism is complex with the underlying mechanisms still unclear. However, Chen et al. in his review describes four signal pathways involved in TNFα-mediated lipid metabolism [55]. The effects of TNFα’s on lipid metabolism occur in different cells, tissues, and organs and include a number of metabolic processes. TNFα induces lipolysis, increasing free fatty acid (FFA) production. In addition, TNFα regulates cholesterol metabolism and other adipocyte-derived adipokines such as leptin, adiponectin, etc. which may also alter lipid metabolism [55].
A number of studies, in cell culture, rodent and human clinical models have confirmed the relationship between TNFα and lipid metabolism [55]. In clinical patients with dyslipidemia, TNFα levels were altered. Compared with healthy subjects, patients with hyperlipoproteinemia showed higher TNFα levels and raised total cholesterol (T-C), triglycerides (TAG), and LDL-C concentrations. Furthermore, after treatment with fenofibrate, T-C, TAG and VLDL-C decreased, which correlated with decreasing TNFα concentrations [56]. It has also been shown that cholesterol-lowering drugs such as simvastatin and atorvastatin decrease TNFα concentrations [57,58], and that blockading TNFα production improves lipid metabolism [59]. Lastly, administration of TNFα interferes with plasma lipid levels [55,60,61]. Feingold et al. have shown that an increase in hepatic VLDL-TAG secretion induced by TNFα is due to both the stimulation of hepatic de novo fatty acid synthesis and an increase in lipolysis [60].
4.1. TNFα and Dietary Fatty Acids
Several studies have investigated the effect of dietary fatty acids on TNFα concentrations and TNFA gene expression in cell, animal and human models (Table 1). Plasma TNFα levels and TNFA gene expression increased in 3T3-L1 adipocytes incubated with the SFA palmitic acid, whereas incubation with the MUFA, oleic acid and n-3 PUFA, DHA had no effect [62]. In rodent studies, supplementing the diet with n-3 PUFA decreased TNFA gene expression in mice [63]. In another study, rats were fed a standard diet (18% energy from protein, 76% as carbohydrate (CHO) and 6% of energy as lipid) or a high-fat cafeteria diet (9% energy as protein, 29% as CHO and 62% as lipid). The high fat diet increased both body weight and fat mass, however when these rats were supplemented with EPA they gained less weight, decreased their food intake and increased leptin production. TNFA gene expression was also increased by the high fat diet, but not in the rats supplemented with EPA [64].
Similar results were reported in human studies; Caucasians supplemented with ALA in the form of flaxseed oil in domestic food preparation for four weeks, experienced a 30% reduction in TNFα production. When these subjects were exposed to further supplementation with fish oil (9 g/day) for an additional four weeks, TNFα production was reduced by up to 70%. There was a significant inverse exponential relation between TNFα synthesis and mononuclear cell content of EPA [65]. Similarly, Endres et al. [66] used a radioimmunoassay to measure TNFα produced in vitro by stimulated peripheral-blood mononuclear cells. They reported that supplementation with n-3 PUFA (18 g/day) for six weeks decreased TNFα levels, but these levels returned to baseline levels once supplementation was stopped [66]. However, Grimble et al. demonstrated that the change in TNFα production in response to n-3 PUFA supplementation depended on the subjects’ plasma TNFα levels prior to supplementation, with subjects with lower pre-supplementation TNFα levels showing the greatest decrease in TNFα levels post-supplementation [67]. It is possible that the inherent inflammatory status, potentially due to a pre-existing condition such as obesity, could determine the extent of the inflammatory response to different dietary fatty acids. In addition to the independent influence of dietary fatty acids on TNFα production, variation in the TNFA gene may also contribute to the individual variability observed in TNFα production and TNFA gene expression.
4.2. TNFA Gene Variants, Obesity and Serum Lipids
Several SNPs have been identified in the promoter region of the TNFA gene, however the TNFA –308 G > A (rs1800629) and –238 G > A (rs361525) SNPs are most commonly associated with measures of adiposity, obesity risk and serum lipids (Table 2). The A allele of the functional –308 G > A SNP results in a 2-fold increase in TNFA transcription, with a subsequent increase in TNFα production [20]. Several studies have reported that carriers of the pro-inflammatory –308 A allele (AA and GA genotypes) reported a higher body mass index (BMI) and/or percent body fat than those with the GG genotype [68,69,70,71,72,73]. In a recent meta-analysis by Yu et al., including 48 eligible studies, the –308 GA + AA genotypes were associated with an increased risk of obesity (OR, 1.19; 95% CI, 1.02–1.39) [21]. This result was consistent with the study by Sookoian et al., who performed a meta-analysis of 31 observational studies with a total of 3562 individuals and showed that individuals with the GA + AA genotypes had a 23% elevated risk of obesity compared with the GG genotype (OR, 1.23; 95% CI, 1.05–1.45) [74]. In comparison to the –308 G > A SNP, only a few studies have investigated the association between the –238 G > A SNP and obesity (Table 2). In our laboratory it was found that black South African women with the –238 A allele had greater body fat % than those with the GG genotype [5]. To our knowledge only two papers have found an independent association between the –308 G > A SNP and serum lipid concentrations. In Caucasian men, the –308 A allele was associated with increased TAG [75], and in Polish Caucasian men and women the AA genotype was associated with lower high-density lipoprotein cholesterol (HDL-C) concentrations compared to the GG genotype [76].

Table 1.
Dietary fatty acids and TNFα and IL-6 production.
| Study type | Dietary fatty acid | Effect on gene expression | Effect on plasma levels | Reference | |
|---|---|---|---|---|---|
| TNFα | |||||
| 3T3-L1 adipocytes. Incubation at 24 and 48 h with 50 or 500 μM fatty acid | Cell culture | SFA (PA) | Increase | Increase | [62] |
| MUFA (OA) | No effect | No effect | |||
| Human macrophages treated with n-3 PUFA | Cell culture | EPA & DHA | Decrease | Decrease | [77] |
| Male Wistar rats, high fat diet, 1 g/kg per day EPA, 5 weeks | Rodent | n-3 PUFA (EPA) | Prevent over expression | Not examined | [64] |
| NZB/NZW F1 Lupus-prone female mice, 10% fat, fed ad lib for lifespan | Rodent | n-3 PUFA | Decrease | Not examined | [63] |
| Caucasians. Supplemented normal diet with 18 g fish oil daily for 6 weeks | Human intervention (9) | n-3 PUFA | Decrease | [66] | |
| Caucasians. Supplemented normal diet with 6 g fish oil daily for 12 weeks | Human intervention (111) | n-3 PUFA | Decrease in subjects with lower levels of TNFα before supplementation | [67] | |
| Caucasians. Supplemented normal diet with flaxseed oil, and flaxseed oil and butter spread for 8 weeks. At week 4, diets were supplemented with fish oil, (1.62 g EPA, 1.08 g DHA)/day | Human intervention (28) | n-3 PUFA (EPA & DHA) | Decrease | [65] | |
| IL-6 | |||||
| 3T3-L1 adipocytes. Incubation at 24 h with 250 μM fatty acid | Cell culture | SFA (PA) | Increase | Increase | [31] |
| SFA (DA) | No effect | No effect | |||
| n-3 PUFA (DHA) | No effect | No effect | |||
| Human macrophages treated with n-3 PUFA | Cell culture | EPA & DHA | Decrease | Decrease | [77] |
| Male c57bl/10sCn mice fed ad lib either high fat control diet (soybean oil) or a high PA diet for 16 weeks | Rodent | SFA (PA) | Increase | Not examined | [78] |
| Male Sprague-Dawley rats fed ad lib one of 3 diets: SFA, MUFA, or PUFA for 4 weeks | Rodent | SFA (coconut oil) | Not examined | Increase IL-6 release from adipocytes | [79] |
| MUFA (olive oil) | Not examined | Decrease IL-6 release from adipocytes | |||
| PUFA (sunflower oil) | Not examined | Moderate IL-6 release from adipocytes | |||
| Abdominally overweight Caucasians. Fed either SFA-rich diet (19% SFA and 11% MUFA) or MUFA-rich diet (20% MUFA and 11% SFA) for 8 weeks | Human intervention (20) | SFA | Increase IL-6 gene expression | [80] | |
| MUFA | Decrease IL-6 gene expression | ||||
| African Americans, Caucasians, Chinese and Hispanics men and women. Relationship between dietary intake (food frequency questionnaire) and biomarkers of inflammation and endothelial activation | Human (5677) | n-3 PUFA | Decrease in IL-6 levels | [81] | |
| Relationship between plasma fatty acids and inflammatory marker levels | Human (1123) | n-3 PUFA (DHA) | Low plasma levels of DHA associated with increased IL-6 levels | [82] | |
| n-6 PUFA (AA) | Low plasma levels of AA associated with increased IL-6 levels | ||||
The number of subjects (n) is in parentheses. IL, interleukin; SFA, saturated fatty acid; TNFA, tumor necrosis factor alpha; saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; (n-3) PUFA, omega-3 polyunsaturated fatty acid; (n-6) PUFA, ALA, α-linolenic acid; LA, linoleic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. AA, arachidonic acid; DA, lauric acid.

Table 2.
Studies investigating associations between TNFA and IL-6 single nucleotide polymorphisms and obesity and serum lipids.
| SNP | Study cohort | Genotype frequency | Result | Reference |
|---|---|---|---|---|
| TNFA | ||||
| Obesity | ||||
| TNFA –308 G > A | Caucasian N-W (154) and obese (154) | N-W, GG: 75.8%; GA + AA: 24.2% Obese, GG: 70.8%; GA + AA: 29.2% |
| [71] |
| Caucasian women (378) | GG: 72.2%; GA + AA: 27.7% |
| [70] | |
| Caucasian BMI < 27.3 (44), BMI 27.3–31.9 (44), BMI 31.9–36.5 (44) and BMI > 36.5 (44) | BMI < 27.3, GG: 75%; GA + AA: 25% BMI 27.3–31.9, GG: 68.2%; GA + AA: 31.8% BMI 31.9–36.5, GG: 77%; GA + AA: 33% BMI > 36.5, GG: 47.7%; GA + AA: 52.3% |
| [68] | |
| Caucasian (1392) | GG: 67.6%; GA + AA: 32.3% |
| [69] | |
| Caucasian normal wt. (79) and obese (115) | N-W, GG: 73.4%; GA + AA: 24.2% Obese, GG: 75.6%; GA + AA: 26% |
| [83] | |
| Korean normal wt. (82) and obese (153) | N-W, GG: 82.9%; GA + AA: 17% Obese, GG: 84.3%; GA + AA: 15.7% |
| [84] | |
| Caucasian normotensive (113) and hypertensive (62) | Normotensive, GG: 84.8%; GA + AA: 15% Hypertensive, GG: 67.7%; GA + AA: 32.3% |
| [74] | |
| Caucasian normal weight (64) and overweight (65) | Not shown |
| [72] | |
| Caucasian men (262) | GG: 56.4%; GA + AA: 43.5% |
| [73] | |
| Meta-analysis (48 eligible studies) |
| [21] | ||
| TNFA –238 G > A | N-W (107) and obese (120) black, and N-W (89) and obese (62) white SA women | Black, GG: 60%; GA: 40%; AA: 0%; A allele: 20% White, GG: 78.5%; GA: 21%; AA: 0.5%; A allele: 11% |
| [5] |
| TNFA –308 G > A & –238 G > A | Iranian men and women (BMI < 25, 25 ≤ BMI < 30, BMI ≥ 30) (239) | Under 18 years, BMI < 85%, GG: 80.8%; GA: 19.2% BMI > 85%, GG: 81.2%; GA: 12.5%; AA: 6.2%. Above 18 years, BMI < 25, GG: 89.3%; GA: 10.7% 25 ≤ BMI < 30, GG: 87.7%; GA: 12.3% BMI ≥ 30, GG: 80.4%; 15.2%; 4.3% |
| [85] |
| Caucasian and African-American non-diabetics (424) | –308 G > A BMI < 25, GG: 73.2%; GA: 22.5%, AA: 4.3% BMI 25–29.9, GG: 66.7%; GA: 27%, AA: 6.3% BMI 30–40, GG: 67.2%; GA: 30.2%; AA: 2.6% BMI > 40, GG: 73.4%; GA: 24.3%; AA: 2.4% –238 G > A BMI < 25, GG: 90.4%; GA: 9.1%, AA: 0.5% BMI 25–29.9, GG: 90.5%; GA: 7.9%, AA: 1.6% BMI 30–40, GG: 85.9%; GA: 14.1% BMI > 40, GG: 91.1%; GA: 8.9% |
| [86] | |
| Serum lipids | ||||
| TNFA –308 G > A | Caucasian obese women (136) and obese men (34) | Obese women, GG: 57.3%; GA + AA: 42.6% Obese men, GG: 64.7%; GA + AA: 35.3% |
| [75] |
| Caucasian obese men (38) and obese women (83) | Obese men, GG: 50%; GA + AA: 50% Obese women, GG: 54.2%; GA + AA: 45.7% |
| [76] | |
| IL-6 | ||||
| Obesity | ||||
| IL-6 –174 G > C | Finnish men and women (1334) | GG: 19.3%; GC: 51.3%; CC: 29.3% | In men BMI was higher in the –174 CC genotype compared to GC and GG | [87] |
| Meta-analysis (48 eligible studies) |
| [21] | ||
| Meta-analysis Caucasians, diabetic and non-diabetic (25635) |
| [88] | ||
| IL-6 –174 G > C & IVS3 +281 G > T and IVS4 +869 A > G | Health men (980) and women (2255) and Meta-analysis (26944) |
| [89] | |
| IL-6 –174 G > C IVS3 +281 G > T & IVS4 +869 A > G | N-W (108) and obese (124) black, and N-W (89) and obese (63) white SA women | -174 G > C N-W black: GG: 97%; GC: 3% Obese black: GG: 95%; GC: 3%; CC: 2% N-W white: GG: 30%; GC: 58%; CC: 11% Obese white: GG: 32%; GC: 46%; CC: 22% IVS3 +281 G > T N-W black: GG: 54%; GT: 38%; TT: 8% Obese black: GG: 55%; GT: 37%; TT:9% N-W white: GG: 35%; GT: 48%; TT: 17% Obese white: GG: 30%; GT: 51%; TT: 19% IVS4 +869 A > G N-W black: AA: 51%; AG: 39%; GG: 10% Obese black: AA: 54%; AG: 40%; GG: 6% N-W white: AA: 42%; AG: 57%; GG: 1% Obese white: AA: 37%; AG: 63% |
| [90] |
| Serum lipids | ||||
| IL-6 –174 G > C | Caucasian men (245) and women (252) | Women, GG: 28%; GC: 47%; CC: 24% Men, GG: 30%; GC: 46%; CC: 24% |
| [91] |
| Finnish men and women (1334) | GG: 19.3%; GC: 51.3%; CC: 29.3% |
| [87] | |
| Spanish Caucasian men (15) and women (17) | GG: 25%; GC: 40.6%; CC: 34.4% |
| [92] | |
| Finnish men and women (2228) | GG: 20.8%; GC: 50.4%; CC: 28.8% |
| [93] | |
| IL-6 -174 G > C, IVS3 +281 G > T & IVS4 +869 A > G | N-W (108) and obese (124) black, and N-W (89) and obese (63) white SA women | –174 G > C N-W black: GG: 97%; GC: 3% Obese black: GG: 95%; GC: 3%; CC: 2% N-W white: GG: 30%; GC: 58%; CC: 11% Obese white: GG: 32%; GC: 46%; CC: 22% IVS3 +281 G > T N-W black: GG: 54%; GT: 38%; TT: 8% Obese black: GG: 55%; GT: 37%; TT:9% N-W white: GG: 35%; GT: 48%; TT: 17% Obese white: GG: 30%; GT: 51%; TT: 19% IVS4 +869 A > G N-W black: AA: 51%; AG: 39%; GG: 10% Obese black: AA: 54%; AG: 40%; GG: 6% N-W white: AA: 42%; AG: 57%; GG: 1% Obese white: AA: 37%; AG: 63% |
| [90] |
Genotype frequency is expressed as a percentage. The number of subjects (n) is in parentheses. IL, interleukin; SFA, TNFA, tumor necrosis factor alpha; N-W; Normal-weight; WHR, waist-hip ratio; TAG, triglycerides; T-C, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; T-C:HDL-C ratio, total cholesterol: high-density lipoprotein cholesterol ratio.
Not all studies have however shown an association between the –308 G > A and –238 G > A SNPs and obesity [3,4,74,83,84,85,86]. Further, to our knowledge, no independent associations have been reported between the –238 G > A SNP and serum lipid concentrations. While the A allele of the –308 G > A and –238 G > A SNPs appear to be associated with the obese phenotype and serum lipid concentrations, it is highly likely that genetic variation in the TNFA gene may provide only a partial explanation with regards the inter-individual variability observed and the heterogeneity of the results in these studies. Other variables such as ethnicity, gender, diet, lifestyle and environmental factors may modulate these associations and contribute to the different results observed.
4.3. TNFA Gene and Diet Interactions on Obesity and Serum Lipids
The TNFA –308 G > A and –238 G > A SNPs have been shown to modulate the relationship between dietary fat intake on obesity and serum lipid profiles in different populations (Table 3). Despite the many studies showing independent association between the TNFA SNPs and obesity (Table 2), to our knowledge only two studies have investigated diet–gene interactions. Nieters et al. found that German Caucasian men and women with the –308 A allele, who were in the highest tertile for intake of the n-6 PUFAs LA and AA (%E), had an increased obesity risk [71]. More recently, we have reported that the odds of obesity for black South African (SA) women with the –308 A allele increased with total dietary fat intake (%E) [3] (Figure 2). This interaction was not observed in white SA women [4].
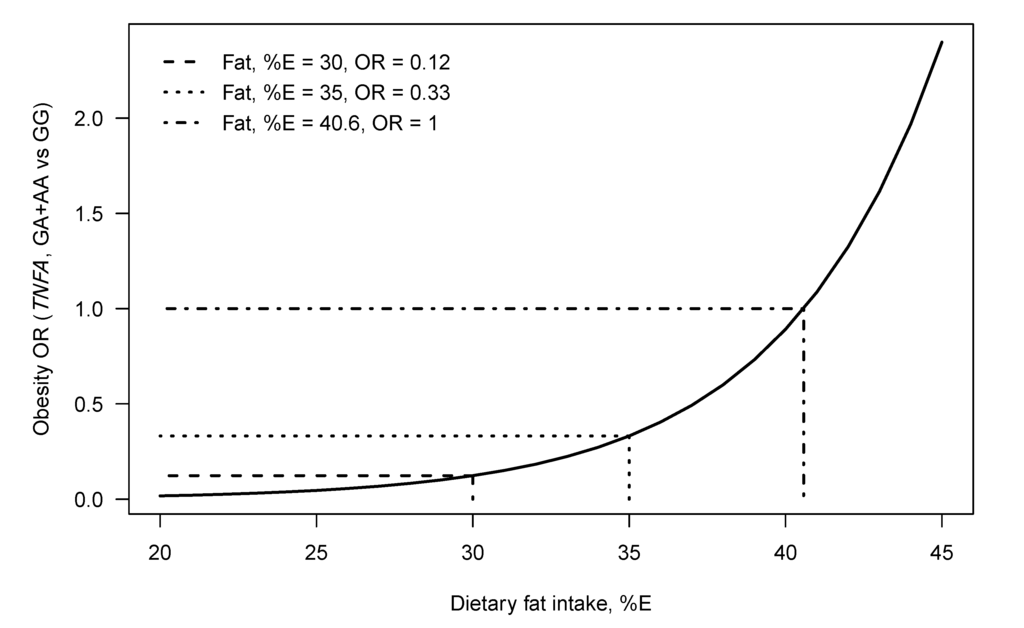
Figure 2.
The modeled relationship between the odds of being obese (odds of being obese vs. being normal weight), TNFA–308 genotype and dietary fat intake (%E) for black SA women. The curve gives the modeled obesity OR for genotype GA+AA versus genotype GG, at each fat intake (%E). Lines show the total dietary fat intake (%E) of equal odds (OR = 1, for the genotype groups), namely 40.6 (%E), the OR for fat intake = 30 (%E) namely 0.12 and the OR for fat intake = 35 (%E), namely 0.33 [3].

Table 3.
Diet–gene interactions between TNFA and IL-6 single nucleotide polymorphisms and dietary fat intake on obesity and serum lipids.
| SNP | Study cohort | Genotype frequency | Diet assessment and fats | Diet-gene association | Reference |
|---|---|---|---|---|---|
| TNFA | |||||
| Obesity | |||||
| TNFA –308 G > A | Caucasian N-W (154) and obese (154) | Normal weight, GG: 75.8%; GA + AA: 24.2% Obese, GG: 70.8%; GA + AA: 29.2% | Food frequency questionnaire measured energy and dietary fatty acid intake. |
| [71] |
| Black South African N-W (105) and obese (118) | Black, GG: 69%; GA: 28%; AA: 3%; A allele: 17% | Food frequency questionnaire measured dietary fatty acid intake. |
| [3] | |
| TNFA –238 G > A | N-W (107) and obese (120) black, and N-W (89) and obese (62) white SA women | Black, GG: 60%; GA: 40%. A allele: 20% White, GG: 78.5%; GA: 21%; AA: 0.5%; A allele: 11% | Food frequency questionnaire measured dietary fatty acid intake. | In black women:
| [5] |
| Serum lipids | |||||
| TNFA –308 G > A | Black South African N-W (105) and obese (118) | Black, GG: 69%; GA: 28%; AA: 3%; A allele: 17% | Food frequency questionnaire measured dietary fatty acid intake. |
| [3] |
| Caucasian South African N-W (88) and obese (60) white SA women | White, GG: 56%; GA: 42%; AA: 2%; A allele: 23% | Food frequency questionnaire measured dietary fatty acid intake. |
| [4] | |
| TNFA –238 G > A | N-W (107) and obese (120) black, and N-W (89) and obese (62) white SA women | Black, GG: 60%; GA: 40%. A allele: 20% White, GG: 78.5%; GA: 21%; AA: 0.5%; A allele: 11% | Food frequency questionnaire measured dietary fatty acid intake. |
| [5] |
| TNFA –308 G > A & –238 G > A | Ethnoracially diverse Canadian diabetic men (53) and women (56) | –308 G > A GG: 63.3%; GA: 32%; AA: 0.05% –238 G > A GG: 75.2%; GA: 21.1%; AA: 0.04% | Three-day food record measured dietary fat intake. |
| [94] |
| TNFA –308 G > A & –238 G > A | Ethnoracially diverse Canadian healthy men (202) and women (393) | –308 G > A, 11% for A allele –238 G > A, 5% for A allele | Food frequency questionnaire measured dietary intake. |
| [95] |
| IL-6 | |||||
| Obesity | |||||
| IL-6 –174 G > C | Obese Caucasians men (181) and women (541) | GG: 28.8%; GC: 50.2%, CC: 18%. | Test meal consisted of 95%E from fat, of which 60% SFA. |
| [96] |
| 737 Spanish men and women | GG: 37.6%; GC: 46.8%; CC: 15.6% | Three years diet intervention assigned to low-fat diet; Mediterranean diet supplemented with virgin olive oil or with nuts. |
| [97] | |
| IL-6 –174 G > C, IVS3 +281 G > T & IVS4 +869 G > A | N-W (107) and obese (120) black, and N-W (89) and obese (62) white SA women | –174 G > C N-W black: GG: 97%; GC: 3% Obese black: GG: 95%; GC: 3%; CC: 2% N-W white: GG: 30%; GC: 58%; CC: 11% Obese white: GG: 32%; GC: 46%; CC: 22% IVS3 +281 G > T N-W black: GG: 54%; GT: 38%; TT: 8% Obese black: GG: 55%; GT: 37%; TT:9% N-W white: GG: 35%; GT: 48%; TT: 17% Obese white: GG: 30%; GT: 51%; TT: 19% IVS4 +869 A > G N-W black: AA: 51%; AG: 39%; GG: 10% Obese black: AA: 54%; AG: 40%; GG: 6% N-W white: AA: 42%; AG: 57%; GG: 1% Obese white: AA: 37%; AG: 63% | Food frequency questionnaire measured dietary fatty acid intake. |
| [98] |
| Serum lipids | |||||
| IL-6 –174 G > C | Spanish Caucasian men and women (32) | GG: 25%; GC: 40.6%; CC: 34.4% | Measured fasting and post-glucose load plasma lipids. |
| [92] |
| IL-6 –174 G > C, IVS3 +281 G > T & IVS4 +869 G > A | N-W (107) and obese (120) black, and N-W (89) and obese (62) white SA women | –174 G > C N-W black: GG: 97%; GC: 3% Obese black: GG: 95%; GC: 3%; CC: 2% N-W white: GG: 30%; GC: 58%; CC: 11% Obese white: GG: 32%; GC: 46%; CC: 22% IVS3 +281 G > T N-W black: GG: 54%; GT: 38%; TT: 8% Obese black: GG: 55%; GT: 37%; TT:9% N-W white: GG: 35%; GT: 48%; TT: 17% Obese white: GG: 30%; GT: 51%; TT: 19% IVS4 +869 A > G N-W black: AA: 51%; AG: 39%; GG: 10% Obese black: AA: 54%; AG: 40%; GG: 6% N-W white: AA: 42%; AG: 57%; GG: 1% Obese white: AA: 37%; AG: 63% | Food frequency questionnaire measured dietary fatty acid intake. |
| [98] |
Genotype frequency is expressed as a percentage. The number of subjects (n) is in parentheses. IL, interleukin; SFA, TNAF, tumor necrosis factor alpha; N-W; Normal-weight; TAG, triglycerides; T-C, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; T-C:HDL-C ratio, total cholesterol: high density lipoprotein cholesterol ratio, SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; P:S ratio, polyunsaturated fatty acid : saturated fatty acid ratio; (n-3) PUFA, omega-3 polyunsaturated fatty acid; (n-6) PUFA, omega-6 polyunsaturated fatty acid; (n-6):(n-3) PUFA ratio, omega-6:omega-3 polyunsaturated fatty acid ratio; ALA, α-linolenic acid; LA, linoleic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Only one study has reported a diet–gene interaction between the –238 G > A SNP, dietary intake and obesity (Table 3). We found that in black SA women, with increasing total fat, SFA and MUFA intake (%E), weight increased in those with the –238 GA genotype, but not the GG genotype [5]. In contrast, a number of studies have shown interactions between the –308 G > A and –238 G > A SNPs and dietary fat intake on serum lipid profiles (Table 3). In ethnically diverse diabetic Canadians, Fontaine-Bisson et al. reported that PUFA intake was inversely associated with HDL-C concentrations in –308 A allele carriers, but positively associated with HDL-C concentrations in –238 A allele carriers [94]. In a combined analysis in healthy non-diabetic Canadians, they also reported that in individuals with the –308 GA + AA and –238 GG genotypes, an inverse relationship was observed between HDL-C concentrations and n-3, n-6 and total PUFA intake [95].
More recently, we reported interactions between dietary fat intake and the TNFA –308 G > A and –238 G > A SNPs on serum lipid profiles in black and white SA women. With increasing dietary fat intake, serum lipids increased in black women with the –308 GA + AA genotypes; however, with increasing n-3 PUFA and ALA intake, total cholesterol:HDL-cholesterol ratio (T-C:HDL-C ratio) decreased in black SA women [3,4]. For the –238 G > A SNP, in black SA women with increasing polyunsaturated:saturated fat ratio and n-6:n-3 ratio, HDL-C concentrations decreased, and T-C:HDL-C ratio increased in those with the –238 GA genotype but not the GG genotype. However, with increasing n-3 PUFA, T-C:HDL-C ratio decreased in those with the –238 GA genotype, but not in those with the GG genotype. In white SA women, with increasing EPA intake, LDL-C concentrations decreased in those with the –238 GG genotype but not the GA genotype [5]. Interactions between the –238 G > A SNP and dietary fat intake on serum lipids differed depending on the type of dietary fatty acid consumed, and were not the same in black and white SA women suggesting that ethnicity as well as diet–gene interactions contribute to the complexity and heterogeneity observed within serum lipid profiles.
5. Interleukin-6
During an acute incident, the cytokine IL-6 acts as an anti-inflammatory cytokine, but in a chronic inflammatory condition, IL-6 is pro-inflammatory, as well as being a key regulator of hepatic C-reactive protein (CRP) production [99,100]. IL-6 is secreted by a variety of cells. However approximately 30% of total IL-6 is produced by AT and macrophages that have infiltrated WAT produce approximately 50% of AT derived IL-6 [99,100,101]. It is therefore not surprising that higher circulating concentrations of IL-6 have been associated with obesity, especially visceral fat deposition [102,103,104], and decrease in response to weight loss [102,105].
IL-6 is associated with lipid metabolism and plays a role in the development of atherosclerosis through a number of different mechanisms causing metabolic and endothelial dysfunction. [13,101]. IL-6 impairs insulin action, elevating lipolysis, and increasing FFA release [106]. The increase in FFAs reduces nitric oxide (NO) bioavailability and impairs vasodilation [107]. Insulin resistance and subsequent hyperglycemia also increases lipoprotein oxidation and subsequent increased expression of adipocytokines [108]. In rats administrated IL-6, serum TAG, T-C levels and hepatic TAG secretion increased [109]. Similarly, in human studies, increased IL-6 levels have been associated with an increase in serum TAG and FFAs, and low HDL-C concentrations [110,111].
5.1. IL-6 and Dietary Fatty Acids
Several studies have investigated the effect of different dietary fatty acids on IL-6 production and IL-6 gene expression in cell, animal and human models, with all studies showing similar results (Table 1). An in vitro study reported that IL-6 plasma levels and IL-6 gene expression increased when 3T3-L1 adipocytes were incubated with the SFA, palmitic acid, whereas no effect was observed for lauric acid (C12:0) and the n-3 PUFA, DHA [31]. Similarly, macrophages treated with EPA or DHA showed a decrease in LPS stimulated IL-6 mRNA and IL-6 production. In agreement with these findings, rats fed a diet high in n-6 PUFA-rich sunflower oil showed moderate IL-6 release from adipocytes, which was less than when fed a SFA-rich diet, but greater than when fed a MUFA-rich diet [79]. In addition, in mice fed a low fat diet or one of two high-fat diets, consisting of either unsaturated soybean oil or saturated palmitic acid for 16 weeks, MCP-1 expression in AT tissue increased for both high-fat diets compared to the low-fat diet. However, the high saturated fat diet also showed a three-fold increase in IL-6 expression in AT not observed in the soybean oil diet [78].
In human studies (Table 1), increased long-chain n-3 PUFA intake and fish consumption were associated with decreased plasma IL-6 concentrations and other inflammatory markers (matrix metalloproteinase-3, CRP, soluble intercellular adhesion molecule-1) in men from the Multi-Ethnic Study of Atherosclerosis cohort [81]. Furthermore, Ferrucci et al. reported that plasma levels of PUFAs, especially n-3 PUFAs, were independently associated with lower levels of pro-inflammatory markers and higher levels of anti-inflammatory markers [82]. A controlled feeding trial investigating the effects of SFA and MUFA-rich diets on serum lipid concentrations and whole-genome microarray gene expression profiles of AT, found that consuming a SFA-enriched diet for eight weeks resulted in increased expression of genes involved in inflammatory processes in AT including IL-6, and NFκB signaling, whereas the MUFA-enriched diet led to a more anti-inflammatory gene expression profile, accompanied by a decrease in serum LDL-C concentration [80].
5.2. IL-6 Gene Variants, Obesity and Serum Lipids
There is growing scientific evidence reporting associations between DNA sequence variants within the IL-6 gene and increased risk of obesity and dyslipidemia (Table 2) [88,91,99,101,112,113]. The most frequently studied IL-6 SNP is –174 G > C (rs1800795). This is a functional SNP, with most studies showing the –174 C allele to be associated with raised IL-6 and CRP concentrations in mostly Caucasian populations [21,91,114,115]. This SNP is rare in individuals of African ancestry, is not informative, and has therefore not been studied in these populations. Association studies between the –174 G > C SNP, obesity and dyslipidemia have yielded conflicting results. A recent meta-analysis found the –174 G > C SNP was associated with obesity [21]. However, this finding was not reproduced in two other meta-analyses [88,89]. The relationship between the –174 G > C SNP appears to be complex in that while the –174 C allele appears to be the risk allele associated with obesity [21], it is the –174 G allele that is associated with raised serum lipids concentrations [87,92,93], despite the –174 C allele being shown to be associated with raised IL-6 and CRP levels [21,91,114,115]. The lack of consistent results in these association studies may be due to interactions between multiple SNPs on the IL-6 gene that may modulate these relationships. This was illustrated by Qi et al., who found no independent association between the –174 G > C SNP and obesity, but did identify an association between an IL-6 haplotype containing the –174 G > C SNP and adiposity in healthy American men and women [89].
A number of studies have reported associations between the –174 G > C SNP and serum lipid profiles (Table 2). Though not consistent for all the studies [93], for most, in Caucasian men and women the –174 G allele was associated with higher T-C, LDL-C, VLDL-C and TAG concentrations compared to the C allele [88,92,93]. Riikola et al. suggested several possible reasons that may explain the inconclusiveness of study results [87]. These include differences in body mass and body composition, as well as metabolic and pharmacological interference in the different study cohorts. They also suggested that the effect of IL-6 on serum lipids may differ depending on the phase of development of atherosclerosis. Age may also impact these associations as subtle allelic effects observable in young populations may be masked by stronger life-long diet and lifestyle covariates in older populations [88].
Preliminary results from our laboratory found no independent associations between the –174 G > C SNP and obesity or serum lipid profiles in white SA women. However, we did find associations between the less researched SNPs IL-6 IVS4 +869 A > G (rs2069845) and IVS3 +281 G > T (rs1554606) and obesity and serum lipid profiles in black and white SA women. The IVS4 +869 G allele was associated with greater waist and fat mass in black SA women, and the IVS3 +281 T allele was associated with lower TAG concentrations in white SA women compared to those with the GG genotype [90] (Table 2).
5.3. IL-6 Gene and Diet Interactions on Obesity and Serum Lipids
As previously described, studies have shown that dietary fat intake modulates the relationship between TNFA gene variants and obesity and serum lipid profiles (Table 3). However, to our knowledge, only two published studies have reported on the relationship between any IL-6 SNP and dietary intake, and both studies investigated IL-6 –174 G > C. Corpeleijn et al. reported that the ability to increase fat oxidation after a high fat load was increased in obese European Caucasians with the –174 C allele [96]. In Spanish men and women with a high CVD risk, the –174 CC genotype was associated with higher levels of adiposity at baseline, however after three years of nutritional intervention, those with the –174 CC genotype following a Mediterranean-style diet, had the greatest reduction in body weight [97].
To our knowledge only a single study has reported an interaction between the –174 G > C SNP and serum lipids levels. In this study, those individuals with the G allele had higher post-glucose load TAG and VLDL-C concentrations, and higher post-glucose load FFA levels than C allele carriers [92].
Preliminary results from our laboratory have recently identified a number of interactions between three IL-6 SNPs (IL-6 –174 G > C, IVS3 +281 G > T, and IVS4 +869 A > G) and dietary fatty acids on obesity and serum lipid levels in both black and white SA women [98] (Table 3). In white SA women, with increasing n-3 PUFA intake and decreasing n-6:n-3 ratio, BMI decreased in those with the –174 C allele, IVS3 +281 T allele and IVS4 +869 AG genotype. In black SA women, with increasing dietary fat intake, adiposity decreased in those with the IVS3 +281 TT and IVS4 +869 GG genotypes and increased in the IVS3 +281 GT + GG and IVS4 +869 AA or AG genotypes (Figure 3). This figure illustrates that the type of fatty acid consumed in the diet interacts with IL-6 genotypes to modulate measures of obesity.
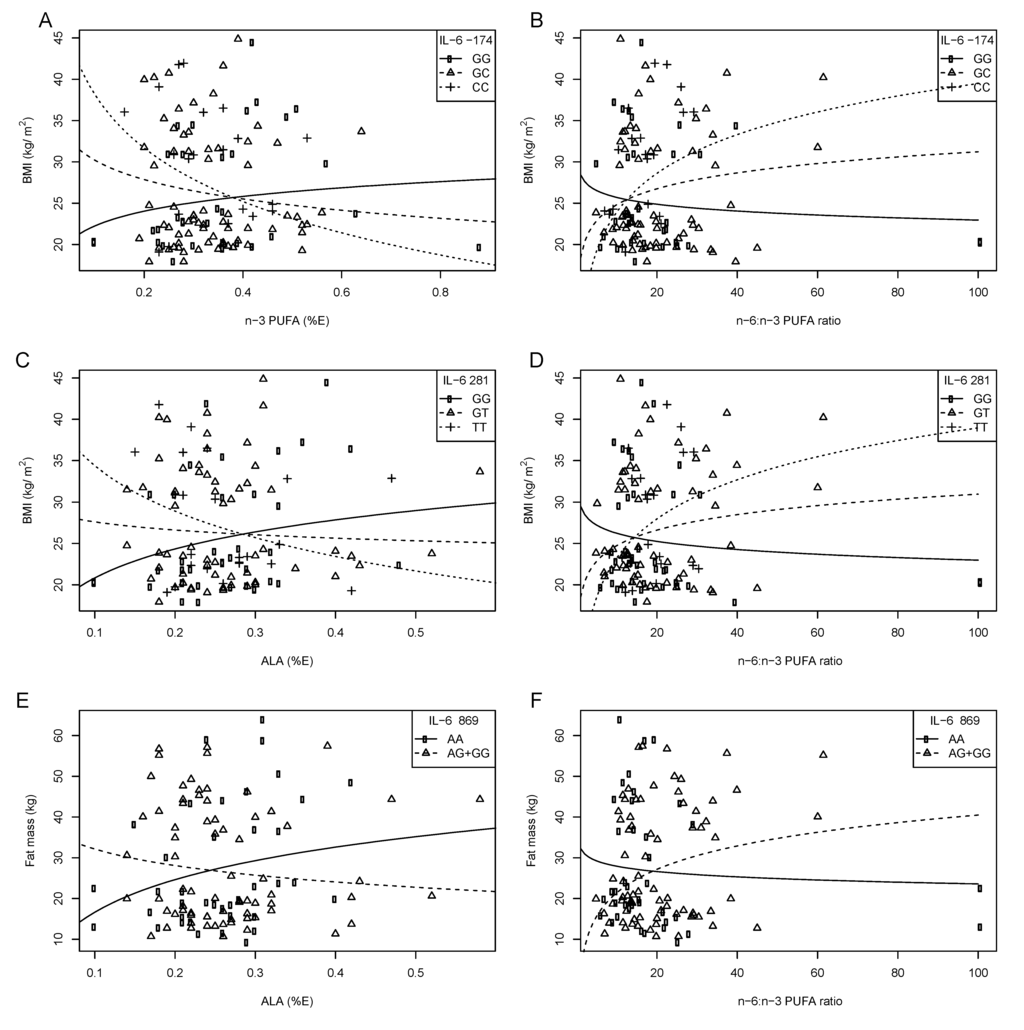
Figure 3.
The relationship between BMI and fat mass, IL-6 –174 G>C, IVS3+281 G>T and IVS4+869 A>G polymorphisms and dietary fat intake in white SA women. Symbols represent observed values for each woman. The lines are modeled relationships for a woman of average age (27.3 years) [98]. (A) With increasing n-3 PUFA intake (%E), BMI decreased in those with the –174 CC or GC genotypes. (B) With increasing n-6:n-3 PUFA ratio, BMI increased equally with each additional –174 C allele. (C) With increasing ALA intake (%E), BMI decreased with each additional IVS3+281 T allele. (D) With increasing n-6:n-3 PUFA ratio, BMI increased with each additional IVS3+281T allele. (E) With increasing ALA intake (%E), fat mass decreased in those with the IVS4+869 AG or GG genotype. (F) With increasing n-6:n-3 PUFA ratio, fat mass increased in those with the IVS4+869 AG or GG genotype; compared to those with the AA genotype.
When analyzing serum lipid profiles in white SA women, we observed that with increasing n-3 PUFA; TAG and T-C:HDL-C ratio decreased and HDL-C increased in those with the –174 C allele, and T-C:HDL-C ratio decreased in the IVS3 +281 TT genotype compared to the GG and GT genotype, with HDL-C increasing with each IVS3 +281 T allele. In contrast, HDL-C decreased with each IVS4 +869 G allele. In black SA women, with increasing total fat intake, TAG and T-C:HDL-C ratio increased in those with the IVS4 +869 G allele and decreased in the AA genotype [98]. These results suggest that both the quantity and quality of dietary fatty acids modulate the relationship between IL-6 SNPs on measures of obesity and serum lipids, and that these effects may differ according to ethnic group.
6. The Role of Ethnicity and Gender as Confounders
It has been reported that while the prevalence of obesity may be high in a given population, the prevalence of obesity-associated comorbidities may also differ between ethnic groups [75,116,117,118,119,120]. As an example; while both African American and black SA women have a higher prevalence of obesity [117,121,122,123], they have less atherogenic lipid profiles than their white counterparts; characterized by low TAG, T-C, and LDL-C concentrations [118,124,125,126].
Differences between ethnicities comprise complex etiologies. Ethnicity incorporates a variety of different components including; genetic variation, diet and lifestyle, as well as cultural, behavioral and socio-demographic conditions [120]. In the example of ethnic differences in lipid profiles described above, ethnic variability may be observed in dietary intake, body fat distribution (VAT vs. SAT) [127,128], sequence variation in genes such as apolipoprotein E (APOE) [129], inflammatory gene expression (TNFA and IL-6) [130,131], and genotype frequencies. Genotype and allele frequencies of SNPs discussed in this review paper have been compared in Table 4 for European, British in England and Scotland, African, and African American populations. These SNPs are polymorphic in all these populations. However, there is a very low frequency of the IL-6 –174 C allele in populations of African descent [132].
Of importance, dietary fatty acid intake may differ [133], as well as physical activity levels. In considering differences between ethnic groups resident in developed and developing countries, attitudes to food as well as the quality of the urban environment will also pay a role [120].
In a systematic review on the influence of ethnicity on the relationship between n-3 PUFA intake and CVD, Patel et al. concluded that ethnicity is a factor that accounts for inconsistencies in study results. Specifically, some of the effects of n-3 PUFA are limited to cultures with very high n-3 PUFA intake, and in turn, ethnicity moderates the efficiency with which PUFAs are derived from the diet [133]. Another key consideration in reviewing dietary intake and diet–gene interactions is the impact that certain genes have on dietary fatty acid metabolism, and how these may differ between ethnic groups. Of interest is the fatty acid desaturase gene (FADS), which codes for enzymes in PUFA metabolism. Lu et al. reported how genetic variation in the FADS1 gene interacted with dietary intake of both n-3 and n-6 PUFA to affect T-C and HDL-C concentrations [134]. Furthermore, it has recently been shown that FADS SNPs altered the capacity of different ethnic groups to synthesize long-chain fatty acids [135,136]. Specifically, Sergeant et al. found that FADS genotype frequencies differed significantly between African Americans and European Americans, and SNPs in the FADS genes meant that African Americans were able to more efficiently convert the n-6 fatty acid LA to the proinflammatory fatty acid AA, resulting in higher circulating AA levels, and potentially a more deleterious impact of a diet high in LA in this ethnic group [136].

Table 4.
TNFA and IL-6 genotype and minor allele frequencies.
| Ensemble 1000 Genomes: phase 1 | ||||
|---|---|---|---|---|
| EUR | GBR | AFR | ASW | |
| TNFA –308 G > A rs1800629 | ||||
| GG | 0.75 | 0.80 | 0.81 | 0.87 |
| GA | 0.24 | 0.17 | 0.18 | 0.13 |
| AA | 0.02 | 0.03 | 0.00 | 0.00 |
| A allele | 0.14 | 0.12 | 0.10 | 0.10 |
| TNFA –238 G > A rs361525 | ||||
| GG | 0.87 | 0.81 | 0.93 | 0.90 |
| GA | 0.13 | 0.19 | 0.07 | 0.10 |
| AA | 0.00 | 0.00 | 0.00 | 0.00 |
| A allele | 0.07 | 0.10 | 0.03 | 0.05 |
| IL-6 –174 G > C rs1800795 | ||||
| GG | 0.36 | 0.39 | 0.95 | 0.78 |
| GC | 0.44 | 0.42 | 0.05 | 0.21 |
| CC | 0.20 | 0.19 | 0.0 | 0.0 |
| C allele | 0.41 | 0.40 | 0.02 | 0.11 |
| IL-6 IVS3 +281 G > T rs1554606 | ||||
| GG | 0.34 | 0.36 | 0.48 | 0.39 |
| GT | 0.45 | 0.44 | 0.46 | 0.57 |
| TT | 0.20 | 0.19 | 0.05 | 0.03 |
| T allele | 0.43 | 0.41 | 0.28 | 0.32 |
| IL-6 IVS4 +869 A > G rs2069845 | ||||
| AA | 0.34 | 0.36 | 0.46 | 0.37 |
| GA | 0.45 | 0.44 | 0.47 | 0.57 |
| GG | 0.20 | 0.19 | 0.05 | 0.04 |
| G allele | 0.43 | 0.41 | 0.29 | 0.33 |
Population frequencies are from the Ensemble public database 1000 Genomes [137,138]. EUR, European; GBR, British in England and Scotland; AFR, African; ASW, Americans of African Ancestry in SW USA.
In this review we have identified for both the TNFA and IL-6 genes, independent associations and diet–gene interactions that differed between ethnic groups. It is likely that the contributing factors described above may partly explain these differences. Like ethnicity, it is likely that the gender (sex) of study participants may impact genotype-phenotype interactions. A number of studies have observed gene–diet–gender interactions, whereby an interaction was identified in one sex, but not another. For example, the FINGEN study, which examined the effect of long chain n-3 PUFA supplementation and APOE genotype on plasma lipids, reported greater TAG lowering effects following dietary intervention in APOE4 males than in females [139]. Further, results from the Framingham Heart Study showed that dietary PUFA intake modulates the effect of the apolipoprotein A1 (APOA1) –75 G > A polymorphism (rs670) on plasma HDL-C concentrations in women but not in men [140]. In addition, Phillips et al. have shown that the TCF7L2 rs7903146 polymorphism influences MetS risk, and is impacted by both gender and dietary SFA intake [141]. Phillips et al. have also identified associations between genetic variants of the apolipoprotein B and APOA1 gene and MetS risk, however the modulation of MetS risk by dietary fat intake observed in the entire cohort was observed in the male high-fat consumers only [142].
7. Conclusions
This review suggests that DNA sequence variations in genes involved in inflammation may interact with environmental exposures, such as dietary intake, to modulate an individual’s susceptibility to developing obesity and its comorbidities. In summary, dietary fatty acids, in particular SFAs and the n-3 and n-6 PUFAs, impact the expression of the cytokine genes TNFA and IL-6, and alter TNFα and IL-6 production. In addition, sequence variants in these genes have also been shown to alter their gene expression and plasma levels, and are associated with obesity, measures of adiposity and serum lipid concentrations. When interactions between dietary fatty acids with TNFA and IL-6 SNPs on obesity and serum lipid were analyzed, it became evident that both the quantity and quality of dietary fatty acids modulate the relationship between TNFA and IL-6 SNPS on obesity and serum lipid profiles, thereby impacting the association between phenotype and genotype. The inter-individual variability in the obese phenotype and the inconsistencies in study results may be better understood by researching these diet–gene interactions more extensively.
Although this review focused on only two of the inflammatory cytokines, many other adipokines and chemokines have been associated with obesity (e.g., adiponectin, IL-1, IL-10, and MCP-1), and are sensitive to dietary fatty acid intake and should be studied further. In addition, it is also important to unravel the molecular mechanisms that govern the impact of dietary fatty acids on inflammation, which remain unclear and appear to act via multiple pathways. While it is known that different groups of dietary fatty acids (SFA, MUFA, and n-3, n-6 and n-9 PUFA) differ in their effect on inflammatory gene expression and plasma levels [27], these results are not conclusive, especially with regards the n-6 PUFA class of fatty acids which have at times been shown to act in both a pro- and anti-inflammatory manner, and appear to be modulated by gene variants.
The nutrigenetic interactions described in this review are complex and it is difficult to assess the magnitude of their impact in managing an individual’s diet. Furthermore, the inter-ethnic variability reported should caution us with regards singular dietary recommendations, as it cannot be assumed that fatty acids and other nutrient metabolism is uniform for all populations. In addition to diet–single polymorphism interactions, it is also important to understand the combined effect of a number of polymorphisms on the same or different genes interacting with the environment. For this reason, the identification and analysis of haplotype-nutrient interactions may provide additional insights in future research. The future study of nutrigenomics offers the opportunity to clarify the underlying molecular mechanisms governing the interactions between dietary fatty acids and the inflammatory phenotype, potentially elucidating the observed differences between different ethnic groups and genders in developing population-specific dietary recommendations.
Conflict of Interest
Yael Joffe is a consultant to a South African genetics company, DNAlysis biotechnology. She is also co-author of the book “It’s not just your genes!”; she receives no profits or royalties from this book. None of the other authors have a conflict of interest.
References
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar]
- Bray, M.S. Implications of gene-behavior interactions: Prevention and intervention for obesity. Obesity 2008, 16 (Suppl. 3), S72–S78. [Google Scholar] [CrossRef]
- Joffe, Y.T.; van der Merwe, L.; Carstens, M.; Collins, M.; Jennings, C.; Levitt, N.S.; Lambert, E.V.; Goedecke, J.H. Tumor necrosis factor-α gene –308 G/A polymorphism modulates the relationship between dietary fat intake, serum lipids, and obesity risk in black South African women. J. Nutr. 2010, 140, 901–907. [Google Scholar] [CrossRef]
- Joffe, Y.T.; van der Merwe, L.; Collins, M.; Carstens, M.; Evans, J.; Lambert, E.V.; Goedecke, J.H. The –308 G/A polymorphism of the tumour necrosis factor-α gene modifies the association between saturated fat intake and serum total cholesterol levels in white South African women. Genes Nutr. 2011, 6, 353–359. [Google Scholar]
- Joffe, Y.T.; van der Merwe, L.; Evans, J.; Collins, M.; Lambert, E.V.; September, A.; Goedecke, J.H. The tumor necrosis factor-α gene –238 G > A polymorphism, dietary fat intake, obesity risk and serum lipid concentrations in black and white South African women. Eur. J. Clin. Nutr. 2012, 66, 1295–1302. [Google Scholar] [CrossRef]
- Stryjecki, C.; Mutch, D.M. Fatty acid-gene interactions, adipokines and obesity. Eur. J. Clin. Nutr. 2011, 65, 285–297. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Nair, S.; Lee, Y.H.; Rousseau, E.; Cam, M.; Tataranni, P.A.; Baier, L.J.; Bogardus, C.; Permana, P.A. Increased expression of inflammation-related genes in cultured preadipocytes/stromal vascular cells from obese compared with non-obese Pima Indians. Diabetologia 2005, 48, 1784–1788. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003, 112, 1796–1808. [Google Scholar]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003, 112, 1821–1830. [Google Scholar]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Berliner, J.; Leitinger, N.; Watson, A.; Huber, J.; Fogelman, A.; Navab, M. Oxidized lipids in atherogenesis: Formation, destruction and action. Thromb. Haemost. 1997, 78, 195–199. [Google Scholar]
- Sugiyama, S.; Okada, Y.; Sukhova, G.K.; Virmani, R.; Heinecke, J.W.; Libby, P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 2001, 158, 879–891. [Google Scholar] [CrossRef]
- Dichtl, W.; Nilsson, L.; Goncalves, I.; Ares, M.P.; Banfi, C.; Calara, F.; Hamsten, A.; Eriksson, P.; Nilsson, J. Very low-density lipoprotein activates nuclear factor-κB in endothelial cells. Circ. Res. 1999, 84, 1085–1094. [Google Scholar] [CrossRef]
- Burzotta, F.; Iacoviello, L.; Di Castelnuovo, A.; Glieca, F.; Luciani, N.; Zamparelli, R.; Schiavello, R.; Donati, M.B.; Maseri, A.; Possati, G.; et al. Relation of the –174 G/C polymorphism of interleukin-6 to interleukin-6 plasma levels and to length of hospitalization after surgical coronary revascularization. Am. J. Cardiol. 2001, 88, 1125–1128. [Google Scholar] [CrossRef]
- Santtila, S.; Savinainen, K.; Hurme, M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1β production in vitro. Scand. J. Immunol. 1998, 47, 195–198. [Google Scholar]
- Terry, C.F.; Loukaci, V.; Green, F.R. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J. Biol. Chem. 2000, 275, 18138–18144. [Google Scholar] [CrossRef]
- Wilson, A.G.; Symons, J.A.; McDowell, T.L.; McDevitt, H.O.; Duff, G.W. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA 1997, 94, 3195–3199. [Google Scholar]
- Yu, Z.; Han, S.; Cao, X.; Zhu, C.; Wang, X.; Guo, X. Genetic polymorphisms in adipokine genes and the risk of obesity: A systematic review and meta-analysis. Obesity 2011, 20, 396–406. [Google Scholar]
- Phillips, C.M. Nutrigenetics and metabolic disease: Current status and implications for personalised nutrition. Nutrients 2013, 5, 32–57. [Google Scholar] [CrossRef]
- Phillips, C.M.; Goumidi, L.; Bertrais, S.; Ferguson, J.F.; Field, M.R.; Kelly, E.D.; Mehegan, J.; Peloso, G.M.; Cupples, L.A.; Shen, J.; et al. Additive effect of polymorphisms in the IL-6, LTA, and TNF-αgenes and plasma fatty acid level modulate risk for the metabolic syndrome and its components. J. Clin. Endocrinol. Metab. 2010, 95, 1386–1394. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids and inflammation: From molecular biology to the clinic. Lipids 2003, 38, 343–352. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar]
- Van den Berghe, W.; Vermeulen, L.; Delerive, P.; De Bosscher, K.; Staels, B.; Haegeman, G. A paradigm for gene regulation: Inflammation, NF-κB and PPAR. Adv. Exp. Med. Biol. 2003, 544, 181–196. [Google Scholar]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jonsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef]
- Suganami, T.; Tanimoto-Koyama, K.; Nishida, J.; Itoh, M.; Yuan, X.; Mizuarai, S.; Kotani, H.; Yamaoka, S.; Miyake, K.; Aoe, S.; et al. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 84–91. [Google Scholar] [CrossRef]
- Fessler, M.B.; Rudel, L.L.; Brown, J.M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 379–385. [Google Scholar] [CrossRef]
- Weatherill, A.R.; Lee, J.Y.; Zhao, L.; Lemay, D.G.; Youn, H.S.; Hwang, D.H. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J. Immunol. 2005, 174, 5390–5397. [Google Scholar]
- Ajuwon, K.M.; Spurlock, M.E. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFα expression in 3T3-L1 adipocytes. J. Nutr. 2005, 135, 1841–1846. [Google Scholar]
- Weigert, C.; Brodbeck, K.; Staiger, H.; Kausch, C.; Machicao, F.; Haring, H.U.; Schleicher, E.D. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-κB. J. Biol. Chem. 2004, 279, 23942–23952. [Google Scholar]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef]
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 2012, 6, 216–234. [Google Scholar] [CrossRef]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef]
- Kelley, D.S.; Taylor, P.C.; Nelson, G.J.; Schmidt, P.C.; Mackey, B.E.; Kyle, D. Effects of dietary arachidonic acid on human immune response. Lipids 1997, 32, 449–456. [Google Scholar] [CrossRef]
- Thies, F.; Miles, E.A.; Nebe-von-Caron, G.; Powell, J.R.; Hurst, T.L.; Newsholme, E.A.; Calder, P.C. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 2001, 36, 1183–1193. [Google Scholar] [CrossRef]
- Thies, F.; Nebe-von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar]
- Erkkila, A.; de Mello, V.D.; Riserus, U.; Laaksonen, D.E. Dietary fatty acids and cardiovascular disease: An epidemiological approach. Prog. Lipid Res. 2008, 47, 172–187. [Google Scholar] [CrossRef]
- Williams, C.M.; Burdge, G. Long-chain n-3 PUFA: Plant v. marine sources. Proc. Nutr. Soc. 2006, 65, 42–50. [Google Scholar] [CrossRef]
- Stulnig, T.M. Immunomodulation by polyunsaturated fatty acids: Mechanisms and effects. Int. Arch. Allergy Immunol. 2003, 132, 310–321. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; West, S.G.; Gillies, P.J.; Kris-Etherton, P.M. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J. Nutr. 2004, 134, 2991–2997. [Google Scholar]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Bray, G.A.; Lovejoy, J.C.; Smith, S.R.; DeLany, J.P.; Lefevre, M.; Hwang, D.; Ryan, D.H.; York, D.A. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J. Nutr. 2002, 132, 2488–2491. [Google Scholar]
- Itariu, B.K.; Zeyda, M.; Hochbrugger, E.E.; Neuhofer, A.; Prager, G.; Schindler, K.; Bohdjalian, A.; Mascher, D.; Vangala, S.; Schranz, M.; et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1137–1149. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar]
- Maury, E.; Noel, L.; Detry, R.; Brichard, S.M. In vitro hyperresponsiveness to tumor necrosis factor-α contributes to adipokine dysregulation in omental adipocytes of obese subjects. J. Clin. Endocrinol. Metab. 2009, 94, 1393–1400. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor α. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef]
- Dandona, P.; Weinstock, R.; Thusu, K.; Abdel-Rahman, E.; Aljada, A.; Wadden, T. Tumor necrosis factor-α in sera of obese patients: Fall with weight loss. J. Clin. Endocrinol. Metab. 1998, 83, 2907–2910. [Google Scholar] [CrossRef]
- Wang, B.; Trayhurn, P. Acute and prolonged effects of TNF-α on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflug. Archiv. Eur. J. Physiol. 2006, 452, 418–427. [Google Scholar]
- Bruun, J.M.; Lihn, A.S.; Verdich, C.; Pedersen, S.B.; Toubro, S.; Astrup, A.; Richelsen, B. Regulation of adiponectin by adipose tissue-derived cytokines: In vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E527–E533. [Google Scholar]
- Ruan, H.; Hacohen, N.; Golub, T.R.; Van Parijs, L.; Lodish, H.F. Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: Nuclear factor-κB activation by TNF-α is obligatory. Diabetes 2002, 51, 1319–1336. [Google Scholar] [CrossRef]
- Chen, X.; Xun, K.; Chen, L.; Wang, Y. TNF-α, a potent lipid metabolism regulator. Cell Biochem. Funct. 2009, 27, 407–416. [Google Scholar] [CrossRef]
- Madej, A.; Okopien, B.; Kowalski, J.; Zielinski, M.; Wysocki, J.; Szygula, B.; Kalina, Z.; Herman, Z. Levels of tumor necrosis factor alpha in serum of patients with hyperlipoproteinemia IIB before and after micronized fenofibrate therapy. Pol. Arch. Med. Wewn. 1998, 99, 308–313. [Google Scholar]
- Ascer, E.; Bertolami, M.C.; Venturinelli, M.L.; Buccheri, V.; Souza, J.; Nicolau, J.C.; Ramires, J.A.; Serrano, C.V., Jr. Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis 2004, 177, 161–166. [Google Scholar] [CrossRef]
- Marketou, M.E.; Zacharis, E.A.; Nikitovic, D.; Ganotakis, E.S.; Parthenakis, F.I.; Maliaraki, N.; Vardas, P.E. Early effects of simvastatinversus atorvastatin on oxidative stress and proinflammatory cytokines in hyperlipidemic subjects. Angiology 2006, 57, 211–218. [Google Scholar] [CrossRef]
- Popa, C.; Netea, M.G.; Radstake, T.; van der Meer, J.W.; Stalenhoef, A.F.; van Riel, P.L.; Barerra, P. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann. Rheum Dis. 2005, 64, 303–305. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Role of cytokines in inducing hyperlipidemia. Diabetes 1992, 41 (Suppl. 2), 97–101. [Google Scholar]
- Feingold, K.R.; Soued, M.; Staprans, I.; Gavin, L.A.; Donahue, M.E.; Huang, B.J.; Moser, A.H.; Gulli, R.; Grunfeld, C. Effect of tumor necrosis factor (TNF) on lipid metabolism in the diabetic rat. Evidence that inhibition of adipose tissue lipoprotein lipase activity is not required for TNF-induced hyperlipidemia. J. Clin. Invest. 1989, 83, 1116–1121. [Google Scholar] [CrossRef]
- Bradley, R.L.; Fisher, F.F.; Maratos-Flier, E. Dietary fatty acids differentially regulate production of TNF-α and IL-10 by murine 3T3-L1 adipocytes. Obesity 2008, 16, 938–944. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Fernandes, G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem. Biophys. Res. Commun. 1994, 200, 893–898. [Google Scholar] [CrossRef]
- Perez-Matute, P.; Perez-Echarri, N.; Martinez, J.A.; Marti, A.; Moreno-Aliaga, M.J. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: Role of apoptosis, adiponectin and tumour necrosis factor-α. Br. J. Nutr. 2007, 97, 389–398. [Google Scholar] [CrossRef]
- Caughey, G.E.; Mantzioris, E.; Gibson, R.A.; Cleland, L.G.; James, M.J. The effect on human tumor necrosis factor alpha and interleukin 1 β production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996, 63, 116–122. [Google Scholar]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef]
- Grimble, R.F.; Howell, W.M.; O’Reilly, G.; Turner, S.J.; Markovic, O.; Hirrell, S.; East, J.M.; Calder, P.C. The ability of fish oil to suppress tumor necrosis factor αa production by peripheral blood mononuclear cells in healthy men is associated with polymorphisms in genes that influence tumor necrosis factor α production. Am. J. Clin. Nutr. 2002, 76, 454–459. [Google Scholar]
- Brand, E.; Schorr, U.; Kunz, I.; Kertmen, E.; Ringel, J.; Distler, A.; Sharma, A.M. Tumor necrosis factor-α: 308 G/A polymorphism in obese Caucasians. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 581–585. [Google Scholar]
- Herrmann, S.M.; Ricard, S.; Nicaud, V.; Mallet, C.; Arveiler, D.; Evans, A.; Ruidavets, J.B.; Luc, G.; Bara, L.; Parra, H.J.; et al. Polymorphisms of the tumour necrosis factor-α gene, coronary heart disease and obesity. Eur. J. Clin. Invest. 1998, 28, 59–66. [Google Scholar] [CrossRef]
- Hoffstedt, J.; Eriksson, P.; Hellstrom, L.; Rossner, S.; Ryden, M.; Arner, P. Excessive fat accumulation is associated with the TNF α –308 G/A promoter polymorphism in women but not in men. Diabetologia 2000, 43, 117–120. [Google Scholar] [CrossRef]
- Nieters, A.; Becker, N.; Linseisen, J. Polymorphisms in candidate obesity genes and their interaction with dietary intake of n-6 polyunsaturated fatty acids affect obesity risk in a sub-sample of the EPIC-Heidelberg cohort. Eur. J. Nutr. 2002, 41, 210–221. [Google Scholar] [CrossRef]
- Pihlajamaki, J.; Ylinen, M.; Karhapaa, P.; Vauhkonen, I.; Laakso, M. The effect of the –308A allele of the TNF-α gene on insulin action is dependent on obesity. Obes. Res. 2003, 11, 912–917. [Google Scholar] [CrossRef]
- Rosmond, R.; Chagnon, M.; Bouchard, C.; Bjorntorp, P. G-308A polymorphism of the tumor necrosis factor α gene promoter and salivary cortisol secretion. J. Clin. Endocrinol. Metab. 2001, 86, 2178–2180. [Google Scholar] [CrossRef]
- Sookoian, S.; Garcia, S.I.; Gianotti, T.F.; Dieuzeide, G.; Gonzalez, C.D.; Pirola, C.J. The G-308A promoter variant of the tumor necrosis factor-α gene is associated with hypertension in adolescents harboring the metabolic syndrome. Am. J. Hypertens. 2005, 18, 1271–1275. [Google Scholar] [CrossRef]
- Dalziel, B.; Gosby, A.K.; Richman, R.M.; Bryson, J.M.; Caterson, I.D. Association of the TNF-α –308 G/A promoter polymorphism with insulin resistance in obesity. Obes. Res. 2002, 10, 401–407. [Google Scholar] [CrossRef]
- Wybranska, I.; Malczewska-Malec, M.; Niedbal, S.; Naskalski, J.W.; Dembinska-Kiec, A. The TNF-α gene NcoI polymorphism at position –308 of the promoter influences insulin resistance, and increases serum triglycerides after postprandial lipaemia in familiar obesity. Clin. Chem. Lab. Med. 2003, 41, 501–510. [Google Scholar]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef]
- Davis, J.E.; Gabler, N.K.; Walker-Daniels, J.; Spurlock, M.E. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity 2008, 16, 1248–1255. [Google Scholar] [CrossRef]
- Garcia-Escobar, E.; Rodriguez-Pacheco, F.; Garcia-Serrano, S.; Gomez-Zumaquero, J.M.; Haro-Mora, J.J.; Soriguer, F.; Rojo-Martinez, G. Nutritional regulation of interleukin-6 release from adipocytes. Int. J. Obes. (Lond.) 2010, 34, 1328–1332. [Google Scholar] [CrossRef]
- Van Dijk, S.J.; Feskens, E.J.; Bos, M.B.; Hoelen, D.W.; Heijligenberg, R.; Bromhaar, M.G.; de Groot, L.C.; de Vries, J.H.; Muller, M.; Afman, L.A. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am. J. Clin. Nutr. 2009, 90, 1656–1664. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Jenny, N.S.; Mayer-Davis, E.; Jiang, R.; Steffen, L.; Siscovick, D.; Tsai, M.; Herrington, D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 1238–1243. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar]
- Romeo, S.; Sentinelli, F.; Capici, F.; Arca, M.; Berni, A.; Vecci, E.; Di Mario, U.; Baroni, M.G. The G-308A variant of the Tumor Necrosis Factor-alpha (TNF-α) gene is not associated with obesity, insulin resistance and body fat distribution. BMC Med. Genet. 2001, 2, 10. [Google Scholar]
- Um, J.Y.; Park, J.H.; Kim, H.M. Gene polymorphisms in tumor necrosis factor locus and waist-hip ratio in obese Koreans. Clin. Chim. Acta 2003, 338, 117–122. [Google Scholar] [CrossRef]
- Hedayati, M.; Sharifi, K.; Rostami, F.; Daneshpour, M.S.; Zarif Yeganeh, M.; Azizi, F. Association between TNF-α promoter G-308A and G-238A polymorphisms and obesity. Mol. Biol. Rep. 2011, 39, 825–829. [Google Scholar]
- Walston, J.; Seibert, M.; Yen, C.J.; Cheskin, L.J.; Andersen, R.E. Tumor necrosis factor-α-238 and –308 polymorphisms do not associated with traits related to obesity and insulin resistance. Diabetes 1999, 48, 2096–2098. [Google Scholar] [CrossRef]
- Riikola, A.; Sipila, K.; Kahonen, M.; Jula, A.; Nieminen, M.S.; Moilanen, L.; Kesaniemi, Y.A.; Lehtimaki, T.; Hulkkonen, J. Interleukin-6 promoter polymorphism and cardiovascular risk factors: The Health 2000 Survey. Atherosclerosis 2009, 207, 466–470. [Google Scholar] [CrossRef]
- Huth, C.; Illig, T.; Herder, C.; Gieger, C.; Grallert, H.; Vollmert, C.; Rathmann, W.; Hamid, Y.H.; Pedersen, O.H.; Hansen, T.; et al. Joint analysis of individual participants’ data from 17 studies on the association of the IL6 variant –174G > C with circulating glucose levels, interleukin-6 levels, and body mass index. Ann. Med. 2009, 41, 128–138. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; van Dam, R.M.; Hu, F.B. Interleukin-6 genetic variability and adiposity: Associations in two prospective cohorts and systematic review in 26,944 individuals. J. Clin. Endocrinol. Metab. 2007, 92, 3618–3625. [Google Scholar] [CrossRef]
- Joffe, Y.T.; van der Merwe, L.; Evans, J.; Collins, M.; Lambert, E.V.; September, A.; Goedecke, J.H. Genetic polymorphisms in the Interleukin-6 gene and the risk of obesity and dyslipidaemia in black and white South African women. Genes Nutr. 2013. submitted for publication. [Google Scholar]
- Henningsson, S.; Hakansson, A.; Westberg, L.; Baghaei, F.; Rosmond, R.; Holm, G.; Ekman, A.; Nissbrandt, H.; Eriksson, E. Interleukin-6 gene polymorphism –174G/C influences plasma lipid levels in women. Obesity 2006, 14, 1868–1873. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Broch, M.; Vendrell, J.; Richart, C.; Ricart, W. Interleukin-6 gene polymorphism and lipid abnormalities in healthy subjects. J. Clin. Endocrinol. Metab. 2000, 85, 1334–1339. [Google Scholar] [CrossRef]
- Hulkkonen, J.; Lehtimaki, T.; Mononen, N.; Juonala, M.; Hutri-Kahonen, N.; Taittonen, L.; Marniemi, J.; Nieminen, T.; Viikari, J.; Raitakari, O.; et al. Polymorphism in the IL6 promoter region is associated with the risk factors and markers of subclinical atherosclerosis in men: The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2009, 203, 454–458. [Google Scholar] [CrossRef]
- Fontaine-Bisson, B.; Wolever, T.M.; Chiasson, J.L.; Rabasa-Lhoret, R.; Maheux, P.; Josse, R.G.; Leiter, L.A.; Rodger, N.W.; Ryan, E.A.; Connelly, P.W.; et al. Genetic polymorphisms of tumor necrosis factor-αmodify the association between dietary polyunsaturated fatty acids and fasting HDL-cholesterol and apo A–I concentrations. Am. J. Clin. Nutr. 2007, 86, 768–774. [Google Scholar]
- Fontaine-Bisson, B.; El-Sohemy, A. Genetic polymorphisms of tumor necrosis factor-α modify the association between dietary polyunsaturated fatty acids and plasma high-density lipoprotein-cholesterol concentration in a population of young adults. J. Nutrigenet Nutrigenomics 2008, 1, 215–223. [Google Scholar] [CrossRef]
- Corpeleijn, E.; Petersen, L.; Holst, C.; Saris, W.H.; Astrup, A.; Langin, D.; MacDonald, I.; Martinez, J.A.; Oppert, J.M.; Polak, J.; et al. Obesity-related polymorphisms and their associations with the ability to regulate fat oxidation in obese Europeans: The NUGENOB study. Obesity 2010, 18, 1369–1377. [Google Scholar]
- Razquin, C.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Fernandez-Crehuet, J.; Santos, J.M.; Marti, A. A Mediterranean diet rich in virgin olive oil may reverse the effects of the –174G/C IL6 gene variant on 3-year body weight change. Mol. Nutr. Food Res. 2010, 54 (Suppl. 1), 75–82. [Google Scholar] [CrossRef]
- Joffe, Y.T.; van der Merwe, L.; Evans, J.; Collins, M.; Lambert, E.V.; September, A.; Goedecke, J.H.; UCT/MRC Research Unit for Exercise Science and Sports Medicine, University of Cape Town, Cape Town, South Africa. Unpublished work. 2013.
- Curti, M.L.; Jacob, P.; Borges, M.C.; Rogero, M.M.; Ferreira, S.R. Studies of gene variants related to inflammation, oxidative stress, dyslipidemia, and obesity: Implications for a nutrigenetic approach. J. Obes. 2011, 2011, 497401. [Google Scholar] [CrossRef]
- Harkins, J.M.; Moustaid-Moussa, N.; Chung, Y.J.; Penner, K.M.; Pestka, J.J.; North, C.M.; Claycombe, K.J. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J. Nutr. 2004, 134, 2673–2677. [Google Scholar]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001, 9, 414–417. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Rexrode, K.M.; Pradhan, A.; Manson, J.E.; Buring, J.E.; Ridker, P.M. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann. Epidemiol. 2003, 13, 674–682. [Google Scholar] [CrossRef]
- Bougoulia, M.; Triantos, A.; Koliakos, G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss. Association with cardiovascular risk factors. Hormones 2006, 5, 192–199. [Google Scholar]
- Unger, R.H. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 1995, 44, 863–870. [Google Scholar]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—Dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Lau, D.C.; Dhillon, B.; Yan, H.; Szmitko, P.E.; Verma, S. Adipokines: Molecular links between obesity and atheroslcerosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2031–H2041. [Google Scholar] [CrossRef]
- Nonogaki, K.; Fuller, G.M.; Fuentes, N.L.; Moser, A.H.; Staprans, I.; Grunfeld, C.; Feingold, K.R. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology 1995, 136, 2143–2149. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Stouthard, J.M.; Romijn, J.A.; van der Poll, T.; Endert, E.; Klein, S.; Bakker, P.J.; Veenhof, C.H.; Sauerwein, H.P. Endocrinologic and metabolic effects of interleukin-6 in humans. Am. J. Physiol. 1995, 268, E813–E819. [Google Scholar]
- Humphries, S.E.; Luong, L.A.; Ogg, M.S.; Hawe, E.; Miller, G.J. The interleukin-6 –174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur. Heart J. 2001, 22, 2243–2252. [Google Scholar] [CrossRef]
- Jenny, N.S.; Tracy, R.P.; Ogg, M.S.; Luong, L.A.; Kuller, L.H.; Arnold, A.M.; Sharrett, A.R.; Humphries, S.E. In the elderly, interleukin-6 plasma levels and the –174G > C polymorphism are associated with the development of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 2066–2071. [Google Scholar] [CrossRef]
- Sie, M.P.; Sayed-Tabatabaei, F.A.; Oei, H.H.; Uitterlinden, A.G.; Pols, H.A.; Hofman, A.; van Duijn, C.M.; Witteman, J.C. Interleukin 6 –174 G/C promoter polymorphism and risk of coronary heart disease: Results from the rotterdam study and a meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 212–217. [Google Scholar] [CrossRef]
- Walston, J.D.; Fallin, M.D.; Cushman, M.; Lange, L.; Psaty, B.; Jenny, N.; Browner, W.; Tracy, R.; Durda, P.; Reiner, A. IL-6 gene variation is associated with IL-6 and C-reactive protein levels but not cardiovascular outcomes in the Cardiovascular Health Study. Hum. Genet. 2007, 122, 485–494. [Google Scholar] [CrossRef]
- Joubert, J.; Norman, R.; Bradshaw, D.; Goedecke, J.H.; Steyn, N.P.; Puoane, T. Estimating the burden of disease attributable to excess body weight in South Africa in 2000. S. Afr. Med. J. 2007, 97, 683–690. [Google Scholar]
- Lovejoy, J.C.; de la Bretonne, J.A.; Klemperer, M.; Tulley, R. Abdominal fat distribution and metabolic risk factors: Effects of race. Metab. Clin. Exp. 1996, 45, 1119–1124. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Levitt, N.S.; Evans, J.; Ellman, N.; Hume, D.; Kotze, L.; Tootla, M.; Victor, H.; Keswell, D. The role of adipose tissue in insulin resistance in women of African Ancestry. J. Obes. 2013, 2013, 952916. [Google Scholar] [CrossRef]
- Punyadeera, C.; Crowther, N.J.; van der Merwe, M.T.; Toman, M.; Immelman, A.R.; Schlaphoff, G.P.; Gray, I.P. Metabolic response to a mixed meal in obese and lean women from two South african populations. Obes. Res. 2002, 10, 1207–1216. [Google Scholar] [CrossRef]
- Wells, J.C. Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: Addressing the full range of ethnicity, including those of mixed ethnicity. Obes. Rev. 2012, 13 (Suppl. 2), 14–29. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; Smith, S.R.; de Jonge, L.; Xie, H. Ethnic differences in dietary intakes, physical activity, and energy expenditure in middle-aged, premenopausal women: The Healthy Transitions Study. Am. J. Clin. Nutr. 2001, 74, 90–95. [Google Scholar]
- Goedecke, J.H.; Jennings, C.; Lambert, E.V. Obesity in South Africa; Medical Research Council: Cape Town, South Africa, 2006; pp. 65–79. [Google Scholar]
- Adeboye, B.; Bermano, G.; Rolland, C. Obesity and its health impact in Africa: A systematic review. Cardiovasc. J. Afr. 2012, 23, 512–521. [Google Scholar] [CrossRef]
- Despres, J.P.; Couillard, C.; Gagnon, J.; Bergeron, J.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: The health, risk factors, exercise training, and genetics (HERITAGE) family study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1932–1938. [Google Scholar] [CrossRef]
- Punyadeera, C.; van der Merwe, M.T.; Crowther, N.J.; Toman, M.; Schlaphoff, G.P.; Gray, I.P. Ethnic differences in lipid metabolism in two groups of obese South African women. J. Lipid Res. 2001, 42, 760–767. [Google Scholar]
- Sumner, A.E.; Cowie, C.C. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 2008, 196, 696–703. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Utzschneider, K.; Faulenbach, M.V.; Rizzo, M.; Berneis, K.; Spinas, G.A.; Dave, J.A.; Levitt, N.S.; Lambert, E.V.; Olsson, T.; et al. Ethnic differences in serum lipoproteins and their determinants in South African women. Metabolism 2010, 59, 1341–1350. [Google Scholar] [CrossRef]
- Nazare, J.A.; Smith, J.D.; Borel, A.L.; Haffner, S.M.; Balkau, B.; Ross, R.; Massien, C.; Almeras, N.; Despres, J.P. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: The international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-abdominal adiposity. Am. J. Clin. Nutr. 2012, 96, 714–726. [Google Scholar] [CrossRef]
- Corella, D.; Ordovas, J.M. Single nucleotide polymorphisms that influence lipid metabolism: Interaction with dietary factors. Annu. Rev. Nutr. 2005, 25, 341–390. [Google Scholar] [CrossRef]
- Ness, R.B.; Haggerty, C.L.; Harger, G.; Ferrell, R. Differential distribution of allelic variants in cytokine genes among African Americans and White Americans. Am. J. Epidemiol. 2004, 160, 1033–1038. [Google Scholar] [CrossRef]
- Evans, J.; Goedecke, J.H.; Soderstrom, I.; Buren, J.; Alvehus, M.; Blomquist, C.; Jonsson, F.; Hayes, P.M.; Adams, K.; Dave, J.A.; et al. Depot- and ethnic-specific differences in the relationship between adipose tissue inflammation and insulin sensitivity. Clin. Endocrinol. 2011, 74, 51–59. [Google Scholar] [CrossRef]
- Martin, A.M.; Athanasiadis, G.; Greshock, J.D.; Fisher, J.; Lux, M.P.; Calzone, K.; Rebbeck, T.R.; Weber, B.L. Population frequencies of single nucleotide polymorphisms (SNPs) in immuno-modulatory genes. Hum. Hered. 2003, 55, 171–178. [Google Scholar] [CrossRef]
- Patel, J.V.; Tracey, I.; Hughes, E.A.; Lip, G.Y. Omega-3 polyunsaturated acids and cardiovascular disease: Notable ethnic differences or unfulfilled promise? J. Thromb. Haemost. 2010, 8, 2095–2104. [Google Scholar] [CrossRef]
- Lu, Y.; Feskens, E.J.; Dolle, M.E.; Imholz, S.; Verschuren, W.M.; Muller, M.; Boer, J.M. Dietary n-3 and n-6 polyunsaturated fatty acid intake interacts with FADS1 genetic variation to affect total and HDL-cholesterol concentrations in the Doetinchem Cohort Study. Am. J. Clin. Nutr. 2010, 92, 258–265. [Google Scholar]
- Mathias, R.A.; Fu, W.; Akey, J.M.; Ainsworth, H.C.; Torgerson, D.G.; Ruczinski, I.; Sergeant, S.; Barnes, K.C.; Chilton, F.H. Adaptive evolution of the FADS gene cluster within Africa. PLoS One 2012, 7, e44926. [Google Scholar]
- Sergeant, S.; Hugenschmidt, C.E.; Rudock, M.E.; Ziegler, J.T.; Ivester, P.; Ainsworth, H.C.; Vaidya, D.; Case, L.D.; Langefeld, C.D.; Freedman, B.I.; et al. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br. J. Nutr. 2012, 107, 547–555. [Google Scholar] [CrossRef]
- 1000 Genomes. Available online: http://browser.1000genomes.org (accessed on 1 February 2013).
- Flicek, P.; Amode, M.R.; Barrell, D.; Beal, K.; Brent, S.; Carvalho-Silva, D.; Clapham, P.; Coates, G.; Fairley, S.; Fitzgerald, S.; et al. Ensembl 2012. Nucl. Acids Res. 2012, 40, D84–D90. [Google Scholar] [CrossRef]
- Caslake, M.J.; Miles, E.A.; Kofler, B.M.; Lietz, G.; Curtis, P.; Armah, C.K.; Kimber, A.C.; Grew, J.P.; Farrell, L.; Stannard, J.; et al. Effect of sex and genotype on cardiovascular biomarker response to fish oils: The FINGEN Study. Am. J. Clin. Nutr. 2008, 88, 618–629. [Google Scholar]
- Ordovas, J.M.; Corella, D.; Cupples, L.A.; Demissie, S.; Kelleher, A.; Coltell, O.; Wilson, P.W.; Schaefer, E.J.; Tucker, K. Polyunsaturated fatty acids modulate the effects of the APOA1 G-A polymorphism on HDL-cholesterol concentrations in a sex-specific manner: The Framingham Study. Am. J. Clin. Nutr. 2002, 75, 38–46. [Google Scholar]
- Phillips, C.M.; Goumidi, L.; Bertrais, S.; Field, M.R.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; Roche, H.M. Dietary saturated fat, gender and genetic variation at the TCF7L2 locus predict the development of metabolic syndrome. J. Nutr. Biochem. 2012, 23, 239–244. [Google Scholar] [CrossRef]
- Phillips, C.M.; Goumidi, L.; Bertrais, S.; Field, M.R.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; Roche, H.M. Gene-nutrient interactions and gender may modulate the association between ApoA1 and ApoB gene polymorphisms and metabolic syndrome risk. Atherosclerosis 2011, 214, 408–414. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).