Homocysteine Lowering by Folate-Rich Diet or Pharmacological Supplementations in Subjects with Moderate Hyperhomocysteinemia
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
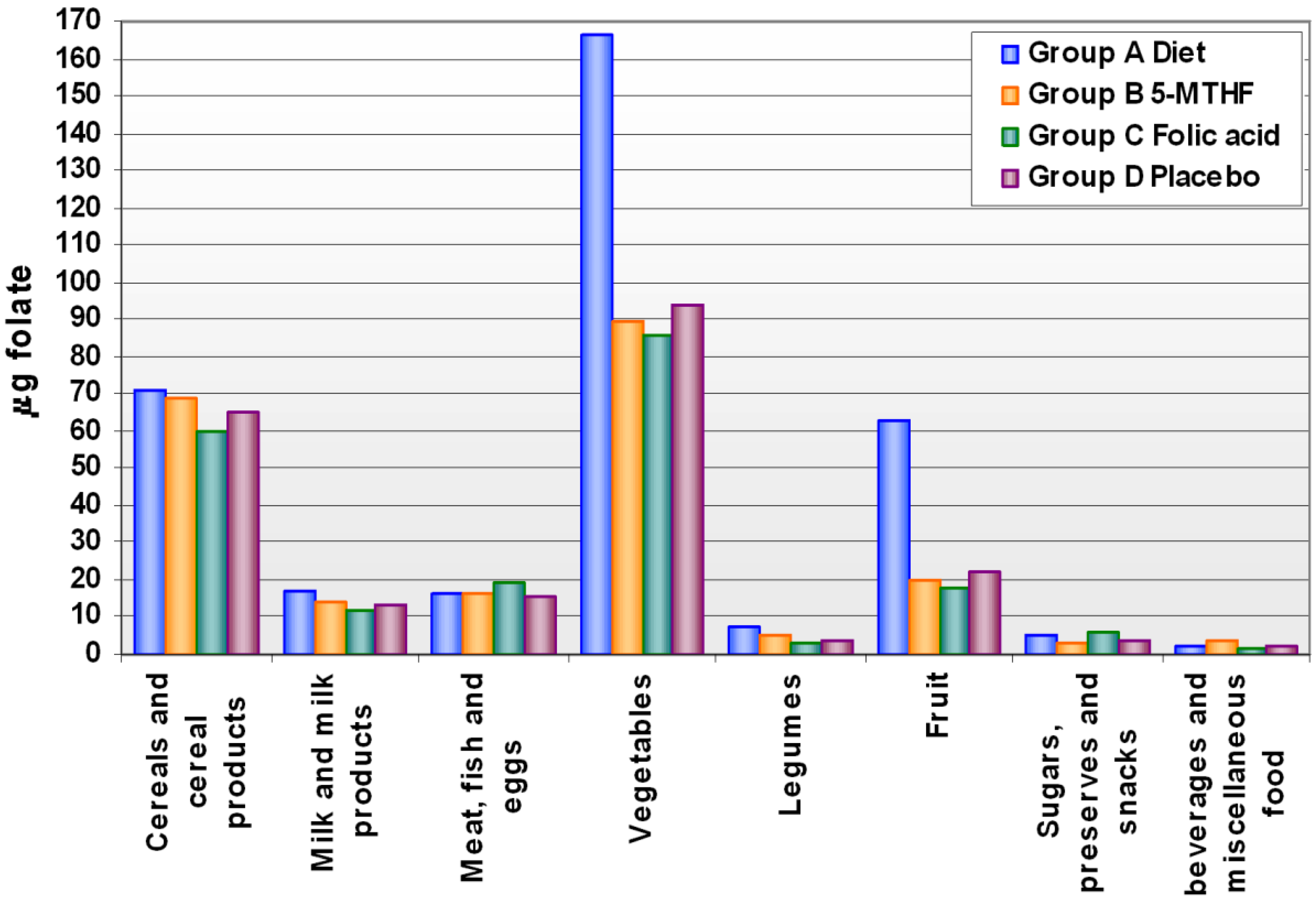
2.2. Dietary Assessment
2.3. Study Design
- habitual diet (usually containing about 220 μg of natural folate) + 200 µg from natural folate-rich diet, daily; subjects were advised to consume their habitual diet plus additional natural folate-rich foods to achieve an additional intake of 200 µg/day of folate; this was obtained throughout a “score diet of at least 10 points” (1 point = 20 µg);
- habitual diet + [6S]5-methyltetrahydrofolate 200 µg (340 DFEs), daily;
- habitual diet + folic acid 200 µg , (340 DFEs) daily;
- habitual diet + placebo.
2.4. Supplements
2.5. Specimen Collection and Biochemical Measurements
2.6. Statistical Analysis
3. Results
| n = 149 | Treatment group | ||||
|---|---|---|---|---|---|
| Enriched Diet (n = 35) | % 5-MTHF (n = 39) | Folic acid (n = 37) | Placebo (n = 38) | p 2 | |
| Age (years) | 41 (33, 51) | 41 (25, 50) | 40 (28, 50) | 41 (31, 51) | 0.87 |
| Sex, male (%) | 54.3 | 59.0 | 62.2 | 57.9 | 0.93 |
| BMI (kg/m2) | 23.8 (21.6, 26.5) | 23.4 (21.5, 25.9) | 24.3 (21.0, 26.8) | 23.7 (22.1, 25.8) | 0.83 |
| Weight (kg) | 70.5 (60.5, 84.5) | 66.0 (55.5, 79) | 71.5 (58, 83) | 67.0 (56.5, 79.0) | 0.64 |
| Height (cm) | 171 (161, 177) | 168 (162, 179) | 172 (165, 176) | 168 (160, 179) | 0.95 |
| Current smokers (%) | 28.6 | 33.3 | 29.7 | 36.8 | 0.87 |
| High educational level (%) | 74.3 | 76.9 | 83.8 | 63.2 | 0.22 |
| Health care professionals (%) | 28.6 | 38.5 | 40.5 | 26.3 | 0.34 |
| Physical active (%) | 37.1 | 43.6 | 32.4 | 52.6 | 0.29 |
| Vegetables eaters (%) | 51.4 | 41.0 | 43.2 | 52.6 | 0.81 |
| Wine drinkers (%) | 51.4 | 69.2 | 56.8 | 65.8 | 0.28 |
| Coffee drinkers (%) | 22.9 | 30.8 | 40.5 | 26.3 | 0.23 |
| MTHFR polymorphism (%) | 0.98 | ||||
| Homozygotes for wild-type | 42.9 | 33.3 | 32.4 | 34.2 | |
| Heterozygotes | 31.4 | 38.5 | 40.5 | 39.5 | |
| Homozygotes for variant allele | 25.7 | 28.2 | 27.1 | 26.3 | |
| n = 149 | Treatment group | ||||
|---|---|---|---|---|---|
| Enriched diet (n = 35) | % 5-MTHF (n = 39) | Folic acid (n = 37) | Placebo (n = 38) | p 2 | |
| Homocystein (μmol/L) | 14.3 (11, 17.3) | 14 (11.1, 18.1) | 13.3 (11.6, 15.3) | 14.8 (10.1, 17.2) | 0.96 |
| Red blood cells (millions) | 4.64 (4.32, 5.15) | 4.89 (4.43, 5.34) | 4.91 (4.60, 5.19) | 4.76 (4.43, 4.97) | 0.32 |
| Hemoglobin (g/dL) | 13.5 (12.9, 14.7) | 13.8 (13.0, 15.2) | 14.7 (13.2, 15.1) | 14.4 (13.6, 14.7) | 0.42 |
| Haematocrit (%) | 42.2 (39.3, 45.9) | 42.4 (40.0, 47.2) | 44.6 (40.5, 46.3) | 43.5 (40.5, 44.9) | 0.65 |
| Creatinine (mg/dL) | 0.8 (0.7, 0.9) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.8 (0.7, 0.9) | 0.53 |
| Serum B6 (ng/mL) | 9.0 (6.4, 12.8) | 9.3 (6.2, 10.9) | 8.0 (5.1, 12.5) | 8.7 (6.7, 11.9) | 0.55 |
| Serum B12 (pg/mL) | 388 (329, 494) * | 310 (252, 437) | 307 (250, 388) | 311 (225, 386) | 0.04 |
| Treatment group | Pre-treatment Homocysteine 1 (μmol/L) | Post-treatment Homocysteine 1 (μmol/L) | % Change after treatment 2 | p 3 | p 4 | Homocysteine decrease >25% (%) |
|---|---|---|---|---|---|---|
| Enriched Diet (n = 35) | 13.9 (12.2, 15.9) | 11.1 (9.5, 12.9) | −20.1 | 0.002 | <0.001 | 42.9 |
| 5-MTHF (n = 39) | 13.9 (12.2, 15.9) | 11.2 (9.8, 12.8) | −19.4 | 0.001 | 0.002 | 46.2 |
| Folic acid (n = 37) | 13.7 (12.5, 15.1) | 10.7 (9.6, 11.9) | −21.9 | <0.001 | <0.001 | 48.7 |
| Placebo (n = 38) | 14.6 (12.7, 16.8) | 15.3 (13.2, 17.7) | 4.8 | 0.23 | 10.5 |
| Treatment group | MTHFR polymorphism | Pre-treatment Homocysteine 1 (μmol/L) | Post-treatment Homocysteine 1 (μmol/L) | % Change after treatment 2 | p 3 | p 4 |
|---|---|---|---|---|---|---|
| Enriched Diet | TT (n = 9) | 15.1 (10.6, 21.4) | 11.3 (7.8, 16.3) | −25.2 | 0.46 | 0.07 |
| CT/CC (n = 26) | 13.5 (11.7, 15.7) | 11.0 (9.2, 13.2) | −18.5 | 0.01 | ||
| 5-MTHF | TT (n = 11) | 12.8 (9.1, 18.2) | 11.8 (8.9, 15.6) | −7.8 | 0.47 | 0.37 |
| CT/CC (n = 28) | 14.3 (12.4, 16.5) | 11.0 (9.4, 12.8) | −23.1 | 0.002 | ||
| Folic acid | TT (n = 10) | 15.3 (11.6, 20.3) | 10.6 (8.1, 14.0) | −30.7 | 0.35 | 0.01 |
| CT/CC (n = 27) | 13.2 (12.0, 14.5) | 10.7 (9.4, 12.1) | −18.9 | 0.001 | ||
| Placebo | TT (n = 10) | 17.4 (13.1, 23.2) | 21.0 (16.1, 27.4) | 20.7 | 0.07 | 0.15 |
| CT/CC (n = 28) | 13.7 (11.7, 16.1) | 13.7 (11.6, 16.1) | 0.21 | 0.62 |
| Treatment group | Pre-treatment RBC folate 1 | Post-treatment RBC folate 1 | % Change after treatment 2 | p 3 | p 4 |
|---|---|---|---|---|---|
| Enriched Diet (n = 33) | 225 (210, 241) | 247 (223, 275) | 9.7 | 0.12 | 0.02 |
| 5-MTHF (n = 38) | 218 (205, 233) | 226 (205, 250) | 3.8 | 0.53 | 0.24 |
| Folic Acid (n = 37) | 218 (199, 239) | 253 (228, 280) | 16.1 | 0.01 | 0.01 |
| Placebo (n = 37) | 227 (211, 243) | 216 (191, 243) | −4.8 | 0.35 |
4. Discussion
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Clarke, R.; Halsey, J.; Bennett, D.; Lewington, S. Homocysteine and vascular disease: Review of published results of the homocysteine-lowering trials. J. Inherit. Metab. Dis. 2011, 34, 83–91. [Google Scholar] [CrossRef]
- Dangour, A.D.; Withouse, P.J.; Rafferty, K.; Mitchell, S.A.; Smith, L.; Hawkesworth, S.; Vellas, B. B-vitamins and fatty acids in the prevention and treatment of Alzheimer’s disease and dementia: A systematic review. J. Alzheimers Dis. 2010, 22, 205–224. [Google Scholar]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of folic acid, with or without other B vitamins, on cognitive disorders: Meta-analysis of randomized trials. Am. J. Med. 2010, 123, 522–527.e2. [Google Scholar]
- Kim, Y.I. Folate and colorectal cancer: An evidence based critical review. Mol. Nutr. Food Res. 2007, 51, 267–292. [Google Scholar] [CrossRef]
- Eussen, S.J.P.M.; Vollset, S.E.; Ingland, J.; Meyer, K.; Fredriksen, A.; Ueland, P.M.; Jenab, M.; Slimani, N.; Boffetta, P.; Overvad, K.; et al. Plasma folate, related genetic variants and colorectal cancer risk in EPIC. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1328–1340. [Google Scholar] [CrossRef]
- De-Regie, L.M.; Fernandez-Gaxiola, A.C.; Donswell, T.; Pena-Rosas, J.P. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2010, CD007950. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant and child development. Food Nutr. Bull. 2008, 29 (2 Suppl.), S101–S111. [Google Scholar]
- Brouwer, I.A.; van Dusseldorp, M.; West, C.E.; Steegers-Theunissen, R.P.M. Bioavailability and bioefficacy of folate and folic acid in man. Nutr. Res. Rev. 2001, 14, 267–293. [Google Scholar] [CrossRef]
- Dhonukshe-Rutten, R.A.M.; de Vries, J.H.M.; de Bree, A.; van der Put, N.; van Staveren, W.A.; de Groot, L.C.P.G.M. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur. J. Clin. Nutr. 2009, 63, 18–30. [Google Scholar] [CrossRef]
- Turrini, A.; Leclercq, C.; D’Amicis, A. Patterns of food and nutrient intakes in Italy and their application to the development of food-based dietary guidelines. Br. J. Nutr. 1999, 81 (Suppl. 2), S83–S89. [Google Scholar]
- Institute of Medicine, DRI Dietary References Intake for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline; National Academy Press: Washington, DC, USA, 1998.
- Araki, A.; Sako, Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detector. J. Chromatogr. 1987, 422, 43–52. [Google Scholar] [CrossRef]
- De Stefano, V.; Zappacosta, B.; Persichilli, S.; Rossi, E.; Casorelli, I.; Paciaroni, K.; Chiusolo, P.; Leone, A.M.; Giardina, B.; Leone, G.; et al. Prevalence of mild hyperhomocisteinemia and association with thrombophilic genotypes (Factor V Leiden and factor II G20210A) in Italian patients with venous thromboembolic disease. Br. J. Haematol. 1999, 106, 564–568. [Google Scholar] [CrossRef]
- Wright, A.J.A.; Finglas, P.M.; Southon, S. Erythrocyte folate analysis: Saponin added during lysis of whole blood can increase apparent folate concentrations, depending on hemolysate pH. Clin. Chem. 2000, 46, 1978–1986. [Google Scholar]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar]
- Fohr, I.P.; Prinz-Langenohl, R.; Brönstrup, A.; Bohlmann, A.M.; Nau, H.; Berthold, H.K.; Pietrzik, K. 5,10-Methylenetetrahydrofolate reductase genotype determines the plasma homocysteine-lowering effect of supplementation with 5-methyltetrahydrofolate or folic acid in healthy young women. Am. J. Clin. Nutr. 2002, 75, 275–282. [Google Scholar]
- Venn, B.J.; Green, T.J.; Moser, R.; Mann, J.I. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: A randomized placebo-controlled study. Am. J. Clin. Nutr. 2003, 77, 658–662. [Google Scholar]
- Prinz-Langenohl, R.; Brämswig, S.; Tobolski, O.; Smulders, Y.M.; Smith, D.E.C.; Finglas, P.M.; Pietrzik, K. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C→T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 2009, 158, 2014–2021. [Google Scholar] [CrossRef]
- Brouwer, I.A.; van Dusseldorp, M.; West, C.E.; Meyboom, S.; Thomas, C.M.G.; Duran, M.; van het Hof, K.H.; Eskes, T.K.; Hautvast, J.G.; et al. Dietary folate from vegetables and citrus fruit decreases plasma homocysteine concentrations in humans in a dietary controlled study. J. Nutr. 1999, 129, 1135–1139. [Google Scholar]
- Cuskelly, G.J.; McNulty, H.; Scott, J.M. Effect of increasing dietary folate on red-cell folate: Implications for prevention of neural tube defects. Lancet 1996, 347, 657–659. [Google Scholar] [CrossRef]
- Homocysteine Lowering Trialists’ Collaboration. Lowering blood homocysteine with folic acid-based supplements: Meta-analysis of randomised trials. BMJ 1998, 316, 894–898.
- Zappacosta, B.; Persichilli, S.; Iacoviello, L.; di Castelnuovo, A.; Graziano, M.; Gervasoni, J.; Leoncini, E.; Cimino, G.; Mastroiacovo, P. Folate, vitamin B12 and homocysteine status in an Italian blood donor population. Nutr. Metab. Cardiovasc. Dis. 2011. [Google Scholar] [CrossRef]
- Ward, M.; McNulty, H.; McPartlin, J.; Strain, J.J.; Weir, D.G.; Scott, J.M. Plasma homocysteine, a risk factor for cardiovascular disease, is lowered by physiological doses of folic acid. Quart. J. Med. 1997, 90, 519–524. [Google Scholar] [CrossRef]
- Venn, B.J.; Mann, J.L.; Williams, S.M.; Riddell, L.J.; Chisholm, A.; Harper, M.J.; Aitken, W.; Rossaak, J.L. Assessment of three levels of folic acid on serum folate and plasma homocysteine: A randomised placebo-controlled double-blind dietary intervention trial. Eur. J. Clin. Nutr. 2002, 56, 748–754. [Google Scholar] [CrossRef]
- Ashfield-Watt, P.A.L.; Pullin, C.H.; Whiting, J.M.; Clark, Z.E.; Moat, S.J.; Newcombe, R.G.; Burr, M.L.; Lewis, M.J.; Powers, H.J.; McDowell, I.F.W. Methylenetetrahydrofolate reductase 677C→T genotype modulates homocysteine responses to a folate-rich diet or a low dose folic acid supplement: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 180–186. [Google Scholar]
- Ashfield-Watt, P.A.L.; Whiting, J.M.; Clark, Z.E.; Moat, S.J.; Newcombe, R.G.; Burr, M.L.; McDowell, I.F.W. A comparison of the effect of advice to eat either “5-a-day” fruit and vegetables or folic acid-fortified foods on plasma folate and homocysteine. Eur. J. Clin. Nutr. 2003, 57, 316–323. [Google Scholar] [CrossRef]
- Bogers, R.P.; Dagnelie, P.C.; Bast, A.; van Leeuwen, M.; van Klaveren, J.D.; van den Brandt, P.A. Effect of increased vegetable and fruit consumption on plasma folate and homocysteine concentrations. Nutrition 2007, 23, 97–102. [Google Scholar] [CrossRef]
Supplementary
| Folate intake μg/day | Diet n = 35 | 5-MTHF n = 39 | Folic acid n = 37 | Placebo n = 38 |
|---|---|---|---|---|
| Baseline | 205.2 ± 54.9 | 220.5 ± 44.9 | 202.5 ± 66.8 | 220.5 ± 53.9 |
| After supplemetation | 350.9 ± 76.1 | 237.2 ± 75.5 | 236.2 ± 75.7 | 218.5 ± 88.3 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zappacosta, B.; Mastroiacovo, P.; Persichilli, S.; Pounis, G.; Ruggeri, S.; Minucci, A.; Carnovale, E.; Andria, G.; Ricci, R.; Scala, I.; et al. Homocysteine Lowering by Folate-Rich Diet or Pharmacological Supplementations in Subjects with Moderate Hyperhomocysteinemia. Nutrients 2013, 5, 1531-1543. https://doi.org/10.3390/nu5051531
Zappacosta B, Mastroiacovo P, Persichilli S, Pounis G, Ruggeri S, Minucci A, Carnovale E, Andria G, Ricci R, Scala I, et al. Homocysteine Lowering by Folate-Rich Diet or Pharmacological Supplementations in Subjects with Moderate Hyperhomocysteinemia. Nutrients. 2013; 5(5):1531-1543. https://doi.org/10.3390/nu5051531
Chicago/Turabian StyleZappacosta, Bruno, Pierpaolo Mastroiacovo, Silvia Persichilli, George Pounis, Stefania Ruggeri, Angelo Minucci, Emilia Carnovale, Generoso Andria, Roberta Ricci, Iris Scala, and et al. 2013. "Homocysteine Lowering by Folate-Rich Diet or Pharmacological Supplementations in Subjects with Moderate Hyperhomocysteinemia" Nutrients 5, no. 5: 1531-1543. https://doi.org/10.3390/nu5051531
APA StyleZappacosta, B., Mastroiacovo, P., Persichilli, S., Pounis, G., Ruggeri, S., Minucci, A., Carnovale, E., Andria, G., Ricci, R., Scala, I., Genovese, O., Turrini, A., Mistura, L., Giardina, B., & Iacoviello, L. (2013). Homocysteine Lowering by Folate-Rich Diet or Pharmacological Supplementations in Subjects with Moderate Hyperhomocysteinemia. Nutrients, 5(5), 1531-1543. https://doi.org/10.3390/nu5051531





