Myths, Artifacts, and Fatal Flaws: Identifying Limitations and Opportunities in Vitamin C Research
Abstract
:1. Introduction
2. Review of Studies Using Ascorbic Acid
2.1. Ascorbic Acid in Human Cell Culture
2.1.1. Ascorbate Stability in Cell Culture
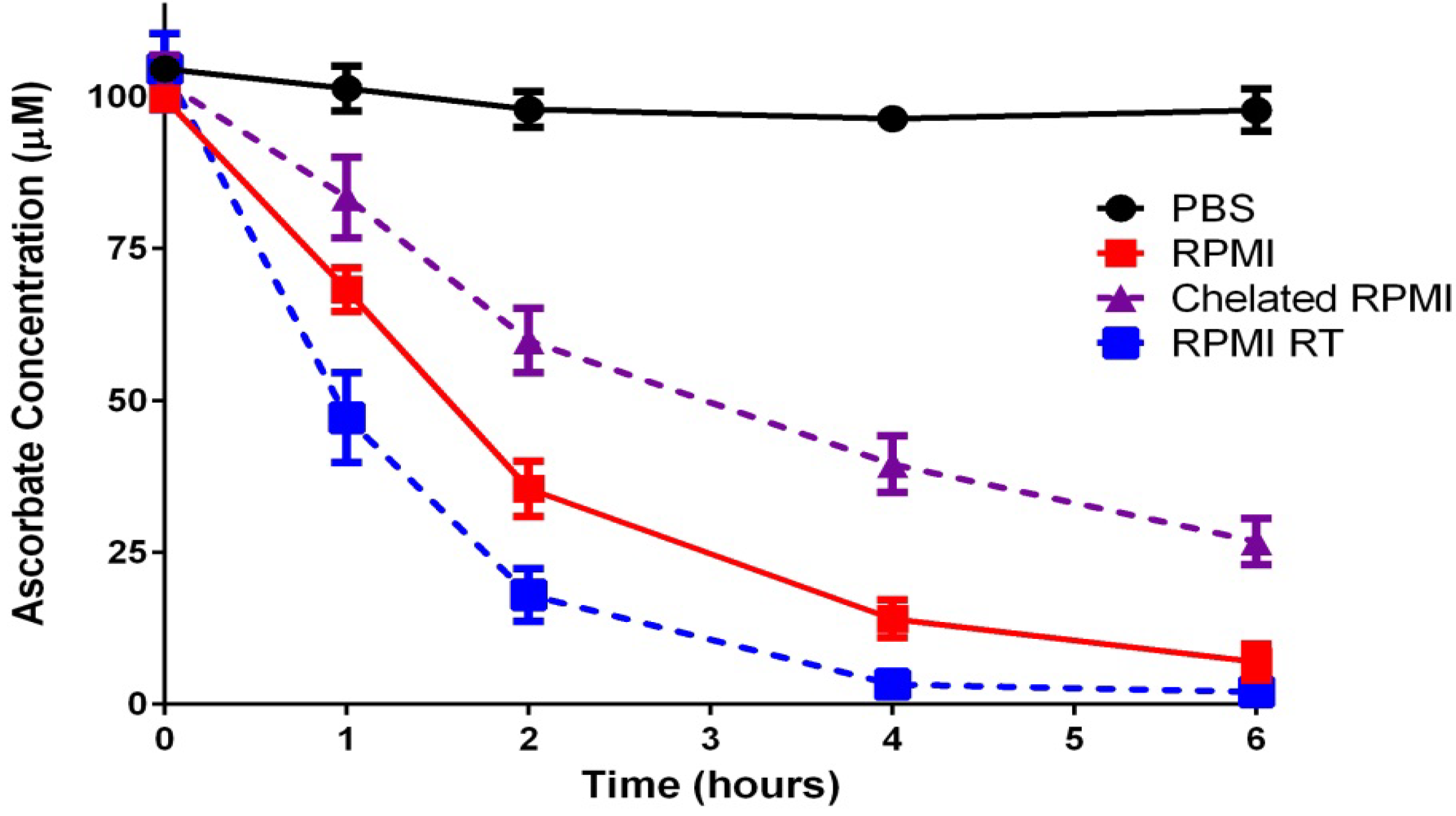
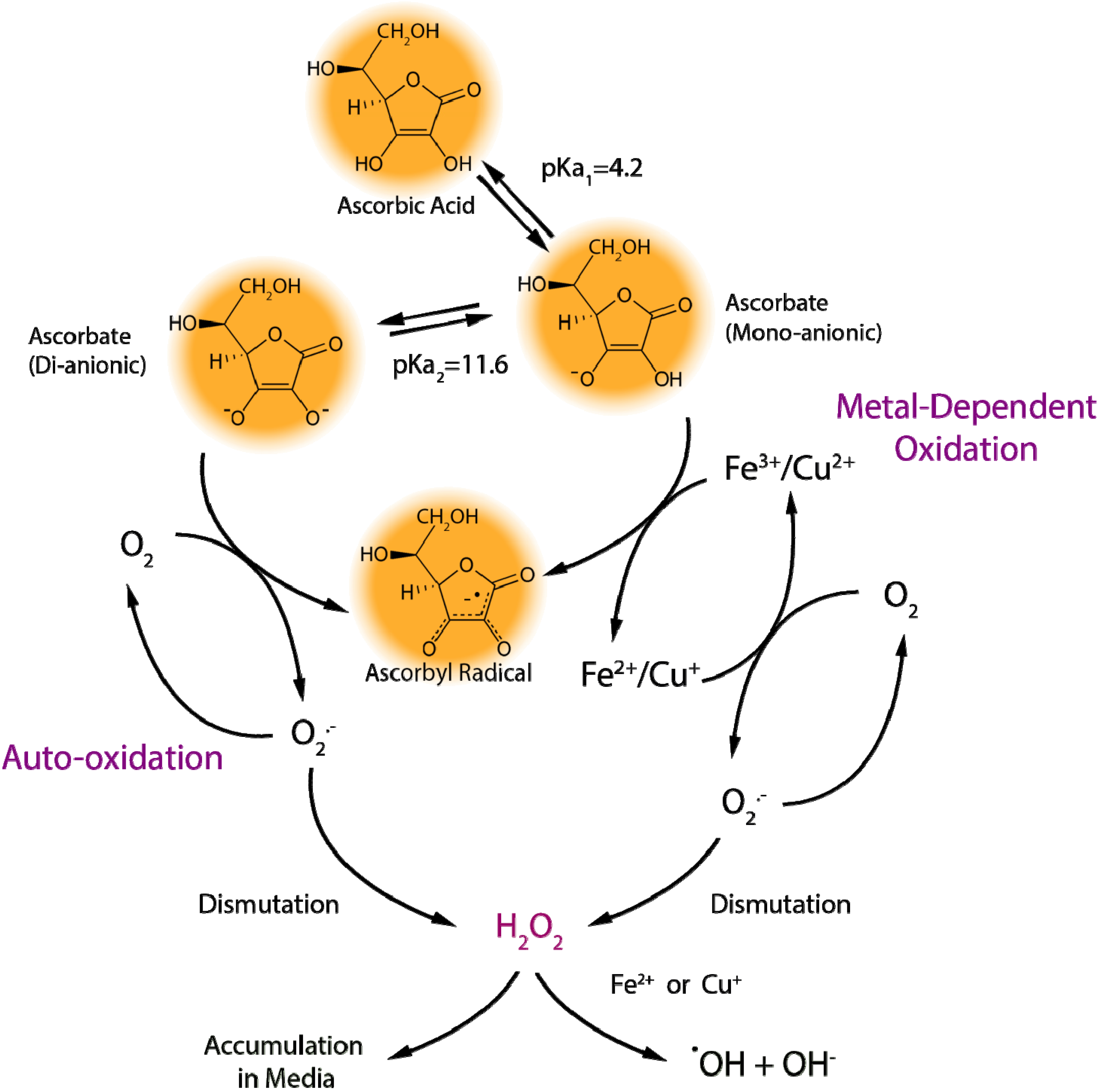
2.1.2. “Cellular Scurvy”
2.1.3. Proper Use of Ascorbate in Cell Culture
2.2. Animal Studies Involving Ascorbic Acid
2.2.1. When a Vitamin in not a Vitamin
2.2.2. Transporter Troubles
| Species/Strain | GULO Status | Maintenance Dose (mg/kg Body Weight) * | Saturation Dose (mg/kg Body Weight) | Comparisons to Humans and Other Models |
|---|---|---|---|---|
| Mouse (wild-type) | Functional | n/a | n/a | Expresses GLUT4 on erythrocytes [78] |
| Sfx Mouse | Complete deletion | ~20 | >100 | Spontaneous mutant model that develops spontaneous bone fractures and bone fragility not seen in other GULO knockout mice [71,79] |
| GULO−/− Mouse | Exon 3 & 4 deleted | ~20 | ~600 | Genetically engineered mouse model that displays blood vessel fragility [80] Possible muscle weakness independent of vitamin C status [81] |
| SMP30−/− mouse | Functional | ~20 | ~240 | Not a GULO knockout [82]; may have residual synthesis activity Multiple aging effects independent of vitamin C status [50] |
| Rat (wild-type) | Functional | n/a | n/a | Expresses GLUT4 on erythrocytes [78] Preferential absorption of dehydroascorbic acid in gut [63] Lack of active ascorbate transport in intestine [49,50,51] |
| ODS Rat | GULO point mutation | ~10 | ~200 | Spontaneous mutant model that develops hind limb bone disorders [83] Vitamin C deficiency lowers blood pressure [84] Vitamin C deficiency protects from ischemic injury [85] GULO mRNA and protein still expressed [86] |
| Guinea Pig | Multiple exons lost | ~2 | ~27 | GULO gene deletion during evolution [55] Expresses GLUT1 on erythrocytes, similar to humans [78] Active ascorbate absorption in gut, similar to humans [49,50,51] Vitamin C deficiency exaggerates cardiovascular decline with age, similar to humans [87,88,89] Other similarities to human vitamin C supplementation [50] |
| Human | Multiple exons lost | ~0.15 | ~3 |
2.2.3. Choose Models with Care
2.3. Human Studies with Vitamin C
2.3.1. Vitamin C RCTs: Failures in Design
2.3.2. Technical Issues of Human Studies
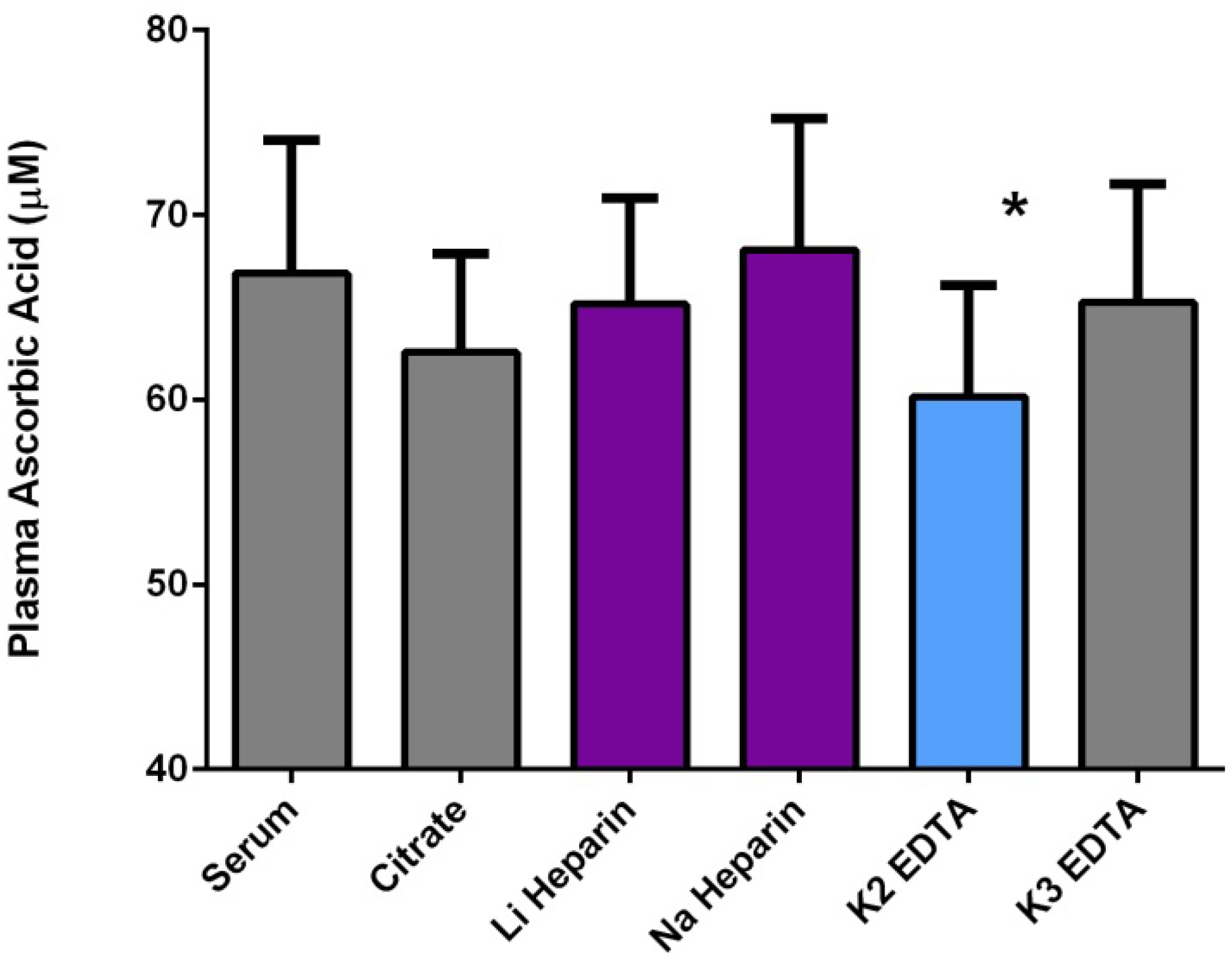
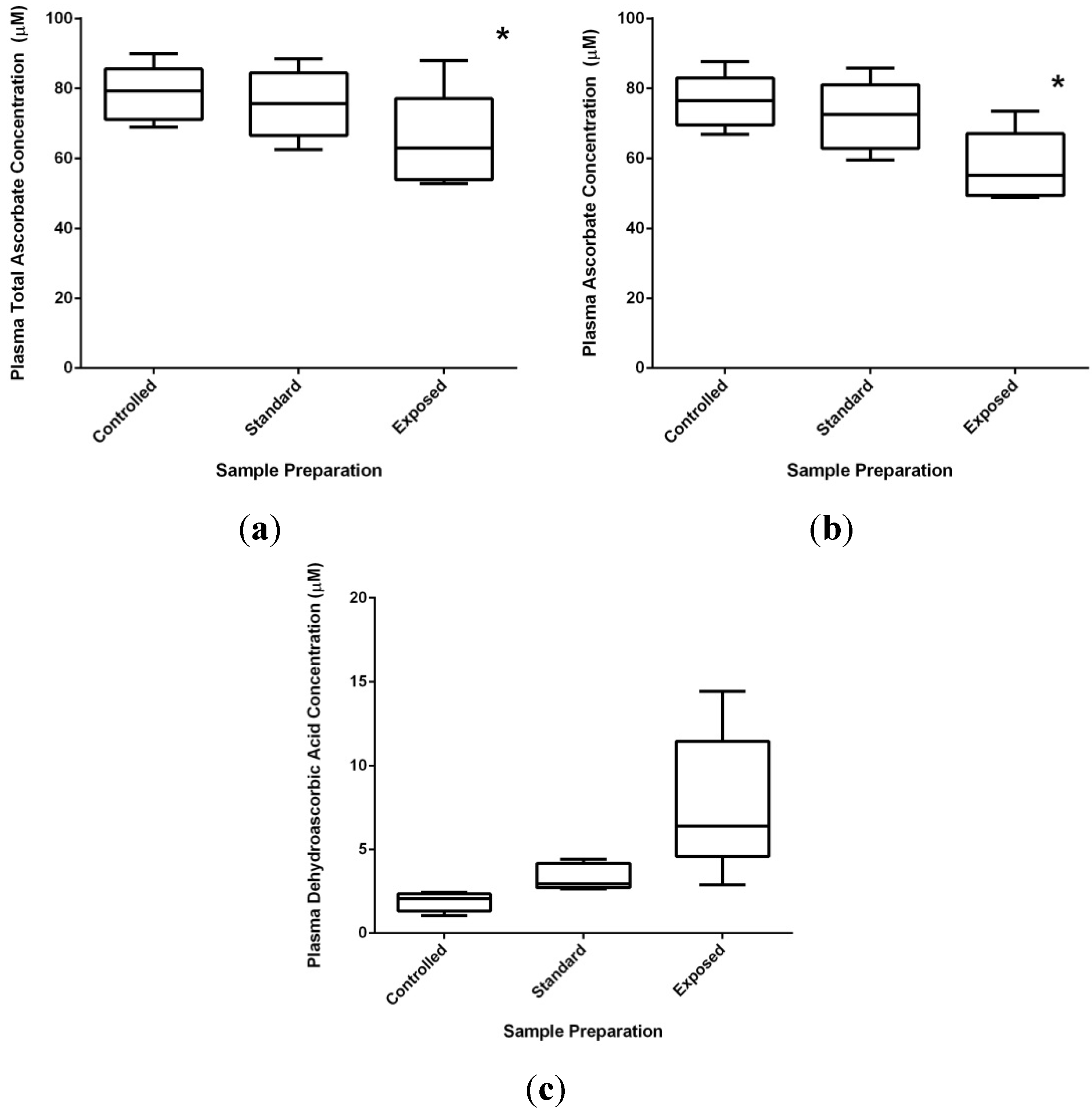
2.3.3. Health Effects of Vitamin C: Reality versus Mythology
2.3.4. Supplementing C: From Formulation to Dose
3. Methods
3.1. Ascorbate in Cell Culture Media
3.2. Human Subjects and Blood Collection
3.3. Vitamin C and Urate Analysis
3.4. Statistics
4. Conclusions
Conflicts of Interest
References
- Frei, B.; Birlouez-Aragon, I.; Lykkesfeldt, J. Authors’ perspective: What is the optimum intake of vitamin C in humans? Crit. Rev. Food Sci. Nutr. 2012, 52, 815–829. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Poulsen, H.E. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br. J. Nutr. 2010, 103, 1251–1259. [Google Scholar] [CrossRef]
- Michels, A.J.; Frei, B.B. Vitamin C. In Biochemical, Physiological, and Molecular Aspects of Human Nutrition, 3rd ed.; Stipanuk, M.H., Caudill, M.A., Eds.; Elsevier/Saunders: St. Louis, MO, USA, 2012; pp. 626–654. [Google Scholar]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R., III. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003, 540, 3–6. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef]
- Frikke-Schmidt, H.; Lykkesfeldt, J. Keeping the intracellular vitamin C at a physiologically relevant level in endothelial cell culture. Anal. Biochem. 2010, 397, 135–137. [Google Scholar] [CrossRef]
- Chepda, T.; Cadau, M.; Girin, P.; Frey, J.; Chamson, A. Monitoring of ascorbate at a constant rate in cell culture: Effect on cell growth. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 26–30. [Google Scholar] [CrossRef]
- Wee, L.M.; Long, L.H.; Whiteman, M.; Halliwell, B. Factors affecting the ascorbate- and phenolic-dependent generation of hydrogen peroxide in Dulbecco’s Modified Eagles Medium. Free Radic. Res. 2003, 37, 1123–1130. [Google Scholar] [CrossRef]
- Clement, M.V.; Ramalingam, J.; Long, L.H.; Halliwell, B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid. Redox Signal. 2001, 3, 157–163. [Google Scholar]
- Cazzola, M.; Bergamaschi, G.; Dezza, L.; Arosio, P. Manipulations of cellular iron metabolism for modulating normal and malignant cell proliferation: Achievements and prospects. Blood 1990, 75, 1903–1919. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Buettner, G.R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: Ascorbate as a test for catalytic metals. J. Biochem. Biophys. Methods 1988, 16, 27–40. [Google Scholar] [CrossRef]
- Minetti, M.; Forte, T.; Soriani, M.; Quaresima, V.; Menditto, A.; Ferrari, M. Iron-induced ascorbate oxidation in plasma as monitored by ascorbate free radical formation. No spin-trapping evidence for the hydroxyl radical in iron-overloaded plasma. Biochem. J. 1992, 282, 459–465. [Google Scholar]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar]
- Chen, K.; Suh, J.; Carr, A.C.; Morrow, J.D.; Zeind, J.; Frei, B. Vitamin C suppresses oxidative lipid damage in vivo, even in the presence of iron overload. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1406–E1412. [Google Scholar]
- Berger, T.M.; Polidori, M.C.; Dabbagh, A.; Evans, P.J.; Halliwell, B.; Morrow, J.D.; Roberts, L.J., II; Frei, B. Antioxidant activity of vitamin C in iron-overloaded human plasma. J. Biol. Chem. 1997, 272, 15656–15660. [Google Scholar]
- Halliwell, B. Vitamin C: Antioxidant or pro-oxidant in vivo? Free Radic. Res. 1996, 25, 439–454. [Google Scholar] [CrossRef]
- Long, L.H.; Halliwell, B. Artefacts in cell culture: Pyruvate as a scavenger of hydrogen peroxide generated by ascorbate or epigallocatechin gallate in cell culture media. Biochem. Biophys. Res. Commun. 2009, 388, 700–704. [Google Scholar] [CrossRef]
- Long, L.H.; Halliwell, B. Artefacts in cell culture: α-Ketoglutarate can scavenge hydrogen peroxide generated by ascorbate and epigallocatechin gallate in cell culture media. Biochem. Biophys. Res. Commun. 2011, 406, 20–24. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Ascorbate free radical as a marker of oxidative stress: An EPR study. Free Radic. Biol. Med. 1993, 14, 49–55. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef]
- Bode, A.M.; Cunningham, L.; Rose, R.C. Spontaneous decay of oxidized ascorbic acid (dehydro-l-ascorbic acid) evaluated by high-pressure liquid chromatography. Clin. Chem. 1990, 36, 1807–1809. [Google Scholar]
- Linster, C.L.; van Schaftingen, E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar]
- Washko, P.W.; Wang, Y.; Levine, M. Ascorbic acid recycling in human neutrophils. J. Biol. Chem. 1993, 268, 15531–15535. [Google Scholar]
- Rumsey, S.C.; Kwon, O.; Xu, G.W.; Burant, C.F.; Simpson, I.; Levine, M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997, 272, 18982–18989. [Google Scholar] [CrossRef]
- Vera, J.C.; Rivas, C.I.; Fischbarg, J.; Golde, D.W. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 1993, 364, 79–82. [Google Scholar] [CrossRef]
- Wilson, J.X. The physiological role of dehydroascorbic acid. FEBS Lett. 2002, 527, 5–9. [Google Scholar] [CrossRef]
- Pedraza, C.E.; Chien, Y.C.; McKee, M.D. Calcium oxalate crystals in fetal bovine serum: Implications for cell culture, phagocytosis and biomineralization studies in vitro. J. Cell. Biochem. 2008, 103, 1379–1393. [Google Scholar] [CrossRef]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Preventable effect of l-threonate, an ascorbate metabolite, on androgen-driven balding via repression of dihydrotestosterone-induced dickkopf-1 expression in human hair dermal papilla cells. BMB Rep. 2010, 43, 688–692. [Google Scholar] [CrossRef]
- Smith, A.R.; Visioli, F.; Hagen, T.M. Vitamin C matters: Increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J. 2002, 16, 1102–1104. [Google Scholar]
- Huang, A.; Vita, J.A.; Venema, R.C.; Keaney, J.F., Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J. Biol. Chem. 2000, 275, 17399–17406. [Google Scholar] [CrossRef]
- Bergsten, P.; Amitai, G.; Kehrl, J.; Dhariwal, K.R.; Klein, H.G.; Levine, M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J. Biol. Chem. 1990, 265, 2584–2587. [Google Scholar]
- Martin, A.; Frei, B. Both intracellular and extracellular vitamin C inhibit atherogenic modification of LDL by human vascular endothelial cells. Arterioscler. Thromb. Vas. Biol. 1997, 17, 1583–1590. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Meredith, M.E. Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem. Biophys. Res. Commun. 2012, 426, 148–152. [Google Scholar] [CrossRef]
- Visioli, F.; Smith, A.; Zhang, W.; Keaney, J.F., Jr.; Hagen, T.; Frei, B. Lipoic acid and vitamin C potentiate nitric oxide synthesis in human aortic endothelial cells independently of cellular glutathione status. Redox Rep. 2002, 7, 223–227. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef]
- Bruick, R.K.; McKnight, S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001, 294, 1337–1340. [Google Scholar] [CrossRef]
- Flashman, E.; Davies, S.L.; Yeoh, K.K.; Schofield, C.J. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem. J. 2010, 427, 135–142. [Google Scholar] [CrossRef]
- Myllyharju, J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003, 22, 15–24. [Google Scholar] [CrossRef]
- Knowles, H.J.; Raval, R.R.; Harris, A.L.; Ratcliffe, P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003, 63, 1764–1768. [Google Scholar]
- Murad, S.; Grove, D.; Lindberg, K.A.; Reynolds, G.; Sivarajah, A.; Pinnell, S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 2879–2882. [Google Scholar] [CrossRef]
- Diliberto, E.J., Jr.; Daniels, A.J.; Viveros, O.H. Multicompartmental secretion of ascorbate and its dual role in dopamine β-hydroxylation. Am. J. Clin. Nutr. 1991, 54, 1163S–1172S. [Google Scholar]
- Monfort, A.; Wutz, A. Breathing-in epigenetic change with vitamin C. EMBO Rep. 2013, 14, 337–346. [Google Scholar] [CrossRef]
- Ladurner, A.; Schmitt, C.A.; Schachner, D.; Atanasov, A.G.; Werner, E.R.; Dirsch, V.M.; Heiss, E.H. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic. Biol. Med. 2012, 52, 2082–2090. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Qiao, H. Transfer of ascorbic acid across the vascular endothelium: Mechanism and self-regulation. Am. J. Physiol. Cell Physiol. 2009, 297, C169–C178. [Google Scholar] [CrossRef]
- Wu, F.; Schuster, D.P.; Tyml, K.; Wilson, J.X. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic. Biol. Med. 2007, 42, 124–131. [Google Scholar] [CrossRef]
- Yu, R.; Schellhorn, H.E. Recent applications of engineered animal antioxidant deficiency models in human nutrition and chronic disease. J. Nutr. 2013, 143, 1–11. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Braun, L.; Csala, M.; Poussu, A.; Garzo, T.; Mandl, J.; Banhegyi, G. Glutathione depletion induces glycogenolysis dependent ascorbate synthesis in isolated murine hepatocytes. FEBS Lett. 1996, 388, 173–176. [Google Scholar] [CrossRef]
- Chan, T.S.; Wilson, J.X.; O’Brien, P.J. Glycogenolysis is directed towards ascorbate synthesis by glutathione conjugation. Biochem. Biophys. Res. Commun. 2004, 317, 149–156. [Google Scholar] [CrossRef]
- Banhegyi, G.; Braun, L.; Csala, M.; Puskas, F.; Mandl, J. Ascorbate metabolism and its regulation in animals. Free Radic. Biol. Med. 1997, 23, 793–803. [Google Scholar] [CrossRef]
- Yang, H. Conserved or lost: Molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis. Biochem. Genet. 2013, 51, 413–425. [Google Scholar] [CrossRef]
- Spencer, R.P.; Purdy, S.; Hoeldtke, R.; Bow, T.M.; Markulis, M.A. Studies on intestinal absorption of l-ascorbic acid-1-C-14. Gastroenterology 1963, 44, 768–773. [Google Scholar]
- Hornig, D.; Weber, F.; Wiss, O. Site of intestinal absorption of ascorbic acid in guinea pigs and rats. Biochem. Biophys. Res. Commun. 1973, 52, 168–172. [Google Scholar] [CrossRef]
- Hornig, D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975, 258, 103–118. [Google Scholar] [CrossRef]
- Stevenson, N.R.; Brush, M.K. Existence and characteristics of Na positive-dependent active transport of ascorbic acid in guinea pig. Am. J. Clin. Nutr. 1969, 22, 318–326. [Google Scholar]
- Bianchi, J.; Wilson, F.A.; Rose, R.C. Dehydroascorbic acid and ascorbic acid transport systems in the guinea pig ileum. Am. J. Physiol. 1986, 250, G461–G468. [Google Scholar]
- Mellors, A.J.; Nahrwold, D.L.; Rose, R.C. Ascorbic acid flux across mucosal border of guinea pig and human ileum. Am. J. Physiol. 1977, 233, E374–E379. [Google Scholar]
- Stevenson, N.R. Active transport of l-ascorbic acid in the human ileum. Gastroenterology 1974, 67, 952–956. [Google Scholar]
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101. [Google Scholar] [CrossRef]
- Tsao, C.S.; Leung, P.Y.; Young, M. Effect of dietary ascorbic acid intake on tissue vitamin C in mice. J. Nutr. 1987, 117, 291–297. [Google Scholar]
- Rucker, R.B. Allometric scaling, metabolic body size and interspecies comparisons of basal nutritional requirements. J. Anim. Physiol. Anim. Nutr. 2007, 91, 148–156. [Google Scholar] [CrossRef]
- Bush, M.J.; Verlangieri, A.J. An acute study on the relative gastro-intestinal absorption of a novel form of calcium ascorbate. Res. Commun. Chem. Pathol. Pharmacol. 1987, 57, 137–140. [Google Scholar]
- Johnston, C.S.; Luo, B. Comparison of the absorption and excretion of three commercially available sources of vitamin C. J. Am. Diet. Assoc. 1994, 94, 779–781. [Google Scholar] [CrossRef]
- Vissers, M.C.; Bozonet, S.M.; Pearson, J.F.; Braithwaite, L.J. Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: Tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit). Am. J. Clin. Nutr. 2011, 93, 292–301. [Google Scholar] [CrossRef]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. A randomized steady-state bioavailability study of synthetic versus natural (Kiwifruit-Derived) vitamin C. Nutrients 2013, 5, 3684–3695. [Google Scholar] [CrossRef]
- Iwama, M.; Shimokado, K.; Maruyama, N.; Ishigami, A. Time course of vitamin C distribution and absorption after oral administration in SMP30/GNL knockout mice. Nutrition 2011, 27, 471–478. [Google Scholar] [CrossRef]
- Mohan, S.; Kapoor, A.; Singgih, A.; Zhang, Z.; Taylor, T.; Yu, H.; Chadwick, R.B.; Chung, Y.S.; Donahue, L.R.; Rosen, C.; et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J. Bone Miner. Res. 2005, 20, 1597–1610. [Google Scholar] [CrossRef]
- Meredith, M.E.; Harrison, F.E.; May, J.M. Differential regulation of the ascorbic acid transporter SVCT2 during development and in response to ascorbic acid depletion. Biochem. Biophys. Res. Commun. 2011, 414, 737–742. [Google Scholar] [CrossRef]
- Boyer, J.C.; Campbell, C.E.; Sigurdson, W.J.; Kuo, S.M. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem. Biophys. Res. Commun. 2005, 334, 150–156. [Google Scholar] [CrossRef]
- Maulen, N.P.; Henriquez, E.A.; Kempe, S.; Carcamo, J.G.; Schmid-Kotsas, A.; Bachem, M.; Grunert, A.; Bustamante, M.E.; Nualart, F.; Vera, J.C. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J. Biol. Chem. 2003, 278, 9035–9041. [Google Scholar] [CrossRef]
- MacDonald, L.; Thumser, A.E.; Sharp, P. Decreased expression of the vitamin C transporter SVCT1 by ascorbic acid in a human intestinal epithelial cell line. Br. J. Nutr. 2002, 87, 97–100. [Google Scholar] [CrossRef]
- Corpe, C.P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; Wang, Y.; et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Investig. 2010, 120, 1069–1083. [Google Scholar] [CrossRef]
- Savini, I.; Rossi, A.; Pierro, C.; Avigliano, L.; Catani, M.V. SVCT1 and SVCT2: Key proteins for vitamin C uptake. Amino Acids 2008, 34, 347–355. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Kinet, S.; Manel, N.; Mongellaz, C.; Prohaska, R.; Battini, J.L.; Delaunay, J.; Sitbon, M.; Taylor, N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 2008, 132, 1039–1048. [Google Scholar] [CrossRef]
- Beamer, W.G.; Rosen, C.J.; Bronson, R.T.; Gu, W.; Donahue, L.R.; Baylink, D.J.; Richardson, C.C.; Crawford, G.C.; Barker, J.E. Spontaneous fracture (sfx): A mouse genetic model of defective peripubertal bone formation. Bone 2000, 27, 619–626. [Google Scholar] [CrossRef]
- Maeda, N.; Hagihara, H.; Nakata, Y.; Hiller, S.; Wilder, J.; Reddick, R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc. Natl. Acad. Sci. USA 2000, 97, 841–846. [Google Scholar] [CrossRef]
- Ward, M.S.; Lamb, J.; May, J.M.; Harrison, F.E. Behavioral and monoamine changes following severe vitamin C deficiency. J. Neurochem. 2013, 124, 363–375. [Google Scholar] [CrossRef]
- Kondo, Y.; Inai, Y.; Sato, Y.; Handa, S.; Kubo, S.; Shimokado, K.; Goto, S.; Nishikimi, M.; Maruyama, N.; Ishigami, A. Senescence marker protein 30 functions as gluconolactonase in l-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc. Natl. Acad. Sci. USA 2006, 103, 5723–5728. [Google Scholar] [CrossRef]
- Horio, F.; Ozaki, K.; Yoshida, A.; Makino, S.; Hayashi, Y. Requirement for ascorbic acid in a rat mutant unable to synthesize ascorbic acid. J. Nutr. 1985, 115, 1630–1640. [Google Scholar]
- Horio, F.; Hayashi, K.; Mishima, T.; Takemori, K.; Oshima, I.; Makino, S.; Kakinuma, A.; Ito, H. A newly established strain of spontaneously hypertensive rat with a defect of ascorbic acid biosynthesis. Life Sci. 2001, 69, 1879–1890. [Google Scholar]
- Vergely, C.; Perrin, C.; Laubriet, A.; Oudot, A.; Zeller, M.; Guilland, J.C.; Rochette, L. Postischemic myocardial recovery and oxidative stress status of vitamin C deficient rat hearts. Cardiovasc. Res. 2001, 51, 89–99. [Google Scholar] [CrossRef]
- Nishikimi, M.; Koshizaka, T.; Kondo, K.; Ozawa, T.; Yagi, K. Expression of the mutant gene for l-gulono-gamma-lactone oxidase in scurvy-prone rats. Experientia 1989, 45, 126–129. [Google Scholar] [CrossRef]
- Fernandez, M.L.; McNamar, D.J. Dietary fat-mediated changes in hepatic apoprotein B/E receptor in the guinea pig: Effect of polyunsaturated, monounsaturated, and saturated fat. Metabolism 1989, 38, 1094–1102. [Google Scholar] [CrossRef]
- Bell, J.P.; Mosfer, S.I.; Lang, D.; Donaldson, F.; Lewis, M.J. Vitamin C and quinapril abrogate LVH and endothelial dysfunction in aortic-banded guinea pigs. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1704–H1710. [Google Scholar]
- Wolkart, G.; Wenzl, M.V.; Beretta, M.; Stessel, H.; Schmidt, K.; Mayer, B. Vascular tolerance to nitroglycerin in ascorbate deficiency. Cardiovasc. Res. 2008, 79, 304–312. [Google Scholar] [CrossRef]
- Liang, W.J.; Johnson, D.; Jarvis, S.M. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001, 18, 87–95. [Google Scholar] [CrossRef]
- Liang, W.J.; Johnson, D.; Ma, L.S.; Jarvis, S.M.; Wei, J.L. Regulation of the human vitamin C transporters expressed in COS-1 cells by protein kinase C [corrected]. Am. J. Physiol. Cell Physiol. 2002, 283, C1696–C1704. [Google Scholar]
- Subramanian, V.S.; Marchant, J.S.; Boulware, M.J.; Said, H.M. A C-terminal region dictates the apical plasma membrane targeting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J. Biol. Chem. 2004, 279, 27719–27728. [Google Scholar] [CrossRef]
- Mardones, L.; Zuniga, F.A.; Villagran, M.; Sotomayor, K.; Mendoza, P.; Escobar, D.; Gonzalez, M.; Ormazabal, V.; Maldonado, M.; Onate, G.; et al. Essential role of intracellular glutathione in controlling ascorbic acid transporter expression and function in rat hepatocytes and hepatoma cells. Free Radic. Biol. Med. 2012, 52, 1874–1887. [Google Scholar] [CrossRef]
- Nualart, F.J.; Rivas, C.I.; Montecinos, V.P.; Godoy, A.S.; Guaiquil, V.H.; Golde, D.W.; Vera, J.C. Recycling of vitamin C by a bystander effect. J. Biol. Chem. 2003, 278, 10128–10133. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Qiao, H.; Koury, M.J. Maturational loss of the vitamin C transporter in erythrocytes. Biochem. Biophys. Res. Commun. 2007, 360, 295–298. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Blanc, L.; Boyer-Clavel, M.; Jacquet, C.; Vidal, M.; Sitbon, M.; Taylor, N. The Glut1 and Glut4 glucose transporters are differentially expressed during perinatal and postnatal erythropoiesis. Blood 2008, 112, 4729–4738. [Google Scholar] [CrossRef]
- Rumsey, S.C.; Daruwala, R.; Al-Hasani, H.; Zarnowski, M.J.; Simpson, I.A.; Levine, M. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J. Biol. Chem. 2000, 275, 28246–28253. [Google Scholar]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef]
- Roberts, L.J., II; Traber, M.G.; Frei, B. Vitamins E and C in the prevention of cardiovascular disease and cancer in men. Free Radic. Biol. Med. 2009, 46, 1558. [Google Scholar] [CrossRef]
- Dehghan, M.; Akhtar-Danesh, N.; McMillan, C.R.; Thabane, L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr. J. 2007, 6, 41. [Google Scholar] [CrossRef]
- Brubacher, D.; Moser, U.; Jordan, P. Vitamin C concentrations in plasma as a function of intake: A meta-analysis. Int. J. Vitam. Nutr. Res. 2000, 70, 226–237. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar]
- Michels, A.J.; Hagen, T.M.; Frei, B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu. Rev. Nutr. 2013, 33, 45–70. [Google Scholar] [CrossRef]
- Harding, A.H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer—Norfolk prospective study. Arch. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846. [Google Scholar] [CrossRef]
- Kim, H.; Bae, S.; Yu, Y.; Kim, Y.; Kim, H.R.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. The analysis of vitamin C concentration in organs of gulo−/− mice upon vitamin C withdrawal. Immune Netw. 2012, 12, 18–26. [Google Scholar] [CrossRef]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am. J. Clin. Nutr. 2013, 97, 800–807. [Google Scholar] [CrossRef]
- Taylor, A.; Jacques, P.F.; Nowell, T.; Perrone, G.; Blumberg, J.; Handelman, G.; Jozwiak, B.; Nadler, D. Vitamin C in human and guinea pig aqueous, lens and plasma in relation to intake. Curr. Eye Res. 1997, 16, 857–864. [Google Scholar] [CrossRef]
- Dietrich, M.; Block, G.; Benowitz, N.L.; Morrow, J.D.; Hudes, M.; Jacob, P., III; Norkus, E.P.; Packer, L. Vitamin C supplementation decreases oxidative stress biomarker f2-isoprostanes in plasma of nonsmokers exposed to environmental tobacco smoke. Nutr. Cancer 2003, 45, 176–184. [Google Scholar] [CrossRef]
- Block, G.; Jensen, C.D.; Dalvi, T.B.; Norkus, E.P.; Hudes, M.; Crawford, P.B.; Holland, N.; Fung, E.B.; Schumacher, L.; Harmatz, P. Vitamin C treatment reduces elevated C-reactive protein. Free Radic. Biol. Med. 2009, 46, 70–77. [Google Scholar] [CrossRef]
- Dhariwal, K.R.; Washko, P.W.; Levine, M. Determination of dehydroascorbic acid using high-performance liquid chromatography with coulometric electrochemical detection. Anal. Biochem. 1990, 189, 18–23. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: Reliable reduction with tris[2-carboxyethyl]phosphine hydrochloride. Anal. Biochem. 2000, 282, 89–93. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Ascorbate and dehydroascorbic acid as biomarkers of oxidative stress: Validity of clinical data depends on vacutainer system used. Nutr. Res. 2012, 32, 66–69. [Google Scholar] [CrossRef]
- Karlsen, A.; Blomhoff, R.; Gundersen, T.E. Stability of whole blood and plasma ascorbic acid. Eur. J. Clin. Nutr. 2007, 61, 1233–1236. [Google Scholar] [CrossRef]
- Chung, W.Y.; Chung, J.K.; Szeto, Y.T.; Tomlinson, B.; Benzie, I.F. Plasma ascorbic acid: Measurement, stability and clinical utility revisited. Clin. Biochem. 2001, 34, 623–627. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Lykkesfeldt, J. Does Vitamin C deficiency increase lifestyle-associated vascular disease progression? Evidence based on experimental and clinical studies. Antioxid. Redox Signal. 2013. [Google Scholar] [CrossRef]
- Cook, J.D.; Reddy, M.B. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. Am. J. Clin. Nutr. 2001, 73, 93–98. [Google Scholar]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J. Am. Soc. Nephrol. 2004, 15, 3225–3232. [Google Scholar] [CrossRef]
- Thomas, L.D.; Elinder, C.G.; Tiselius, H.G.; Wolk, A.; Akesson, A. Ascorbic acid supplements and kidney stone incidence among men: A prospective study. JAMA Intern. Med. 2013, 173, 386–388. [Google Scholar] [CrossRef]
- Taylor, E.N.; Fung, T.T.; Curhan, G.C. DASH-style diet associates with reduced risk for kidney stones. J. Am. Soc. Nephrol. 2009, 20, 2253–2259. [Google Scholar] [CrossRef]
- Singer, R.F. Vitamin C supplementation in kidney failure: Effect on uraemic symptoms. Nephrol. Dial. Transpl. 2011, 26, 614–620. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Azzi, A.; Blumberg, J.; Bray, T.; Dickinson, A.; Frei, B.; Jialal, I.; Johnston, C.S.; Kelly, F.J.; Kraemer, K.; et al. Vitamins E and C are safe across a broad range of intakes. Am. J. Clin. Nutr. 2005, 81, 736–745. [Google Scholar]
- Rees, D.C.; Kelsey, H.; Richards, J.D. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ 1993, 306, 841–842. [Google Scholar] [CrossRef]
- Hemila, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 1, CD000980. [Google Scholar]
- Douglas, R.M.; Hemila, H. Vitamin C for preventing and treating the common cold. PLoS Med. 2005, 2, e168. [Google Scholar] [CrossRef]
- Sasazuki, S.; Sasaki, S.; Tsubono, Y.; Okubo, S.; Hayashi, M.; Tsugane, S. Effect of vitamin C on common cold: Randomized controlled trial. Eur. J. Clin. Nutr. 2006, 60, 9–17. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C. Synthetic or food-derived vitamin C—Are they equally bioavailable? Nutrients 2013, 5, 4284–4304. [Google Scholar]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Tannenbaum, S.R. Preventive action of vitamin C on nitrosamine formation. Int. J. Vitam. Nutr. Res. Suppl. 1989, 30, 109–113. [Google Scholar]
- Kesinger, N.G.; Stevens, J.F. Covalent interaction of ascorbic acid with natural products. Phytochemistry 2009, 70, 1930–1939. [Google Scholar] [CrossRef]
- Miranda, C.L.; Reed, R.L.; Kuiper, H.C.; Alber, S.; Stevens, J.F. Ascorbic acid promotes detoxification and elimination of 4-hydroxy-2(E)-nonenal in human monocytic THP-1 cells. Chem. Res. Toxicol. 2009, 22, 863–874. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef]
- Yin, R.; Mao, S.Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef]
- Chung, T.L.; Brena, R.M.; Kolle, G.; Grimmond, S.M.; Berman, B.P.; Laird, P.W.; Pera, M.F.; Wolvetang, E.J. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 2010, 28, 1848–1855. [Google Scholar]
- Kirkwood, J.S.; Lebold, K.M.; Miranda, C.L.; Wright, C.L.; Miller, G.W.; Tanguay, R.L.; Barton, C.L.; Traber, M.G.; Stevens, J.F. Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J. Biol. Chem. 2012, 287, 3833–3841. [Google Scholar] [CrossRef]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One 2012, 7, e29794. [Google Scholar]
- Welsh, J.L.; Wagner, B.A.; van’t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J., II; et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Michels, A.J.; Frei, B. Myths, Artifacts, and Fatal Flaws: Identifying Limitations and Opportunities in Vitamin C Research. Nutrients 2013, 5, 5161-5192. https://doi.org/10.3390/nu5125161
Michels AJ, Frei B. Myths, Artifacts, and Fatal Flaws: Identifying Limitations and Opportunities in Vitamin C Research. Nutrients. 2013; 5(12):5161-5192. https://doi.org/10.3390/nu5125161
Chicago/Turabian StyleMichels, Alexander J., and Balz Frei. 2013. "Myths, Artifacts, and Fatal Flaws: Identifying Limitations and Opportunities in Vitamin C Research" Nutrients 5, no. 12: 5161-5192. https://doi.org/10.3390/nu5125161
APA StyleMichels, A. J., & Frei, B. (2013). Myths, Artifacts, and Fatal Flaws: Identifying Limitations and Opportunities in Vitamin C Research. Nutrients, 5(12), 5161-5192. https://doi.org/10.3390/nu5125161





