The Relationship between Zinc Intake and Serum/Plasma Zinc Concentration in Children: A Systematic Review and Dose-Response Meta-Analysis
1. Introduction
2. Methods
2.1. Search Strategy
| No. | Search Term | Results |
|---|---|---|
| 1 | randomised controlled trial.pt. | 280,821 |
| 2 | controlled clinical trial.pt. | 79,998 |
| 3 | randomised.ab. | 196,604 |
| 4 | placebo.ab. | 117,891 |
| 5 | clinical trials as topic.sh. | 146,242 |
| 6 | randomly.ab. | 145,491 |
| 7 | trial.ab. | 203,467 |
| 8 | randomised.ab. | 38,423 |
| 9 | 6 or 3 or 7 or 2 or 8 or 1 or 4 or 5 | 734,511 |
| 10 | (animals not (human and animals)).sh. | 4,482,479 |
| 11 | 9 not 10 | 642,665 |
| 12 | (cohort* or “case control*” or cross-sectional* or “cross sectional” or case-control* or prospective or “systematic review*”).mp. | 768,885 |
| 13 | exp meta-analysis/ or expmulticenter study/ or follow-up studies/ or prospective studies/ or intervention studies/ or epidemiologic studies/ or case-control studies/ or exp cohort studies/ or longitudinal studies/ or cross-sectional studies/ | 1,013,635 |
| 14 | 13 or 12 | 1,203,767 |
| 15 | 14 not 10 | 1,154,385 |
| 16 | 11 or 15 | 1,599,094 |
| 17 | ((zinc or Zn or zinc sulphate or zinc gluconate or zinc acetate or methionine or zinc isotope*) adj3 (intake* or diet* or supplement* or deplet* or status or serum or plasma or leukocyte or concentration* or expos* or fortif* or urine or hair)).ti,ab. | 16,681 |
| 18 | Nutritional Support/ or Dietary Supplements/ or nutritional requirements/ or Breast feeding/ or exp infant food/ or bottle feeding/ or infant formula/ | 63,098 |
| 19 | exp Nutritional Status/ or exp Deficiency Diseases/ or supplementation/ or diet supplementation/ or dietary intake/ or exp diet restriction/ or exp mineral intake/ or Diet/ or Food, Fortified/ or nutrition assessment/ or Nutritive Value/ | 176,014 |
| 20 | (intake* or diet* or supplement* or deplet* or status or serum or plasma or leukocyte or concentration* or expos* or fortif* or urine or hair).ti,ab. | 3,166,092 |
| 21 | 18 or 19 or 20 | 3,263,114 |
| 22 | zinc/ | 41,027 |
| 23 | 22 and 21 | 20,745 |
| 24 | 23 or 17 | 26,943 |
| 25 | 24 and 16 | 2410 |
2.2. Criteria for the Consideration of Studies for This Review
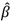 and SE (
and SE ( 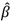 ) for the assumed linear relation on the loge–loge scale. Studies were excluded if they were a group RCT (community trial), or were commentaries, reviews, or duplicate publications from the same study. Studies were excluded if children were hospitalised, had severe protein-energy malnutrition or a chronic disease or if supplemental zinc was provided for less than 6 weeks.
) for the assumed linear relation on the loge–loge scale. Studies were excluded if they were a group RCT (community trial), or were commentaries, reviews, or duplicate publications from the same study. Studies were excluded if children were hospitalised, had severe protein-energy malnutrition or a chronic disease or if supplemental zinc was provided for less than 6 weeks.2.3. Selection of Articles
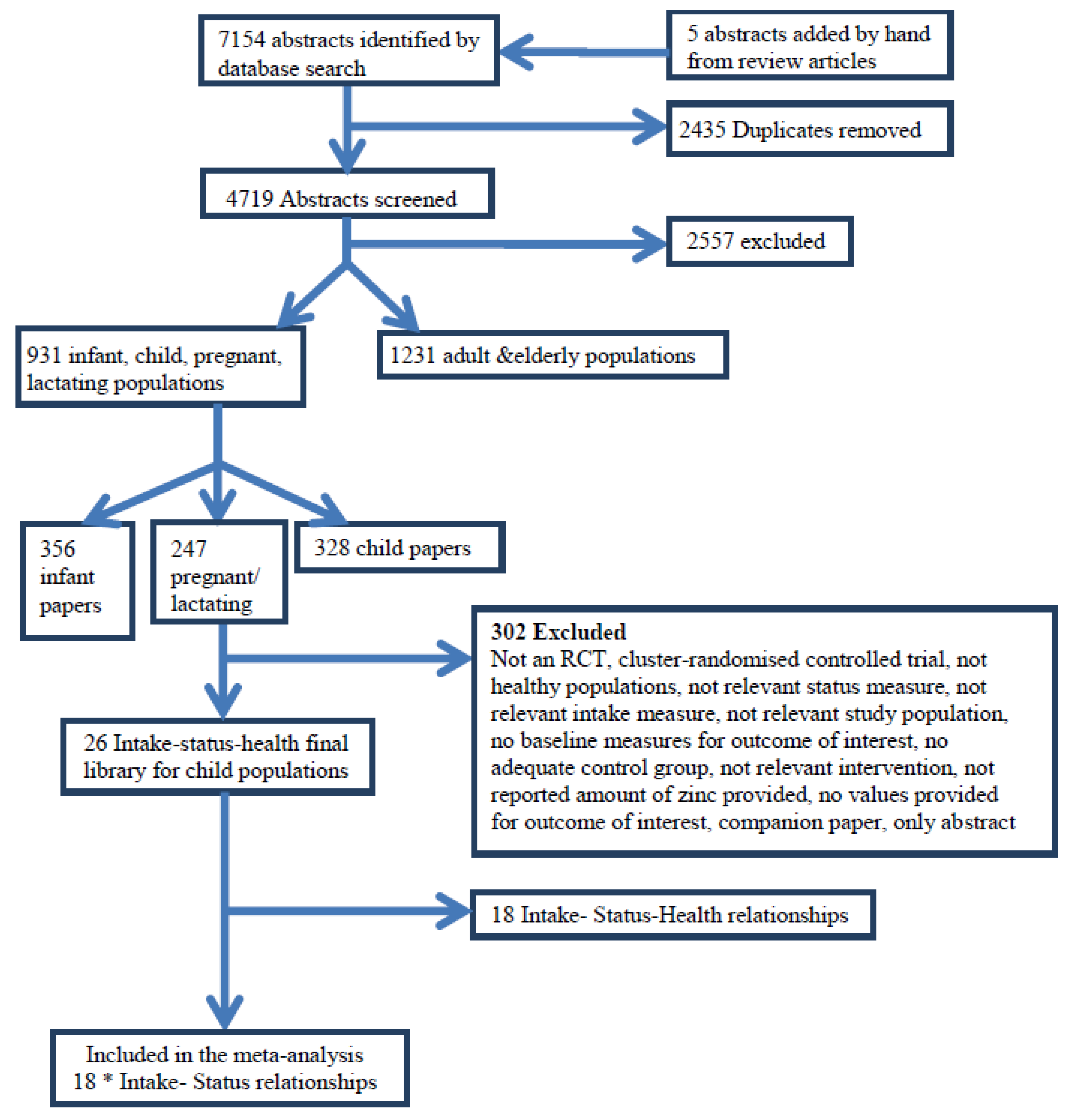
2.4. Data Extraction
| First Author, Year, Country | Participants | Treatment Groups (n) | Mean Zn Intake (mg/day) | Mean (SD) Plasma/Serum Zn (µmol/L) | Duration | Zinc Status Biomarker [Analytical Method] | Main Results |
|---|---|---|---|---|---|---|---|
| Mahloudji, 1975, Iran [23] | Males & females | Fe only (12); | 5.65; | 8.95 (1.80) | 8 months | Plasma Zn [AAS] | No significant difference between plasma Zn of the supplemented and placebo groups |
| aged 6–12 years | Fe + 20 mg/day Zn (13) | 25.65 | 8.50 (1.93) | ||||
| Hambidge, 1979, USA [24] | Males & females | Male placebo (15); | 6.3; | 11.06 (2.23) | 9 months | Plasma Zn [AES] | Plasma Zn significantly higher in Zn supplemented compared to placebo (girls and combined sexes only p < 0.05) |
| aged 33–90 months | Male Zn FM 2.57 mg/day (20); | 9.27; | 11.85 (2.23) | ||||
| Female placebo (14); | 6.3; | 10.61 (1.81) | |||||
| Female Zn FM 2.57 mg/day (11) | 9.27 | 11.96 (1.81) | |||||
| Walravens, 1983, USA [25] | Males & females | Placebo (16); | 4.6; | 11.32 (2.14) | 12 months | Plasma Zn [AES] | No significant difference between plasma Zn of the supplemented and placebo groups |
| aged 2–6 years | 10 mg/day Zn (16) | 15.9 | 10.86 (2.14) | ||||
| Gibson, 1989, Canada [26] | Males | Placebo (21); | 6.4; | 15.8 (3.5) | 6 months | Serum Zn [AAS] | No significant correlation between serum Zn and dietary Zn levels |
| aged 59–95 months | 10 mg Zn/day (18) | 16.7 | 17.9 (3.4) | ||||
| Cavan, 1993, Guatemala [27] | Males & females, | Placebo (74); | 5.65; | 14.9 (2.1) | 25 weeks | Plasma Zn [AAS] | Plasma Zn significantly higher in Zn supplemented compared to placebo ( p < 0.01) |
| mean age 81.5 (±7.0) months 1 | 10 mg Zn/day (71) | 15.65 | 16.2 (2.9) | ||||
| Friis, 1997, Zimbabwe [28] | Males and females | Placebo (121); | 5.65; | 10.89 (2.5) | 12 months | Serum Zn [AAS] | The decline in zinc concentration was significantly lower in the Zn supplemented group compared to the placebo group ( p < 0.02) |
| aged 11–17 years | 30–50 mg/day Zn (122) | 45.65 2 | 11.71 (2.4) | ||||
| Rosado, 1997, Mexico [29] | Males & females | Placebo (55); | 5.65; | 14.4 (4.45) | 12 months | Plasma Zn [AAS] | Plasma Zn increased significantly in the Zn supplemented group over the 12 months period (p < 0.01) |
| aged 18–36 months | 20 mg Zn/day (54) | 25.65 | 16.8 (5.88) | ||||
| Ruz, 1997, Chile [30] | Males & females | Placebo (33); | 6.4; | 17.7 (1.9) | 6 months | Plasma Zn [AAS] | No significant difference between plasma Zn of the supplemented and placebo groups |
| aged 27–50 months | 10 mg/day Zn (36) | 17.1 | 17.6 (2.2) | ||||
| Sandstead, 1998, China [31] (3 regions) | Males & females | Chonqing MN, no Zn (35); | 5.65; | 19.83 (4.12) | 10 weeks | Plasma Zn [AAS] | Plasma Zn significantly higher in Zn supplemented compared to placebo (p < 0.05) in Chonqing and Quindgdao groups. |
| aged 6–9 years | 20 mg/day Zn + MN (35); | 25.65; | 23.6 (4.12) | ||||
| Quindgdao MN, no Zn (36); | 5.65; | 20.42 (4.08) | |||||
| 20mg/day Zn + MN (36); | 25.65; | 22.97 (4.08) | |||||
| Shanghai MN, no Zn (37); | 5.65; | 17.9 (2.75) | |||||
| 20 mg/day Zn + MN (37) | 25.65 | 17.97 (2.75) | |||||
| Clark, 1999, UK [32] | Peripubertal females, | Placebo (19); | 6.6; | 12.6 (1.0) | 6 weeks | Serum Zn [no method given] | Serum Zn significantly higher in Zn supplemented compared to placebo ( p < 0.001) |
| mean age 12.2 (±0.3) years | 15 mg Zn/day (23) | 21.6 | 16.7 (4.9) | ||||
| Smith, 1999, Belize [33] | Males & females | Placebo (10); | 5.65; | 11.7 (0.68) | 6 months | Serum Zn [AAS] | Serum Zn significantly higher in Zn supplemented compared to placebo (p < 0.001) |
| aged 22–66 months | 70 mg Zn/day (12) | 75.65 | 13.5 (0.68) | ||||
| Munoz, 2000, Mexico [34] | Males & females | Placebo (54); | 5.65; | 14.3 (4.7) | 6 months | Plasma Zn [AAS] | Serum Zn significantly higher in Zn supplemented compared to placebo (p < 0.0001) |
| aged 18–36 months | 20 mg/day Zn (47) | 25.65 | 16.8 (5.6) | ||||
| Lopez de Romana, 2005, Peru [35] | Males & females | Fe FM (12); | 4.71; | 11.87 (1.88) | 70 days | Plasma Zn [ICP-MS] | No significant differences in plasma Zn were found between treatments |
| aged 3–4 years | Fe + 3 mg/day Zn FM (10); | 8.72; | 11.65 (1.25) | ||||
| Fe + 9 mg/day Zn FM (12); | 15.7 | 12.60 (1.51) | |||||
| Silva, 2006, Brazil [36] | Males & females aged 12–59 months 3 | Placebo (30); 10 mg/day Zn (28) | 5.65; 15.65 | 8.0 (0.58)13.4 (0.25) | 4 months | Serum Zn [AAS] | Serum Zn significantly higher in Zn supplemented compared to placebo (p < 0.05) |
| Sandstead, 2008, USA (Mexican Americans) [37] | Males & females | MN, no Zn (25); | 5.65; | 15.4 (1.5) | 10 weeks | Plasma Zn [AAS] | Mean plasma Zn increased significantly in both groups compared to baseline (p < 0.05) |
| aged 6–7 years | 20 mg/day Zn + MN (25) | 25.65 | 15.6 (1.2) | ||||
| Wuehler, 2008, Ecuador [38] | Males & females | Placebo (56); | 5.65; | 10.6 (1.6) | 6 months | Plasma Zn [ICP-MS] | The mean change in plasma zinc concentrations from baseline increased progressively with higher doses of supplemental Zn (p < 0.001) |
| aged 12–30 months | 3 mg Zn/day (50); | 8.65; | 12.3 (1.6) | ||||
| 7 mg Zn/day (52); | 12.65; | 13.3 (1.7) | |||||
| 10 mg Zn/day (54) | 15.65 | 14.0 (1.7) 4 | |||||
| de Oliveira, 2009, Brazil [39] | Pubescent males, | Placebo (26); | 5.65; | 16.9 (2.1) | 12 weeks | Plasma Zn [ICP-MS] | Plasma Zn significantly higher in Zn supplemented compared to placebo (p < 0.05) |
| mean age 13 (±0.4) years | 22 mg Zn/day (21) | 27.65 | 18.7 (3.5) | ||||
| Uckarde, 2009, Turkey [40] | Males & females | Placebo (109); | 5.65; | 19.19 (1.80) | 10 weeks | Serum Zn [CS] | Both supplemented and placebo groups had significantly higher serum Zn at follow up (p < 0.05) |
| aged 8–9 years | 15 mg/day Zn (109) | 20.65 | 19.50 (2.41) |
2.5. Data Synthesis
2.6. Pre-Specified Potential Factors Modifying the Association
2.7. Statistical Analyses
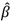 ) and the standard error (SE (
) and the standard error (SE ( 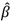 )) of this regression coefficient. The transformations used to derive this common single-study estimate from the available summary statistics per study have been described elsewhere [41]. In short, we estimated an intake-status regression coefficient (
)) of this regression coefficient. The transformations used to derive this common single-study estimate from the available summary statistics per study have been described elsewhere [41]. In short, we estimated an intake-status regression coefficient ( 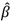 ) for each individual study, based on the assumption of a linear relation on the loge–loge-scale (natural logarithm of intake vs. natural logarithm of status). This shape of this linear relationship on the loge–loge-scale corresponds to a monotonic concave function on the original scale for β < 1. This shape is assumed to be realistic for the biological relationship between zinc intake and plasma/serum zinc concentrations. As the true dose-response curve is unknown, this approximation provides a practical methodology to estimate the dose-response relationship. We calculated the overall pooled
) for each individual study, based on the assumption of a linear relation on the loge–loge-scale (natural logarithm of intake vs. natural logarithm of status). This shape of this linear relationship on the loge–loge-scale corresponds to a monotonic concave function on the original scale for β < 1. This shape is assumed to be realistic for the biological relationship between zinc intake and plasma/serum zinc concentrations. As the true dose-response curve is unknown, this approximation provides a practical methodology to estimate the dose-response relationship. We calculated the overall pooled 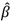 and SE (
and SE ( 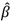 ) using random effects meta-analysis, which estimates the between-study variance using the method of DerSimonian and Laird and used this estimate to modify the weights used to calculate the summary estimate. Residual heterogeneity between studies was evaluated using the I2 statistic. Pre-specified potential factors that could modify the association were explored using stratified random effects meta-analyses. The statistical transformations to obtain
) using random effects meta-analysis, which estimates the between-study variance using the method of DerSimonian and Laird and used this estimate to modify the weights used to calculate the summary estimate. Residual heterogeneity between studies was evaluated using the I2 statistic. Pre-specified potential factors that could modify the association were explored using stratified random effects meta-analyses. The statistical transformations to obtain 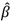 ’s and SE (
’s and SE ( 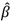 )’s were performed using GenStat version 13-SP2 (VSN International Ltd. [42]) and the meta-analysis was performed using STATA version 11.0 (College Station, TX, USA), with statistical significance defined as p < 0.05.
)’s were performed using GenStat version 13-SP2 (VSN International Ltd. [42]) and the meta-analysis was performed using STATA version 11.0 (College Station, TX, USA), with statistical significance defined as p < 0.05.2.8. Assessment of Risk of Bias in Included Studies
3. Results
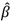 ’s, the overall
’s, the overall 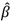 represents the difference in the loge transformed predicted value of serum/plasma zinc status for each one-unit difference in the loge transformed value in zinc intake. Therefore, an overall
represents the difference in the loge transformed predicted value of serum/plasma zinc status for each one-unit difference in the loge transformed value in zinc intake. Therefore, an overall 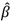 of 0.12 means that for every doubling in zinc intake, the difference in zinc serum or plasma concentration is 2
of 0.12 means that for every doubling in zinc intake, the difference in zinc serum or plasma concentration is 2 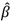 (20.12 = 1.09), which is 9%. This means that a person with a zinc intake of 14 mg/day has a zinc serum/plasma concentration that is 9% higher than a person who has a zinc intake of 7 mg/day.
(20.12 = 1.09), which is 9%. This means that a person with a zinc intake of 14 mg/day has a zinc serum/plasma concentration that is 9% higher than a person who has a zinc intake of 7 mg/day. 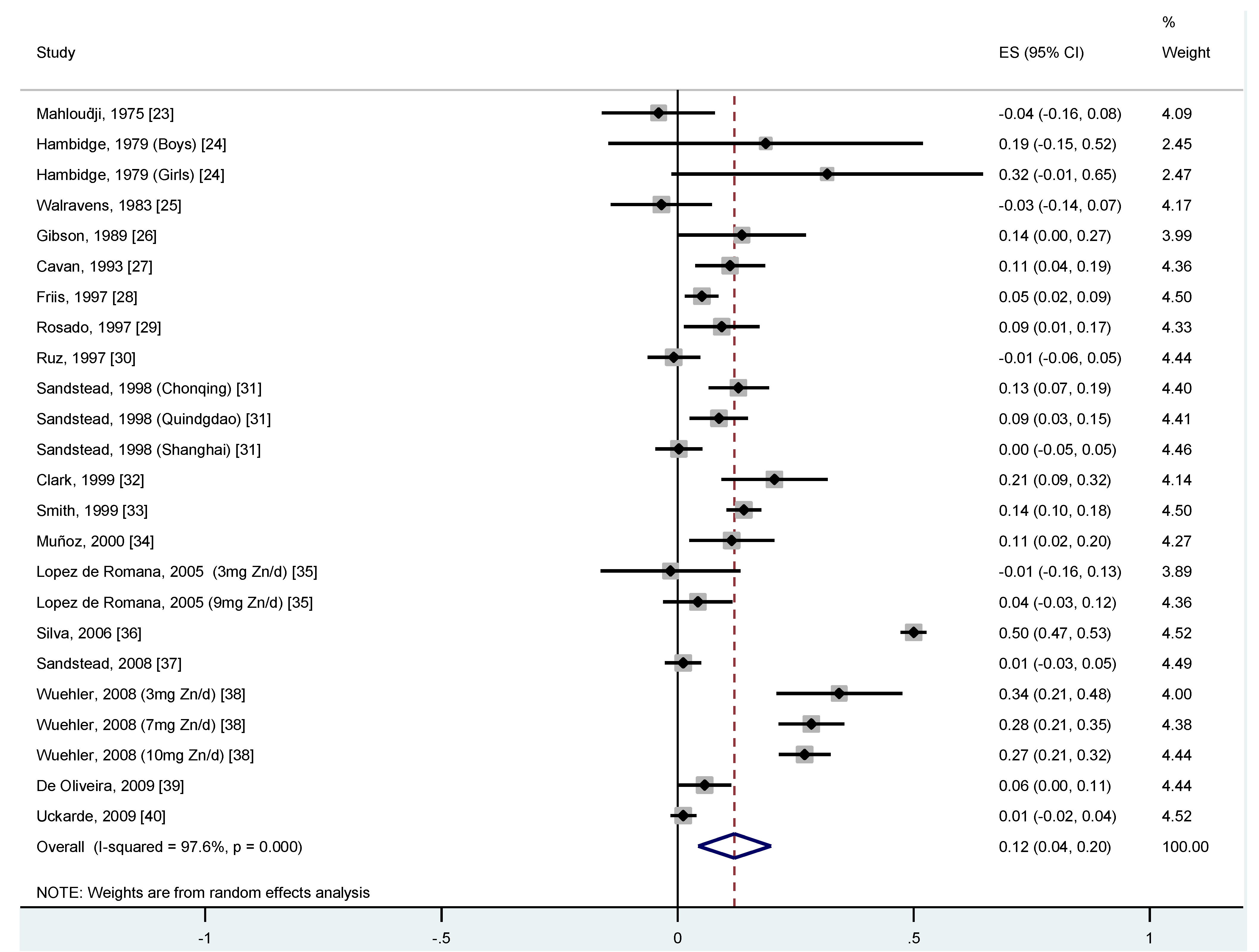
| Author, Year | Adequate Sequence Generation | Allocation Concealment Adequate | Blinding Adequate | Dropouts Adequate and Outcome Data Complete | Funder Adequate | Lack of other Potential Threats to Validity | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Mahloudji, 1975 [23] | Unclear | Yes | Unclear | Unclear | Yes | Unclear | High |
| Hambidge, 1979 [24] | Unclear | Unclear | Yes | Unclear | No | Unclear | High |
| Walravens, 1983 [25] | Unclear | Yes | Yes | Yes | Yes | Yes | Moderate |
| Gibson, 1989 [26] | Unclear | Yes | Yes | Yes | No | Yes | High |
| Cavan, 1993 [27] | Unclear | Yes | Yes | Unclear | Yes | No | High |
| Friis, 1997 [28] | Unclear | Yes | Yes | Yes | Yes | Yes | High |
| Rosado, 1997 [29] | Unclear | Yes | Yes | Unclear | Yes | Yes | High |
| Ruz, 1997 [30] | Unclear | Yes | Yes | Unclear | Yes | Yes | High |
| Sandstead, 1998 [31] | Unclear | Unclear | Yes | Unclear | No | No | High |
| Clark, 1999 [32] | Yes | Yes | Yes | Unclear | No | Unclear | High |
| Smith, 1999 [33] | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| Muñoz, 2000 [34] | Unclear | No | Yes | Yes | Nor | Yes | High |
| Lopez de Romana, 2005 [35] | Unclear | Unclear | Unclear | Yes | Yes | Yes | High |
| Silva, 2006 [36] | Unclear | Unclear | No | Yes | No | Yes | High |
| Sandstead, 2008 [37] | Yes | Yes | Yes | Unclear | No | Yes | Moderate |
| Wuehler, 2008 [38] | Yes | Unclear | Yes | Yes | Yes | Yes | Low |
| de Oliveira, 2009 [39] | Unclear | Unclear | No | Unclear | No | Yes | High |
| Uckarde, 2009 [40] | Unclear | Yes | Yes | Yes | No | Yes | High |
4. Discussion
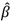 of 0.12 means that for every doubling in zinc intake, the difference in zinc serum or plasma concentration is 9%. In other words, a child with a zinc intake of 14 mg/day has a zinc serum/plasma concentration that is 9% higher than a person who has a zinc intake of 7 mg/day. It is important to note however that, due to homeostatic regulatory mechanisms, the amount of dietary zinc absorbed decreases as intake increases, and plasma zinc concentration is homeostatically controlled within a narrow physiological range, therefore this dose response relationship can only be applied to the range of intakes used to derive this relationship. The studies included in this meta-analysis were different in a number of aspects, such as using various designs, follow-up times, zinc doses, and populations. Therefore, it is no surprise that, when combining these studies in a meta-analysis, a large heterogeneity is observed between the studies (I2 = 97.6% p = 0.0001). This between-study heterogeneity may be caused by methodological factors, such as biological factors, e.g., differences in study population characteristics (age, socio-economic status), differences in doses of provided zinc (amount, one or more doses per day, study duration). We have considered the dose of zinc provided, study duration, age, and supplement type and these factors did not significantly explain the between-study heterogeneity. An individual participant data meta-analysis may have provided a more conclusive explanation of the between-study heterogeneity in this meta-analysis. However, this type of analysis would involve the input of raw individual participant data provided by the original study investigators for re-analysis and combination in a pooled analysis and as such would be a major undertaking in terms of time, costs, and collaboration. Moreover, an inability to include individual participant data from all relevant studies could introduce selection bias. The meta-analytic approach used in this paper is not an attempt to accurately describe the biological relation between actual zinc intake and zinc concentrations in blood under strict experimental conditions and on an individual level, but rather to simulate a dose-response relationship between zinc intake and status that is useful for surveillance studies with a public health point of view and, as such, deliberately incorporates the differences between dietary assessment methods, laboratory assessment methods and participant characteristics to ensure a broad external validity. Thus, the heterogeneity reflects the lack of standardisation of methods and the true heterogeneity between study populations and necessarily enters as uncertainty into the application of such data for public health purposes [45].
of 0.12 means that for every doubling in zinc intake, the difference in zinc serum or plasma concentration is 9%. In other words, a child with a zinc intake of 14 mg/day has a zinc serum/plasma concentration that is 9% higher than a person who has a zinc intake of 7 mg/day. It is important to note however that, due to homeostatic regulatory mechanisms, the amount of dietary zinc absorbed decreases as intake increases, and plasma zinc concentration is homeostatically controlled within a narrow physiological range, therefore this dose response relationship can only be applied to the range of intakes used to derive this relationship. The studies included in this meta-analysis were different in a number of aspects, such as using various designs, follow-up times, zinc doses, and populations. Therefore, it is no surprise that, when combining these studies in a meta-analysis, a large heterogeneity is observed between the studies (I2 = 97.6% p = 0.0001). This between-study heterogeneity may be caused by methodological factors, such as biological factors, e.g., differences in study population characteristics (age, socio-economic status), differences in doses of provided zinc (amount, one or more doses per day, study duration). We have considered the dose of zinc provided, study duration, age, and supplement type and these factors did not significantly explain the between-study heterogeneity. An individual participant data meta-analysis may have provided a more conclusive explanation of the between-study heterogeneity in this meta-analysis. However, this type of analysis would involve the input of raw individual participant data provided by the original study investigators for re-analysis and combination in a pooled analysis and as such would be a major undertaking in terms of time, costs, and collaboration. Moreover, an inability to include individual participant data from all relevant studies could introduce selection bias. The meta-analytic approach used in this paper is not an attempt to accurately describe the biological relation between actual zinc intake and zinc concentrations in blood under strict experimental conditions and on an individual level, but rather to simulate a dose-response relationship between zinc intake and status that is useful for surveillance studies with a public health point of view and, as such, deliberately incorporates the differences between dietary assessment methods, laboratory assessment methods and participant characteristics to ensure a broad external validity. Thus, the heterogeneity reflects the lack of standardisation of methods and the true heterogeneity between study populations and necessarily enters as uncertainty into the application of such data for public health purposes [45].5. Conclusion
Acknowledgments
References
- Brown, K.H.; Wuehler, S.E.; Peerson, J.M. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nutr. Bull. 2001, 22, 113–125. [Google Scholar]
- Caulfield, L.; Black, R. Zinc Deficiency. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, A., Rodgers, A., Murray, C., Eds.; World Health Organization: Geneva, Swtizerland, 2004; Volume 1, pp. 257–280. [Google Scholar]
- Gibson, R.S.; Hess, S.Y.; Hotz, C.; Brown, K.H. Indicators of zinc status at the population level: A review of the evidence. Br. J. Nutr. 2008, 99, S14–S23. [Google Scholar]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar]
- Gibson, R.S. Zinc: The missing link in combating micronutrient malnutrition in developing countries. Proc. Nutr. Soc. 2006, 65, 51–60. [Google Scholar] [CrossRef]
- Junior, J.; Dean, S.; Mayo-Wilson, E.; Imdad, A.; Bhutta, Z. Zinc supplementation for preventing mortality and morbidity, and promoting growth, in children aged 6 months to 12 years of age (protocol). Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Walker, C.L.F.; Ezzati, M.; Black, R.E. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur. J. Clin. Nutr. 2009, 63, 591–597. [Google Scholar] [CrossRef]
- Hess, S.Y.; Lonnerdal, B.; Hotz, C.; Rivera, J.A.; Brown, K.H. Recent advances in knowledge of zinc nutrition and human health. Food Nutr. Bull. 2009, 30, S5–S11. [Google Scholar]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. 2009, 28, 257–265. [Google Scholar]
- Aksglaede, L.; Olsen, L.W.; Sorensen, T.I.A.; Juul, A. Forty years trends in timing of pubertal growth spurt in 157,000 Danish school children. PLoS One 2008, 3, e2728. [Google Scholar]
- King, J.C. Does poor zinc nutriture retard skeletal growth and mineralization in adolescents? Am. J. Clin. Nutr. 1996, 64, 375–376. [Google Scholar]
- Golub, M.S.; Keen, C.L.; Gershwin, M.E.; Styne, D.M.; Takeuchi, P.T.; Ontell, F.; Walter, R.M.; Hendrickx, A.G. Adolescent growth and maturation in zinc-deprived rhesus monkeys. Am. J. Clin. Nutr. 1996, 64, 274–282. [Google Scholar]
- Gibson, R.S.; Heath, A.L.M.; Ferguson, E.L. Risk of suboptimal iron and zinc nutriture among adolescent girls in Australia and New Zealand: Causes, consequences, and solutions. Asia Pac. J. Clin. Nutr. 2002, 11, S543–S552. [Google Scholar] [CrossRef]
- Bougle, D.L.; Sabatier, J.P.; Guaydier-Souquieres, G.; Guillon-Metz, F.; Laroche, D.; Jauzac, P.; Bureau, F. Zinc status and bone mineralisation in adolescent girls. J. Trace Elem. Med. Biol. 2004, 18, 17–21. [Google Scholar] [CrossRef]
- Slemenda, C.W.; Reister, T.K.; Hui, S.L.; Miller, J.Z.; Christian, J.C.; Johnston, C.C. Influences on skeletal mineralization in children and adolescents—Evidence for varying effects of sexual-maturation and physical-activity. J. Pediatr. 1994, 125, 201–207. [Google Scholar] [CrossRef]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040S–2051S. [Google Scholar] [CrossRef]
- Iglesia, I.; Doets, E.L.; Bel-Serrat, S.; Román, B.; Hermoso, M.; Peña Quintana, L.; García-Luzardo, M.D.R.; Santana-Salguero, B.; García-Santos, Y.; Vucic, V.; et al. Physiological and public health basis for assessing micronutrient requirements in children and adolescents. The EURRECA network. Matern. Child Nutr. 2010, 6, 84–99. [Google Scholar] [CrossRef]
- Doets, E.L.; de Wit, L.S.; Dhonukshe-Rutten, R.A.M.; Cavelaars, A.E.J.M.; Raats, M.M.; Timotijevic, L.; Brzozowska, A.; Wijnhoven, T.M.A.; Pavlovic, M.; Totland, T.H.; et al. Current micronutrient recommendations in Europe: Towards understanding their differences and similarities. Eur. J. Nutr. 2008, 47, 17–40. [Google Scholar]
- Ashwell, M.; Lambert, J.P.; Alles, M.S.; Branca, F.; Bucchini, L.; Brzozowska, A.; de Groot, L.C.P.G.M.; Dhonukshe-Rutten, R.A.M.; Dwyer, J.T.; Fairweather-Tait, S.; et al. How we will produce the evidence-based EURRECA toolkit to support nutrition and food policy. Eur. J. Nutr. 2008, 47, 2–16. [Google Scholar] [CrossRef]
- Matthys, C.; van ’t Veer, P.; de Groot, L.; Hooper, L.; Cavelaars, A.E.J.M.; Collings, R.; Donutske-Rutten, R.; Harvey, L.J.; Casgrain, A.; Rollin, F.; et al. EURRECA’s approach for estimating micronutrient requirements. Int. J. Vitam. Nutr. Res. 2011, 81, 256–263. [Google Scholar]
- EURRECA Home Page. Available online: http://www.eurreca.org (accessed on 18 July 2012).
- MEDLINE Home Page. Available online: http://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 10 February 2010).
- Mahloudji, M.; Reinhold, J.G.; Haghshenass, M.; Ronaghy, H.A.; Fox, M.R.; Halsted, J.A. Combined zinc and iron compared with iron supplementation of diets of 6- to 12-year old village schoolchildren in southern Iran. Am. J. Clin. Nutr. 1975, 28, 721–725. [Google Scholar]
- Hambidge, K.M.; Chavez, M.N.; Brown, R.M.; Walravens, P.A. Zinc nutritional status of young middle-income children and effects of consuming zinc-fortified breakfast cereals. Am. J. Clin. Nutr. 1979, 32, 2532–2539. [Google Scholar]
- Walravens, P.A.; Krebs, N.F.; Hambidge, K.M. Linear growth of low income preschool children receiving a zinc supplement. Am. J. Clin. Nutr. 1983, 38, 195–201. [Google Scholar]
- Gibson, R.S.; Smit Vanderkooy, P.D.; MacDonald, A.C.; Goldman, A.; Ryan, B.A.; Berry, M. A growth-limiting, mild zinc-deficiency syndrome in some Southern Ontario boys with low height percentiles. Am. J. Clin. Nutr. 1989, 49, 1266–1273. [Google Scholar]
- Cavan, K.R.; Gibson, R.S.; Grazioso, C.F.; Isalgue, A.M.; Ruz, M.; Solomons, N.W. Growth and body composition of periurban Guatemalan children in relation to zinc status: A longitudinal zinc intervention trial. Am. J. Clin. Nutr. 1993, 57, 344–352. [Google Scholar]
- Friis, H.; Ndhlovu, P.; Mduluza, T.; Kaondera, K.; Sandstrom, B.; Michaelsen, K.F.; Vennervald, B.J.; Christensen, N.O. The impact of zinc supplementation on growth and body composition: A randomized, controlled trial among rural Zimbabwean schoolchildren. Eur. J. Clin. Nutr. 1997, 51, 38–45. [Google Scholar]
- Rosado, J.L.; Lopez, P.; Munoz, E.; Martinez, H.; Allen, L.H. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am. J. Clin. Nutr. 1997, 65, 13–19. [Google Scholar]
- Ruz, M.; Castillo-Duran, C.; Lara, X.; Codoceo, J.; Rebolledo, A.; Alalah, E. A 14-mo zinc-supplementation trial in apparently healthy Chilean preschool children. Am. J. Clin. Nutr. 1997, 66, 1406–1413. [Google Scholar]
- Sandstead, H.H.; Penland, J.G.; Alcock, N.W.; Dayal, H.H.; Chen, X.C.; Li, J.S.; Zhao, F.; Yang, J.J. Effects of repletion with zinc and other micronutrients on neuropsychologic performance and growth of Chinese children. Am. J. Clin. Nutr. 1998, 68, 470S–475S. [Google Scholar]
- Clark, P.J.; Eastell, R.; Barker, M.E. Zinc supplementation and bone growth in pubertal girls. Lancet 1999, 354, 485. [Google Scholar]
- Smith, J.C.; Makdani, D.; Hegar, A.; Rao, D.; Douglass, L.W. Vitamin A and zinc supplementation of preschool children. J. Am. Coll. Nutr. 1999, 18, 213–222. [Google Scholar]
- Munoz, E.C.; Rosado, J.L.; Lopez, P.; Furr, H.C.; Allen, L.H. Iron and zinc supplementation improves indicators of vitamin A status of Mexican preschoolers. Am. J. Clin. Nutr. 2000, 71, 789–794. [Google Scholar]
- Lopez de Romana, D.; Salazar, M.; Hambidge, K.M.; Penny, M.E.; Peerson, J.M.; Krebs, N.F.; Brown, K.H. Longitudinal measurements of zinc absorption in Peruvian children consuming wheat products fortified with iron only or iron and 1 of 2 amounts of zinc. Am. J. Clin. Nutr. 2005, 81, 637–647. [Google Scholar]
- Silva, A.P.R.; Vitolo, M.R.; Zara, L.F.; Castro, C.F.S. Effects of zinc supplementation on 1- to 5-year old children. J. Pediatr. 2006, 82, 227–231. [Google Scholar]
- Sandstead, H.H.; Prasad, A.S.; Penland, J.G.; Beck, F.W.; Kaplan, J.; Egger, N.G.; Alcock, N.W.; Carroll, R.M.; Ramanujam, V.M.; Dayal, H.H.; et al. Zinc deficiency in Mexican American children: Influence of zinc and other micronutrients on T cells, cytokines, and antiinflammatory plasma proteins. Am. J. Clin. Nutr. 2008, 1067–1073. [Google Scholar]
- Wuehler, S.E.; Sempertegui, F.; Brown, K.H. Dose-response trial of prophylactic zinc supplements, with or without copper, in young Ecuadorian children at risk of zinc deficiency. Am. J. Clin. Nutr. 2008, 87, 723–733. [Google Scholar]
- De Oliveira, K.D.J.F.; Donangelo, C.M.; de Oliveira, A.V., Jr.; da Silveira, C.L.P.; Koury, J.C. Effect of zinc supplementation on the antioxidant, copper, and iron status of physically active adolescents. Cell Biochem. Funct. 2009, 27, 162–166. [Google Scholar] [CrossRef]
- Uçkarde, Y.; Ozmert, E.N.; Unal, F.; Yurdakök, K. Effects of zinc supplementation on parent and teacher behaviour rating scores in low socioeconomic level Turkish primary school children. Acta Paediatr. 2009, 98, 731–736. [Google Scholar]
- Souverein, O.W.; Dullemeijer, C.; van ’t Veer, P.; van de Voet, H. Transformations of summary statistics as input in meta-analysis for linear dose-response models on a logarithmic scale: A methodology developed within EURRECA. BMC Med. Res. Methodol. 2012, 12. [Google Scholar] [CrossRef]
- VSN International Ltd. Home Page. Available online: http://www.vsni.co.uk/ (accessed on 18 July 2012).
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews for Interventions, version 5.1.0 (updated March 2011); The Cochrane Collaboration, 2011. Available online: http://www.cochrane-handbook.org (accessed on 18 July 2012).
- Brown, K.H.; Peerson, J.M.; Rivera, J.; Allen, L.H. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2002, 75, 1062–1071. [Google Scholar]
- Hall Moran, V.; Skinner, A.; Warthon Medina, M.; Patel, S.; Dykes, F.; Souverein, O.W.; Dullemeijer, C.; Lowe, N.M. The relationship between zinc intake and serum/plasma zinc concentration in pregnant and lactating women: A systematic review with dose-response meta-analyses. J. Trace Elem. Med. Biol. 2012, 26, 74–79. [Google Scholar]
- Lowe, N.M.; Woodhouse, L.R.; King, J.C. A comparison of the short-term kinetics of zinc metabolism in women during fasting and following a breakfast meal. Br. J. Nutr. 1998, 80, 363–370. [Google Scholar] [CrossRef]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Krebs, N.F. Dietary reference intakes for zinc may require adjustment for phytate intake based upon model predictions. J. Nutr. 2008, 138, 2363–2366. [Google Scholar]
- Gibbs, M.M.; Carriquiry, A.L.; Capanzana, M.V.; Gibson, R.S. Establishing desirable fortificant levels for calcium, iron and zinc in foods for infant and young child feeding: examples from three Asian countries Matern. Child Nutr. 2012. [Google Scholar] [CrossRef]
- Solomons, N.W.; Ruz, M. Zinc and iron interaction: Concepts and perspectives in the developing world. Nutr. Res. 1997, 17, 177–185. [Google Scholar] [CrossRef]
- Walker, C.F.; Kordas, K.; Stoltzfus, R.J.; Black, R.E. Interactive effects of iron and zinc on biochemical and functional outcomes in supplementation trials. Am. J. Clin. Nutr. 2005, 82, 5–12. [Google Scholar]
- Domellof, M.; Cohen, R.J.; Dewey, K.G.; Hernell, O.; Rivera, L.L.; Lonnerdal, B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J. Pediatr. 2001, 138, 679–687. [Google Scholar] [CrossRef]
- Cetin, I.; Koletzko, B.; Moreno, L.A.; Matthys, C. Relevance of European alignment for micronutrients’ recommendation regarding pregnant and lactating women, infants, children and adolescents: an insight into preliminary steps of EURRECA. Matern. Child Nutr. 2010, 6, 3–4. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moran, V.H.; Stammers, A.-L.; Medina, M.W.; Patel, S.; Dykes, F.; Souverein, O.W.; Dullemeijer, C.; Pérez-Rodrigo, C.; Serra-Majem, L.; Nissensohn, M.; et al. The Relationship between Zinc Intake and Serum/Plasma Zinc Concentration in Children: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2012, 4, 841-858. https://doi.org/10.3390/nu4080841
Moran VH, Stammers A-L, Medina MW, Patel S, Dykes F, Souverein OW, Dullemeijer C, Pérez-Rodrigo C, Serra-Majem L, Nissensohn M, et al. The Relationship between Zinc Intake and Serum/Plasma Zinc Concentration in Children: A Systematic Review and Dose-Response Meta-Analysis. Nutrients. 2012; 4(8):841-858. https://doi.org/10.3390/nu4080841
Chicago/Turabian StyleMoran, Victoria Hall, Anna-Louise Stammers, Marisol Warthon Medina, Sujata Patel, Fiona Dykes, Olga W. Souverein, Carla Dullemeijer, Carmen Pérez-Rodrigo, Lluis Serra-Majem, Mariela Nissensohn, and et al. 2012. "The Relationship between Zinc Intake and Serum/Plasma Zinc Concentration in Children: A Systematic Review and Dose-Response Meta-Analysis" Nutrients 4, no. 8: 841-858. https://doi.org/10.3390/nu4080841
APA StyleMoran, V. H., Stammers, A.-L., Medina, M. W., Patel, S., Dykes, F., Souverein, O. W., Dullemeijer, C., Pérez-Rodrigo, C., Serra-Majem, L., Nissensohn, M., & Lowe, N. M. (2012). The Relationship between Zinc Intake and Serum/Plasma Zinc Concentration in Children: A Systematic Review and Dose-Response Meta-Analysis. Nutrients, 4(8), 841-858. https://doi.org/10.3390/nu4080841







