Benefits of Structured and Free Monoacylglycerols to Deliver Eicosapentaenoic (EPA) in a Model of Lipid Malabsorption
Abstract
:1. Introduction
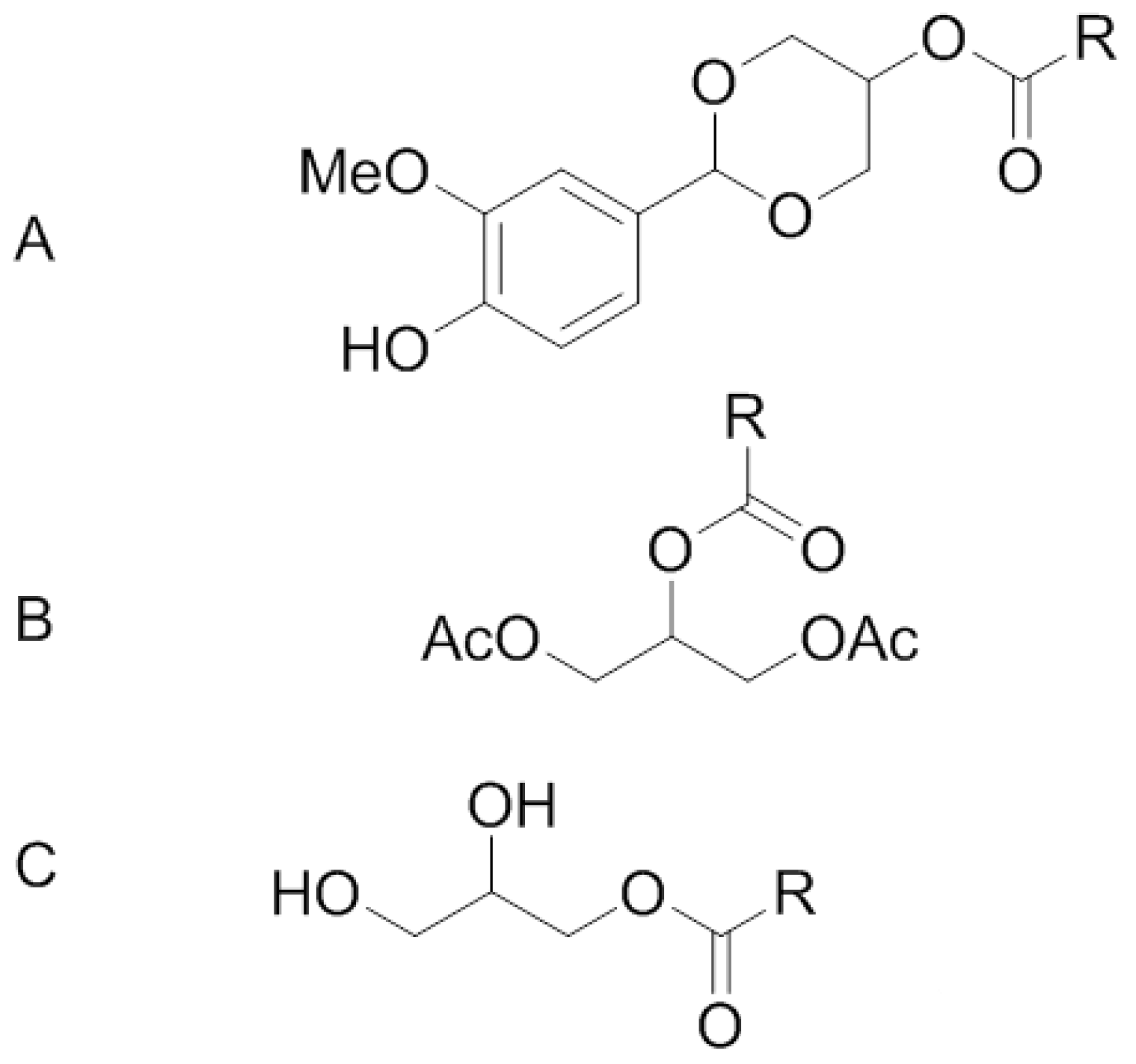
2. Methods and Materials
2.1. animals and Experimental Diet
| Nutrients (g/kg) | FO | FO + O | Vanil + O | Acetyl + O | MAG + O |
|---|---|---|---|---|---|
| Lipids (Total) | 200 | 200 | 200 | 200 | 200 |
| Cocoa butter | 100 | 100 | 100 | 100 | 100 |
| High Oleic Sunflower oil | 72 | 72 | 60 | 70 | 79 |
| Fish oil | 28 | 28 | - | - | - |
| MAG Vanillin Acetal | - | - | 40 | - | - |
| Diacetylated MAG | - | - | - | 30 | - |
| MAG | - | - | - | - | 21 |
| EPA (20:5 n-3) | 4.4 | 4.4 | 4.4 | 4.2 | 4.7 |
| DHA (22:6 n-3) | 2.9 | 2.9 | 7.4 | 3.6 | 3.1 |
| Orlistat | - | 0. 4 | 0. 4 | 0. 4 | 0. 4 |
| Corn starch | 461 | 461 | 461 | 461 | 461 |
| α-Casein | 140 | 140 | 140 | 140 | 140 |
| Sucrose | 100 | 100 | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| Mineral mix AIN-93M | 35 | 35 | 35 | 35 | 35 |
| Vitamin mix AIN-93M | 10 | 10 | 10 | 10 | 10 |
| l-cysteine | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Butylhydroxytoluene | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| Experimental Groups | ||||||
|---|---|---|---|---|---|---|
| FO | FO + O | Vanil + O | Acetyl + O | MAG + O | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Final body weight (g) | 317.7 ± 13.1 | 310.6 ± 7.6 | 314.6 ± 4.2 | 316.5 ± 8.8 | 310.4 ± 13.6 | |
| Weight gain (g/day) | 4.4 ± 0.6 | 4.0 ± 0.3 | 4.2 ± 0.7 | 4.6 ± 0.3 | 4.1 ± 0.5 | |
| Fat Mass (g) | Day 0 | 30.7 ± 1.6 | 30.1 ± 0.4 | 29.0 ± 1.8 | 29.1 ± 0.7 | 29.4 ± 0.8 |
| Day 21 | 54.4 ± 4.0 | 46.1 ± 1.3 | 49.6 ± 2.7 | 52.3 ± 3.0 | 50.2 ± 5.6 | |
| Lean Mass (g) | Day 0 | 179.0 ± 5.6 | 181.2 ± 3.7 | 181.6 ± 3.5 | 178.6 ± 4.6 | 178.6 ± 4.7 |
| Day 21 | 223.8 ± 8.8 | 225.2 ± 5.8 | 226.3 ± 3.1 | 224.8 ± 4.9 | 220.1 ± 7.7 | |
| Lipid Intake (g/day) | 4.1 ± 0.2 | 4.6 ± 0.2 | 5.2 ± 0.1 * | 4.9 ± 0.3 | 4.9 ± 0.3 | |
| Daily Food Intake (g/day) | 20.7 ± 0.8 | 22.8 ± 0.9 | 25.0 ± 0.6 * | 23.9 ± 1.4 | 23.9 ± 1.7 | |
| Fecal Lipid excretion (g/day) | 0.43 ± 0.05 *** | 2.49 ± 0.30 | 3.10 ± 0.17 *** | 1.97 ± 0.21 *** | 3.16 ± 0.31 *** | |
| Apparent Lipid absorption (%) | 89.5 ± 0.8 *** | 44.8 ± 4.7 | 37.1 ± 2.1 *** | 58.5 ± 2.5 *** | 32.9 ± 3.6 *** | |
| EPA Intake (mg/day) | 91.08 ± 3.5 | 100.32 ± 4.2 | 110.0 ± 3.2 | 100.38 ± 6.2 | 112.33 ± 7.5 | |
| DHA Intake (mg/day) | 60.03 ± 3.5 | 66.12 ± 4.2 | 185 ± 3.2 *** | 86.04 ± 6.2 ** | 74.09 ± 5.3 | |
2.2. Experimental Design
2.3. Lipid Extraction
2.4. Fatty Acid Methyl Esters Preparation and Analysis by Gas Chromatography
2.5. Statistical Analyses
3. Results
3.1. Body Weight, Food Intake and Apparent Lipid Absorption
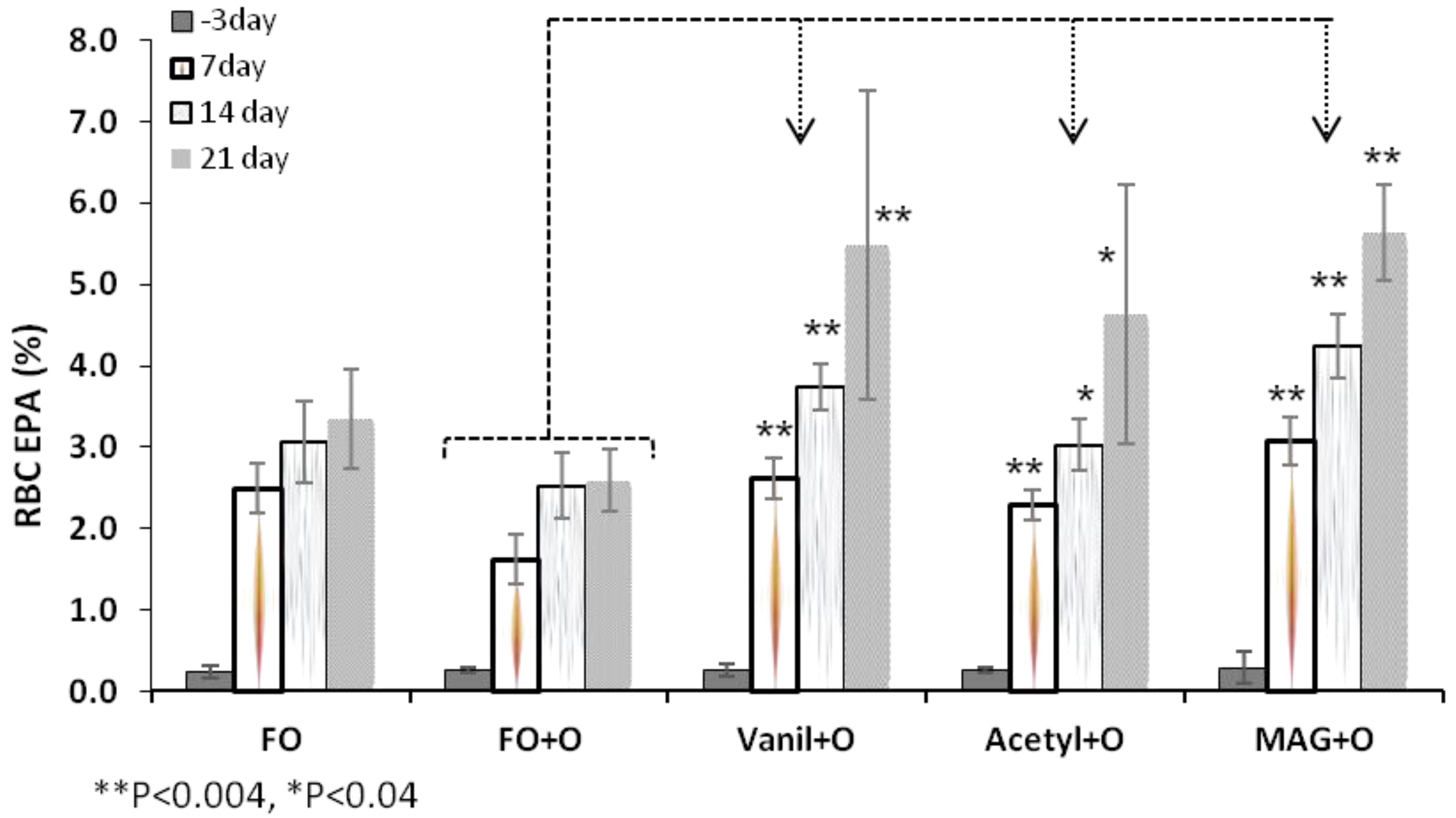
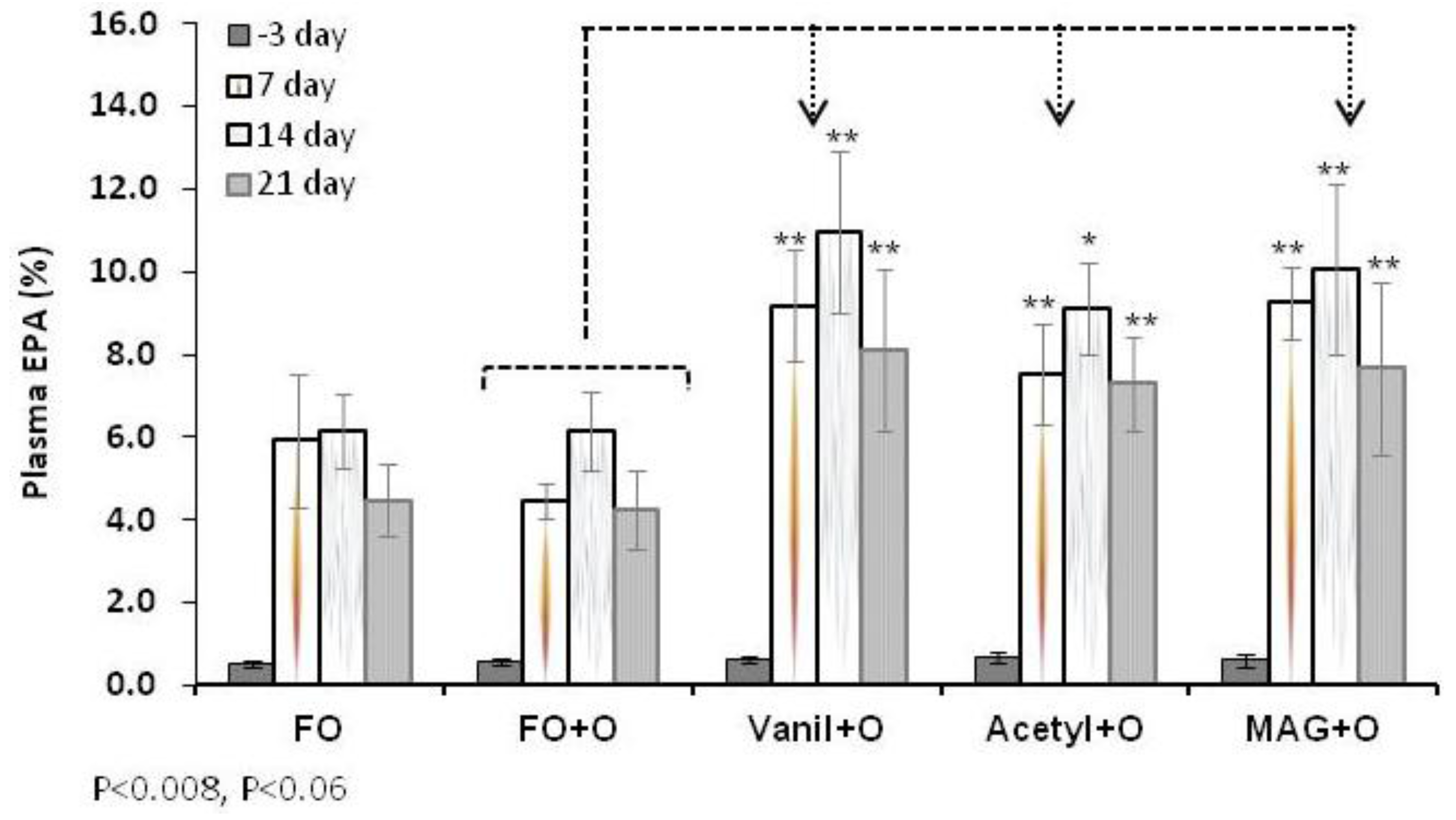
3.2. Incorporation of EPA in RBC, Plasma and Tissues
| Experimental Groups | ||||||
|---|---|---|---|---|---|---|
| FO | FO + O | Vanil + O | Acetyl + O | MAG + O | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| RBC | Day 3 | 0.2 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.2 |
| Day 21 | 3.3 ± 0.6 * | 2.6 ± 0.4 | 5.5 ± 1.9 * | 4.6 ± 1.6 ** | 5.6 ± 0.6 ** | |
| Plasma | Day 3 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| Day 21 | 4.5 ± 0.9 | 4.3 ± 0.9 | 8.5 ± 1.1 ** | 7.0 ± 1.5 ** | 8.5 ± 1.6 ** | |
| Liver | 3.7 ± 0.74 | 3.1 ± 0.92 | 6.5 ± 1.57 ** | 5.2 ± 1.72 ** | 7.9 ± 2.14 ** | |
| Spleen | 1.9 ± 0.57 * | 1.1 ± 0.36 | 2.1 ± 0.8 * | 2.1 ± 0.62 * | 2.6 ± 0.59 ** | |
| Retina | 0.3 ± 0.06 * | 0.2 ± 0.05 | 0.4 ± 0.03 ** | 0.4 ± 0.08 ** | 0.45 ± 0.04 ** | |
| Brain | 0.05 ± 0.01 ** | 0.1 ± 0.03 | 0.2 ± 0.02 ** | 0.1 ± 0.05 | 0.2 ± 0.03 ** | |
3.3. Incorporation of DHA in RBC, Plasma and Tissues
| Experimental Groups | ||||||
|---|---|---|---|---|---|---|
| FO | FO + O | Vanil + O | Acetyl + O | MAG + O | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| RBC | Day 3 | 3.1 ± 0.2 | 3.2 ± 0.2 | 3.2 ± 0.1 | 3.0 ± 0.3 | 3.2 ± 0.2 |
| Day 21 | 4.1 ± 0.8 | 4.0 ± 0.3 | 4.9 ± 0.5 ** | 4.2 ± 0.6 | 4.3 ± 0.3 | |
| Plasma | Day 3 | 2.5 ± 0.2 | 2.3 ± 0.3 | 2.5 ± 0.2 | 2.6 ± 0.2 | 2.9 ± 0.2 |
| Day 21 | 5.7 ± 0.3 ** | 4.6 ± 0.6 | 7.7 ± 0.6 ** | 5.6 ± 0.8 ** | 6.4 ± 1.0 ** | |
| Liver | 8.09 ± 1.13 | 7.41 ± 1.39 | 11.7 ± 3.31 ** | 9.4 ± 0.58 ** | 10.6 ± 1.27 ** | |
| Spleen | 2.1 ± 0.68 | 1.4 ± 0.73 | 2.9 ± 0.91 ** | 2.4 ± 0.5 | 2.3 ± 0.46 * | |
| Retina | 35.1 ± 0.17 | 34.7 ± 1.26 | 35.5 ± 2.44 | 35.2 ± 1.33 | 35.2 ± 2.42 | |
| Brain | 14.1 ± 0.51 | 13.7 ± 0.22 | 14.5 ± 0.22 ** | 14.4 ± 0.64 | 14.6 ± 0.34 ** | |
4. Discussion
5. Conclusion
Acknowledgments
Conflict of Interest
References
- Abia, R.; Pacheco, Y.M.; Perona, J.S.; Montero, E.; Muriana, F.J.; Ruiz-Gutiérrez, V. The metabolic availability of dietary triacylglycerols from two high oleic oils during the postprandial period does not depend on the amount of oleic acid ingested by healthy men. J. Nutr. 2001, 131, 59–65. [Google Scholar]
- Kalivianakis, M.; Elstrodt, J.; Havinga, R. Validation in an animal model of the carbon 13-labeled mixed triglyceride breath test for the detection of intestinal fat malabsorption. J. Pediatr. 1999, 135, 444–450. [Google Scholar]
- Morley, N.; Kuksis, A. Positional specificity of lipoprotein lipase. J. Biol. Chem. 1972, 247, 6389–6393. [Google Scholar]
- Small, D.M. The effects of glycerides structure on absorption and metabolism. Annu. Rev. Nutr. 1991, 11, 413–434. [Google Scholar]
- Straarup, E.M.; Høy, C.E. Structured lipids improve fat absorption in normal and malabsorbing rats. J. Nutr. 2000, 130, 2802–2808. [Google Scholar]
- Christensen, M.S.; Hoy, C.-E.; Becker, C.C.; Redgrave, T.G. Intestinal absorption and lymphatics transport of ecosapentanoic (EPA), docosahexaenoic (DHA), and decanoic acids: Dependance on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 1995, 61, 56–61. [Google Scholar]
- Hunter, E.J. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 2001, 36, 655–668. [Google Scholar] [CrossRef]
- Sukhotnik, S.; Shany, A.; Basheko, Y.; Havari, L.; Chemodanoy, E.; Mogilner, J.; Coran, A.G.; Shaoul, R. Parenteral but not enteral omega-3 fatty acids (Omegaven) modulate intestinal regrowth after massive small bowel resection in rats. J. Parenter. Enteral Nutr. 2010, 34, 503–512. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Bijvelds, M.J.; de Jonge, H.R.; de Lisle, R.C.; Burgerhof, J.G.; Verkade, H.J. Effect of antibiotic treatment on fat absorption in mice with cystic fibrosis. Pediatr. Res. 2012, 71, 1–9. [Google Scholar] [CrossRef]
- Cruz-Hernandez, C.; Oliveira, M.; Pescia, G.; Moulin, J.; Masserey-Elmelegy, I.; Dionisi, F.; Destaillats, F. Lipase inhibitor Orlistat decreases incorporation of eicosapentaenoic and docosahexaenoic acids in rat tissues. Nutr. Res. 2010, 30, 134–140. [Google Scholar]
- Yang, T.; Zhang, H.; Sinclair, A.J.; Xu, X. Diacylglycerols from butterfat: Production by glycerolysis and short-path distillation and analysis of physical properties. J. Am. Oil Chem. Soc. 2004, 81, 979–987. [Google Scholar] [CrossRef]
- Destaillats, F.; Cruz-Hernandez, C.; Nagy, K.; Dionisi, F. Identification of monoacylglycerol regio-isomers by gas chromatography- mass spectrometry. J. Chromatogr. A 2010, 1217, 1543–1548. [Google Scholar] [CrossRef]
- Destaillats, F.; Cruz-Hernandez, C. Fast analysis by gas-liquid chromatography perspective on the resolution of complex fatty acid compositions. J. Chromatogr.A 2007, 1169, 175–178. [Google Scholar] [CrossRef]
- Asakura, L.; Lottenberg, A.M.; Neves, M.Q.; Nunes, V.S.; Rocha, J.C.; Passarelli, M.; Nakandakare, E.R.; Quintão, E.C. Dietary medium-chain triacylglycerol prevents the postprandial rise of plasma triacylglycerols but induces hypercholesterolemia in primary hypertriglyceridemic subjects. Am. J. Clin. Nutr. 2000, 71, 701–705. [Google Scholar]
- Straarup, E.M.; Høy, C. Lymphatic transport of fat in rats with normal- and mal-absorption following intake of fats made from fish oil and decanoic acid: Effects of triacylglycerol structure. Nutr. Res. 2001, 21, 1001–1013. [Google Scholar] [CrossRef]
- Martin, J.C.; Caselli, C.; Broquet, S.; Juanéda, P.; Nour, M.; Sébédio, J.L.; Bernard, A. Lipid effect of cyclic fatty acidmonomers on fatabsorption and transport depends on their positioning within the ingested triacylglycerols. J. Lipid Res. 1997, 38, 1666–1679. [Google Scholar]
- Mu, H.; Høy, C.E. Effects of different medium-chain fatty acids on intestinal absorption of structured triacylglycerols. Lipids 2000, 35, 83–89. [Google Scholar]
- Innis, S.M.; Dyer, R. Dietary triacylglycerols with palmitic acid (16:0) in the 2-position increase 16:0 in the 2-position of plasma and chylomicron triacylglycerols, but reduce phospholipid arachidonic and docosahexaenoic acids, and alter cholesteryl ester metabolism in formula-Fed piglets. J. Nutr. 1997, 127, 1311–1319. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cruz-Hernandez, C.; Thakkar, S.K.; Moulin, J.; Oliveira, M.; Masserey-Elmelegy, I.; Dionisi, F.; Destaillats, F. Benefits of Structured and Free Monoacylglycerols to Deliver Eicosapentaenoic (EPA) in a Model of Lipid Malabsorption. Nutrients 2012, 4, 1781-1793. https://doi.org/10.3390/nu4111781
Cruz-Hernandez C, Thakkar SK, Moulin J, Oliveira M, Masserey-Elmelegy I, Dionisi F, Destaillats F. Benefits of Structured and Free Monoacylglycerols to Deliver Eicosapentaenoic (EPA) in a Model of Lipid Malabsorption. Nutrients. 2012; 4(11):1781-1793. https://doi.org/10.3390/nu4111781
Chicago/Turabian StyleCruz-Hernandez, Cristina, Sagar K. Thakkar, Julie Moulin, Manuel Oliveira, Isabelle Masserey-Elmelegy, Fabiola Dionisi, and Frédéric Destaillats. 2012. "Benefits of Structured and Free Monoacylglycerols to Deliver Eicosapentaenoic (EPA) in a Model of Lipid Malabsorption" Nutrients 4, no. 11: 1781-1793. https://doi.org/10.3390/nu4111781
APA StyleCruz-Hernandez, C., Thakkar, S. K., Moulin, J., Oliveira, M., Masserey-Elmelegy, I., Dionisi, F., & Destaillats, F. (2012). Benefits of Structured and Free Monoacylglycerols to Deliver Eicosapentaenoic (EPA) in a Model of Lipid Malabsorption. Nutrients, 4(11), 1781-1793. https://doi.org/10.3390/nu4111781




