Therapeutic Advances of Curcumin and Nanocurcumin in Glioblastoma: Molecular Targets, Bioavailability, and Drug Delivery
Abstract
1. Introduction
2. Curcumin’s Molecular Mechanisms and Targets in Glioblastoma
2.1. PI3K/AKT/mTOR Pathway Inhibition
2.2. NF-κB Modulation
2.3. STAT3 Activation Suppression
2.4. p53-Mediated Apoptosis Activation
2.5. Reactive Oxygen Species (ROS) Regulation
2.6. Anti-Invasive and Anti-Angiogenic Qualities
2.7. Glioblastoma Stem Cells (GSCs) Activation
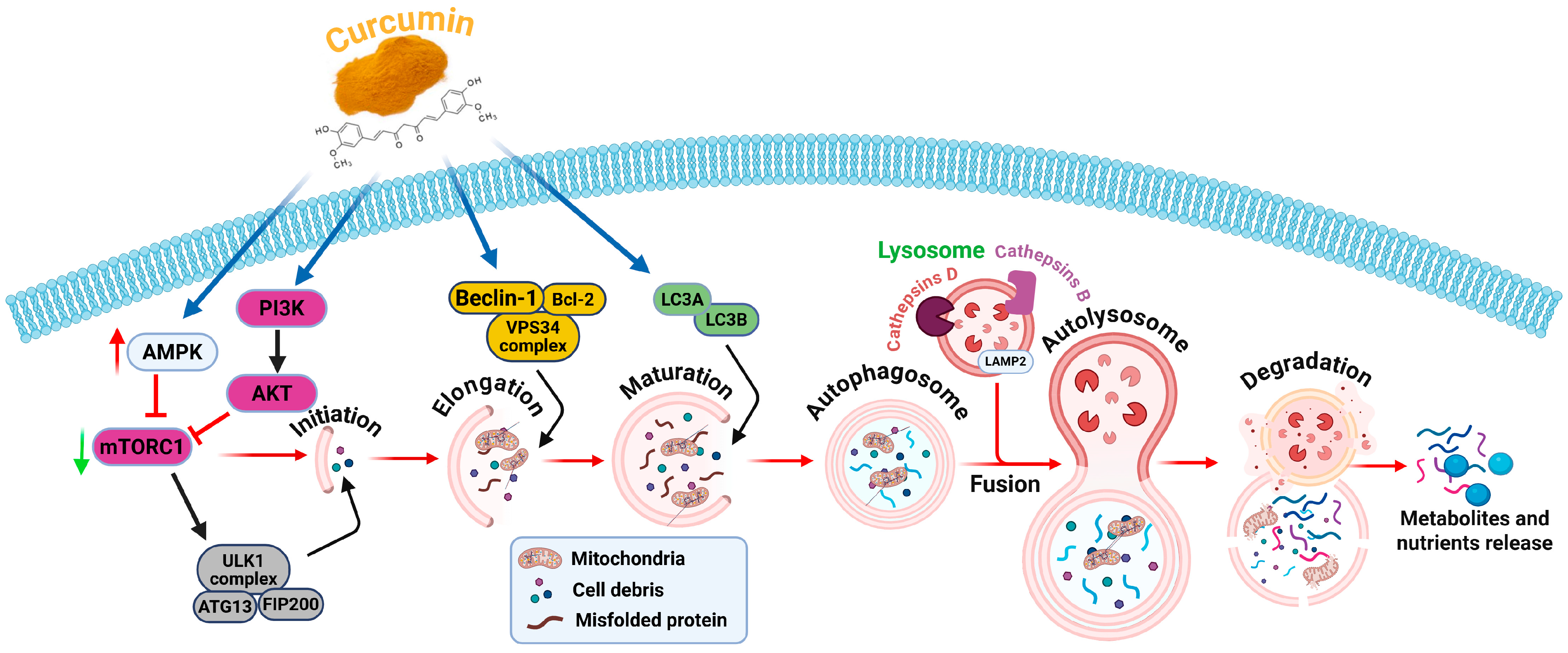
2.8. Autophagy Modulation
2.9. Epigenetic Regulation
2.10. Immunomodulation
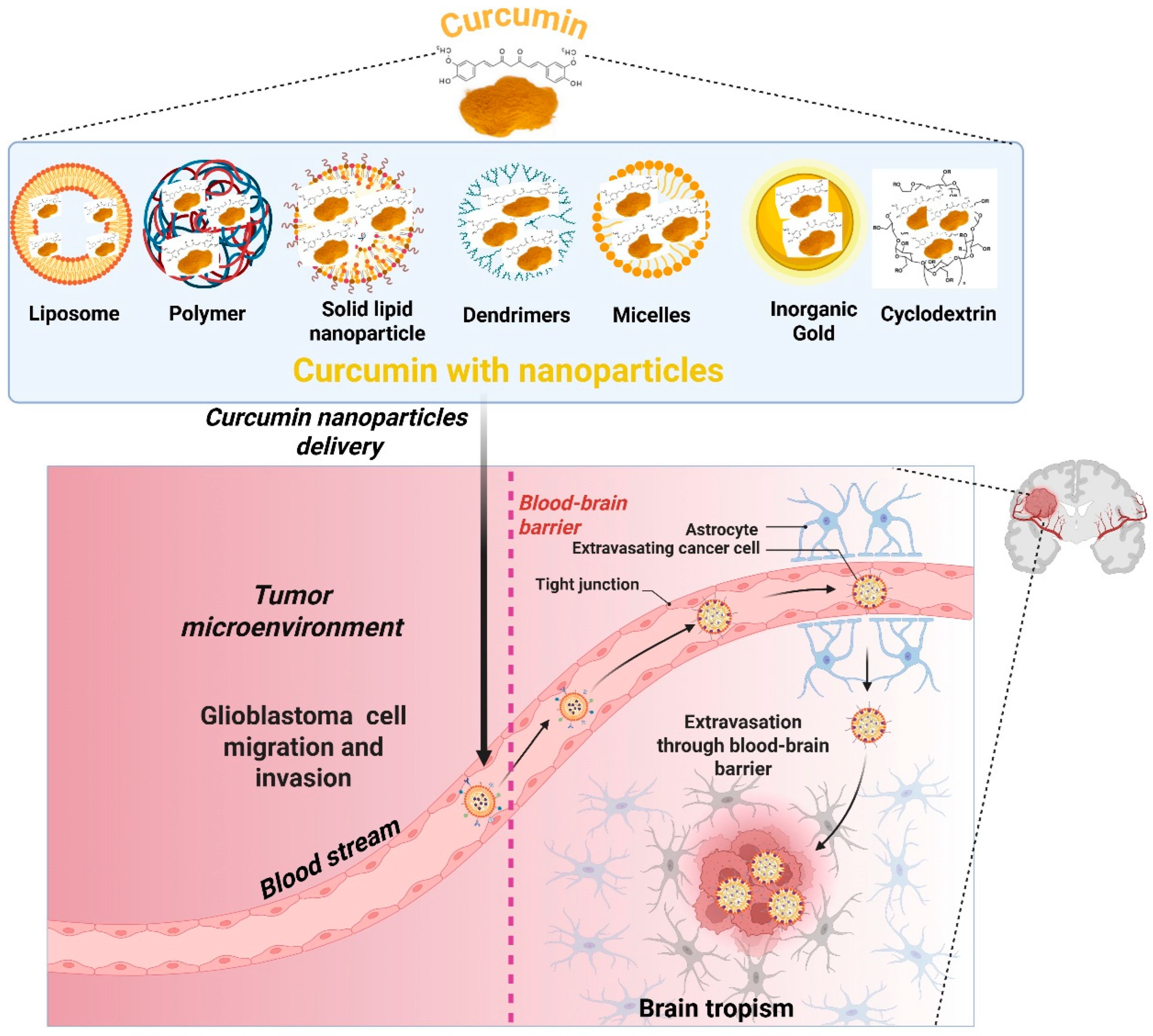
3. Curcumin Delivery Using Nanocarrier Systems for GBM Treatment
3.1. Liposomes
3.2. Nanoparticles of Polymers
3.3. A Solid Lipid Nanoparticle (SLN)
3.4. Dendrimers
3.5. Micelles
3.6. Nanoparticles That Are Inorganic
3.7. Techniques for Penetration of the Blood–Brain Barrier (BBB)
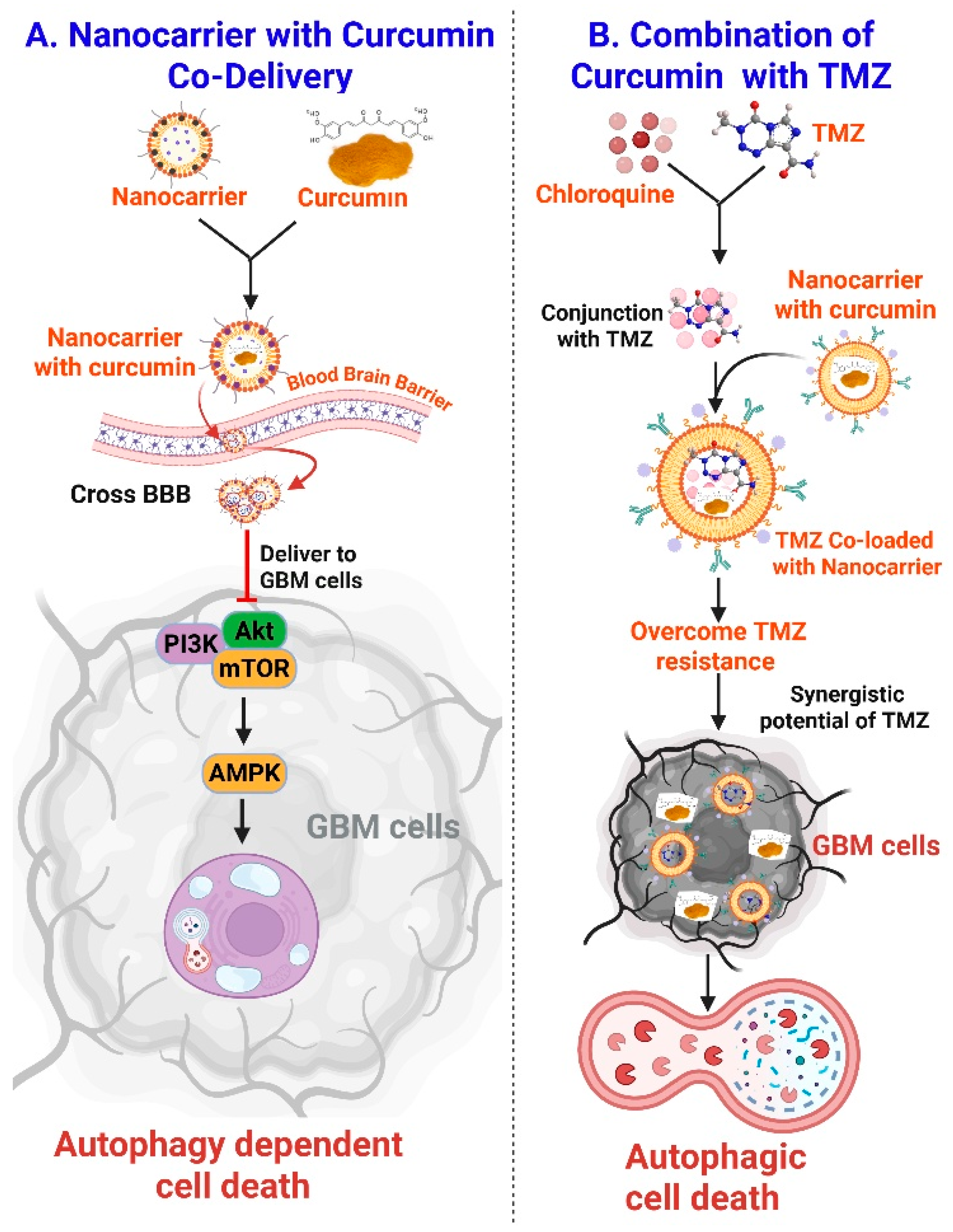
3.8. Curcumin Nanocarrier to Modulate Autophagy in GBM Treatment
4. Recent Preclinical Studies with Curcumin and Nanocurcumin Therapeutic Synergy in Glioblastoma
4.1. GBM Models with Curcumin Monotherapy
4.2. Synergy with Temozolomide
4.3. Improving Radiotherapy
4.4. Combinations of Potential Immunotherapy
4.5. Combinations with Phytochemicals and Natural Compounds
4.6. Targeting Glioma Stem Cells
5. Challenges, Limitations, and Future Directions
5.1. Limitations in Pharmacokinetics and Bioavailability
5.2. Penetration of the Blood–Brain Barrier (BBB)
5.3. Difficulties with Manufacturing and Regulation
5.4. Considerations for Safety and Toxicity
5.5. Insufficient Clinical Experiments
5.6. Microenvironmental Resistance and Tumor Heterogeneity
5.7. Prospects for the Future Direction
6. Critical Perspectives on Curcumin-Loaded Nanocarriers for GBM Therapy
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovács, Á. Glioblastoma: Clinical presentation, multidisciplinary management, and Long-Term outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef]
- Bijalwan, G.; Shrivastav, A.K.; Mallik, S.; Dubey, M.K. Glioblastoma multiforme-a rare type of cancer: A narrative review. Cancer Res. Stat. Treat. 2024, 7, 340–351. [Google Scholar] [CrossRef]
- Munagama, C.L.; Rajendiran, V.; Silva, S. A case report on a common tumour with an uncommon presentation: Glioblastoma. Cureus 2024, 16, e66830. [Google Scholar] [CrossRef]
- Duan, M.; Cao, R.; Yang, Y.; Chen, X.; Liu, L.; Ren, B.; Wang, L.; Goh, B.-C. Blood–brain barrier conquest in glioblastoma nanomedicine: Strategies, clinical advances, and emerging challenges. Cancers 2024, 16, 3300. [Google Scholar] [CrossRef]
- Roney, M.; Huq, A.M.; Rullah, K.; Zamri, N.B.; Mohd Aluwi, M.F.F. Curcumin, a bioactive compound of Turmeric (Curcuma longa) and its derivatives as α-amylase and α-glucosidase inhibitors. Cell Biochem. Biophys. 2025, 83, 53–71. [Google Scholar] [CrossRef]
- Nowacka, A.; Ziółkowska, E.; Smuczyński, W.; Bożiłow, D.; Śniegocki, M. Potential of Curcumin and Its Analogs in Glioblastoma Therapy. Antioxidants 2025, 14, 351. [Google Scholar] [CrossRef]
- Fatima, F.; Chourasiya, N.K.; Mishra, M.; Kori, S.; Pathak, S.; Das, R.; Kashaw, V.; Iyer, A.K.; Kashaw, S.K. Curcumin and its derivatives targeting multiple signaling pathways to elicit anticancer activity: A comprehensive perspective. Curr. Med. Chem. 2024, 31, 3668–3714. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer mechanism of curcumin on human glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Vartholomatos, E.; Zacharopoulou, N.; Tsoumeleka, E.; Gkizas, G.; Bozios, G.; Tsekeris, P.; Chousidis, I.; Leonardos, I. Curcumin and radiotherapy exert synergistic anti-glioma effect in vitro. Biomedicines 2021, 9, 1562. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, A.; Mardani, R.; Roudi, B.; Taghizadeh, M.; Banfshe, H.R.; Ghaderi, A.; Davoodvandi, A.; Shamollaghamsari, S.; Hamblin, M.R.; Mirzaei, H. Combination therapy with nanomicellar-curcumin and temozolomide for in vitro therapy of glioblastoma multiforme via Wnt signaling pathways. J. Mol. Neurosci. 2020, 70, 1471–1483. [Google Scholar] [CrossRef]
- Wen, C.; Cao, L.; Yu, Z.; Liu, G.; Zhang, J.; Xu, X. Advances in lipo-solubility delivery vehicles for curcumin: Bioavailability, precise targeting, possibilities and challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 10835–10854. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, M.; Vengateswaran, H.T.; You, H.W.; Saddhono, K.; Aher, K.B.; Bhavar, G.B. Nanomedicine facilitated cell signaling blockade: Difficulties and strategies to overcome glioblastoma. J. Mater. Chem. B 2024, 12, 1677–1705. [Google Scholar] [CrossRef]
- Kirit, E.; Gokce, C.; Altun, B.; Yilmazer, A.e. Nanotherapeutic Strategies for Overcoming the Blood–Brain Barrier: Applications in Disease Modeling and Drug Delivery. ACS Omega 2025, 10, 32606–32625. [Google Scholar] [CrossRef]
- Khatoon, S.; Kalam, N. Mechanistic insight of curcumin: A potential pharmacological candidate for epilepsy. Front. Pharmacol. 2025, 15, 1531288. [Google Scholar] [CrossRef]
- Musielak, E.; Krajka-Kuźniak, V. Lipidic and Inorganic Nanoparticles for Targeted Glioblastoma Multiforme Therapy: Advances and Strategies. Micro 2025, 5, 2. [Google Scholar] [CrossRef]
- Wu, Y.; Moonshi, S.S.; Ta, H.T. Advancements in Using Polymeric Nanoparticles for Blood–Brain Barrier Penetration in Neurological Disorders. ACS Appl. Bio Mater. 2025, 8, 4416–4431. [Google Scholar] [CrossRef]
- Shetty, N.P.; Prabhakaran, M.; Srivastava, A.K. Pleiotropic nature of curcumin in targeting multiple apoptotic-mediated factors and related strategies to treat gastric cancer: A review. Phytother. Res. 2021, 35, 5397–5416. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Gabrani, R. Current combinatorial therapeutic aspects: The future prospect for glioblastoma treatment. Curr. Med. Sci. 2024, 44, 1175–1184. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.; Wang, L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 2016, 7, 33440. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Liao, W.; Yu, L.; Hu, Z.; Li, M.; Xia, H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch. Biochem. Biophys. 2020, 689, 108412. [Google Scholar] [CrossRef]
- Zoi, V.; Kyritsis, A.P.; Galani, V.; Lazari, D.; Sioka, C.; Voulgaris, S.; Alexiou, G.A. The role of curcumin in cancer: A focus on the PI3K/Akt pathway. Cancers 2024, 16, 1554. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Paralikar, P.; Nagaonkar, D.; Rehman, F.; Alves dos Santos, C. Pharmaceutical applications of curcumin-loaded nanoparticles. In Nanotechnology Applied to Pharmaceutical Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 139–154. [Google Scholar]
- Deng, X.-Z.; Geng, S.-S.; Luo, M.; Chai, J.-J.; Xu, Y.; Chen, C.-L.; Qiu, L.; Ke, Q.; Duan, Q.-W.; Song, S.-M. Curcumin potentiates laryngeal squamous carcinoma radiosensitivity via NF-ΚB inhibition by suppressing IKKγ expression. J. Recept. Signal Transduct. 2020, 40, 541–549. [Google Scholar] [CrossRef]
- Sarkar, S.; Patranabis, S. Immunomodulatory signalling networks in glioblastoma multiforme: A comprehensive review of therapeutic approaches. Hum. Cell 2024, 37, 1355–1377. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Papavassiliou, K.A.; Papavassiliou, A.G. Pivotal role of STAT3 in shaping glioblastoma immune microenvironment. Cells 2019, 8, 1398. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Zamanian, M.Y.; Al-Ani, A.M.; Jabbar, T.L.; Kareem, A.K.; Aghaei, Z.H.; Tahernia, H.; Hjazi, A.; Jissir, S.A.R.; Hakimizadeh, E. Targeting STAT3 signaling pathway by curcumin and its analogues for breast cancer: A narrative review. Anim. Models Exp. Med. 2024, 7, 853–867. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Song, Z.; Pei, Y. Regulation mechanism of curcumin mediated inflammatory pathway and its clinical application: A review. Front. Pharmacol. 2025, 16, 1642248. [Google Scholar] [CrossRef]
- Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Lazzeri, G.; Frati, A.; Fornai, F. The multi-faceted effect of curcumin in glioblastoma from rescuing cell clearance to autophagy-independent effects. Molecules 2020, 25, 4839. [Google Scholar] [CrossRef]
- Kadiyala, P.; Gregory, J.V.; Lowenstein, P.R.; Lahann, J.; Castro, M.G. Targeting gliomas with STAT3-silencing nanoparticles. Mol. Cell. Oncol. 2021, 8, 1870647. [Google Scholar] [CrossRef]
- Sidhar, H.; Giri, R.K. Induction of Bex genes by curcumin is associated with apoptosis and activation of p53 in N2a neuroblastoma cells. Sci. Rep. 2017, 7, 41420. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Salucci, S.; Bavelloni, A.; Stella, A.B.; Fabbri, F.; Vannini, I.; Piazzi, M.; Volkava, K.; Scotlandi, K.; Martinelli, G.; Faenza, I. The cytotoxic effect of curcumin in rhabdomyosarcoma is associated with the modulation of AMPK, AKT/mTOR, STAT, and p53 signaling. Nutrients 2023, 15, 740. [Google Scholar] [CrossRef]
- Ramli, I.; Cheriet, T.; Posadino, A.M.; Giordo, R.; Fenu, G.; Nwachukwu, K.C.; Oyewole, O.A.; Adetunji, C.O.; Calina, D.; Sharifi-Rad, J. Modulating the p53-MDM2 pathway: The therapeutic potential of natural compounds in cancer treatment. EXCLI J. 2024, 23, 1397. [Google Scholar]
- Zepeda-Quiróz, I.; Sánchez-Barrera, H.; Colín-Val, Z.; Robledo-Cadena, D.X.; Rodríguez-Enríquez, S.; López-Marure, R. Curcumin promotes oxidative stress, apoptosis and autophagy in H9c2 rat cardiomyoblasts. Mol. Cell. Toxicol. 2020, 16, 441–453. [Google Scholar] [CrossRef]
- Nainggolan, S.I.; Rajuddin, R.; Kamarlis, R.K.; Hambal, M.; Frengki, F. In silico study of the potential of curcumin and its derivatives for increasing wild-type p53 expression and improving the function of p53 mutant R273H. Vet. World 2025, 18, 715. [Google Scholar] [CrossRef]
- Salehi, A.M.; Hajjari, M.; Pourreza, N.; Khodadadi, A. Investigating How Nano-Curcumin Affects the Expression of the p53 Gene and Inhibits the Cell Cycle in the A549 Lung Cancer Cell Line. Jentashapir J. Cell. Mol. Biol. 2025, 16, e159105. [Google Scholar] [CrossRef]
- Cui, J.; Li, H.; Zhang, T.; Lin, F.; Chen, M.; Zhang, G.; Feng, Z. Research progress on the mechanism of curcumin anti-oxidative stress based on signaling pathway. Front. Pharmacol. 2025, 16, 1548073. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dezfouli, A.B.; Khosravi, M.; Sievert, W.; Stangl, S.; Schwab, M.; Wu, Z.; Steiger, K.; Ma, H.; Multhoff, G. Cannabidiol-induced crosstalk of apoptosis and macroautophagy in colorectal cancer cells involves p53 and Hsp70. Cell Death Discov. 2023, 9, 286. [Google Scholar] [CrossRef]
- Alizadeh, M.; Kheirouri, S. Curcumin reduces malondialdehyde and improves antioxidants in humans with diseased conditions: A comprehensive meta-analysis of randomized controlled trials. BioMedicine 2019, 9, 23. [Google Scholar] [CrossRef]
- Ji, S.; Sun, R.; Xu, K.; Man, Z.; Ji, J.; Pu, Y.; Yin, L.; Zhang, J.; Pu, Y. Prodigiosin induces apoptosis and inhibits autophagy via the extracellular signal-regulated kinase pathway in K562 cells. Toxicol. Vitr. 2019, 60, 107–115. [Google Scholar] [CrossRef]
- Park, J.-Y.; Choi, Y.; Kim, H.-D.; Kuo, H.-H.; Chang, Y.-C.; Kim, C.-H. Matrix Metalloproteinases and Their Inhibitors in the Pathogenesis of Epithelial Differentiation, Vascular Disease, Endometriosis, and Ocular Fibrotic Pterygium. Int. J. Mol. Sci. 2025, 26, 5553. [Google Scholar] [CrossRef]
- Wroński, P.; Wroński, S.; Kurant, M.; Malinowski, B.; Wiciński, M. Curcumin may prevent basement membrane disassembly by matrix metalloproteinases and progression of the bladder cancer. Nutrients 2021, 14, 32. [Google Scholar] [CrossRef]
- Senft, C.; Polacin, M.; Priester, M.; Seifert, V.; Kögel, D.; Weissenberger, J. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer 2010, 10, 491. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Klafke, K.; Figueiró, F.; Terra, S.R.; Paludo, F.J.; Morrone, M.; Bristot, I.J.; Battastini, A.M.; Forcelini, C.M. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015, 358, 220–231. [Google Scholar] [CrossRef]
- Yaroshenko, M.; Christoff, M.; Ścibiorski, M.; Surowiec, K.; Jakubowicz-Gil, J.; Sumorek-Wiadro, J. Natural Compounds That Target Glioma Stem Cells. NeuroSci 2025, 6, 52. [Google Scholar] [CrossRef]
- Naujokat, C.; McKee, D.L. The “Big Five” phytochemicals targeting cancer stem cells: Curcumin, EGCG, sulforaphane, resveratrol and genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef]
- Mattei, V.; Santilli, F.; Martellucci, S.; Delle Monache, S.; Fabrizi, J.; Colapietro, A.; Angelucci, A.; Festuccia, C. The importance of tumor stem cells in glioblastoma resistance to therapy. Int. J. Mol. Sci. 2021, 22, 3863. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.; Gandhi, P.; Kar, S.K. Curcumin and 2-DG synergistically target the glio-oncogenesis trigger IL-6 and down-regulate the stemness in glioblastoma model in-vitro. Adv. Tradit. Med. 2025, 25, 597–609. [Google Scholar] [CrossRef]
- Sabu, A.; Liu, T.-I.; Ng, S.S.; Doong, R.-A.; Huang, Y.-F.; Chiu, H.-C. Nanomedicines targeting glioma stem cells. ACS Appl. Mater. Interfaces 2022, 15, 158–181. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Zhang, M.; Xiang, C.; Niu, R.; He, X.; Luo, W.; Liu, W.; Gu, R. Liposomes as versatile agents for the management of traumatic and nontraumatic central nervous system disorders: Drug stability, targeting efficiency, and safety. Neural Regen. Res. 2025, 20, 1883–1899. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jalouli, M.; Al-Zharani, M.; Hoque Apu, E.; Harrath, A.H. Mechanistic Insights into Autophagy-Dependent Cell Death (ADCD): A Novel Avenue for Cancer Therapy. Cells 2025, 14, 1072. [Google Scholar] [CrossRef]
- Shakeri, A.; Cicero, A.F.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef]
- Wang, N.; Feng, T.; Liu, X.; Liu, Q. Curcumin inhibits migration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR signaling pathway. Acta Pharm. 2020, 70, 399–409. [Google Scholar] [CrossRef]
- Han, J.; Pan, X.-Y.; Xu, Y.; Xiao, Y.; An, Y.; Tie, L.; Pan, Y.; Li, X.-J. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 2012, 8, 812–825. [Google Scholar] [CrossRef]
- Fabianowska-Majewska, K.; Kaufman-Szymczyk, A.; Szymanska-Kolba, A.; Jakubik, J.; Majewski, G.; Lubecka, K. Curcumin from turmeric rhizome: A potential modulator of DNA methylation machinery in breast cancer inhibition. Nutrients 2021, 13, 332. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Li, Y.; Wang, C.; Bian, C.; Wang, H.; Wang, F. Epigenetic regulation of histone modifications in glioblastoma: Recent advances and therapeutic insights. Biomark. Res. 2025, 13, 80. [Google Scholar] [CrossRef]
- Fu, S.; Kurzrock, R. Development of curcumin as an epigenetic agent. Cancer 2010, 116, 4670–4676. [Google Scholar] [CrossRef]
- Miles, F.L.; Mashchak, A.; Filippov, V.; Orlich, M.J.; Duerksen-Hughes, P.; Chen, X.; Wang, C.; Siegmund, K.; Fraser, G.E. DNA methylation profiles of vegans and non-vegetarians in the adventist health study-2 cohort. Nutrients 2020, 12, 3697. [Google Scholar] [CrossRef]
- Sminia, P.; van den Berg, J.; van Kootwijk, A.; Hageman, E.; Slotman, B.J.; Verbakel, W.F. Experimental and clinical studies on radiation and curcumin in human glioma. J. Cancer Res. Clin. Oncol. 2021, 147, 403–409. [Google Scholar] [CrossRef]
- Amini, A.; Khadivar, P.; Ahmadnia, A.; Alipour, M.; Majeed, M.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. Role of curcumin in regulating long noncoding RNA expression in cancer. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Springer: Berlin/Heidelberg, Germany, 2021; pp. 13–23. [Google Scholar]
- Fu, X.; He, Y.; Li, M.; Huang, Z.; Najafi, M. Targeting of the tumor microenvironment by curcumin. Biofactors 2021, 47, 914–932. [Google Scholar] [CrossRef]
- Admasu, T.D.; Yu, J.S. Harnessing Immune Rejuvenation: Advances in Overcoming T Cell Senescence and Exhaustion in Cancer Immunotherapy. Aging Cell 2025, 24, e70055. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, Y.; Huang, W.; Lin, Y.; Li, B. Curcumin Reprograms TAMs from a protumor phenotype towards an antitumor phenotype via inhibiting MAO-A/STAT6 Pathway. Cells 2022, 11, 3473. [Google Scholar] [CrossRef]
- Parker, J.M.; Zhao, L.; Mayberry, T.G.; Cowan, B.C.; Wakefield, M.R.; Fang, Y. From Spice to Survival: The Emerging Role of Curcumin in Cancer Immunotherapy. Cancers 2025, 17, 2491. [Google Scholar] [CrossRef]
- Saberian, E.; Jenčová, J.; Jenča, A.; Jenča, A.; Petrášová, A.; Jenča, J.; Akbarzadehkhayavi, A. Combination Therapy of Curcumin and Cisplatin Encapsulated in Niosome Nanoparticles for Enhanced Oral Cancer Treatment. Indian. J. Clin. Biochem. 2025, 40, 59–66. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Obeid, M.A.; Alsaadi, M.; Aljabali, A.A. Recent updates in curcumin delivery. J. Liposome Res. 2023, 33, 53–64. [Google Scholar] [CrossRef]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood-Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of curcumin-loaded nanocarriers for food, drug and cosmetic purposes. Trends Food Sci. Technol. 2019, 88, 445–458. [Google Scholar] [CrossRef]
- Santhanakrishnan, K.R.; Koilpillai, J.; Narayanasamy, D.; Santhanakrishnan, K. PEGylation in pharmaceutical development: Current status and emerging trends in macromolecular and immunotherapeutic drugs. Cureus 2024, 16, e66669. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Karatug Kacar, A.; Sak, R.; Nurdogan, A.N.; Ergin Kızılcay, G.; Bahadori, F. Dual-targeted protein-coated PLGA nanoparticles for pancreatic cancer therapy: A novel approach using esculetin and curcumin. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 1–20. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-based nanoformulations: A promising adjuvant towards cancer treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef]
- Tan, X.; Kim, G.; Lee, D.; Oh, J.; Kim, M.; Piao, C.; Lee, J.; Lee, M.S.; Jeong, J.H.; Lee, M. A curcumin-loaded polymeric micelle as a carrier of a microRNA-21 antisense-oligonucleotide for enhanced anti-tumor effects in a glioblastoma animal model. Biomater. Sci. 2018, 6, 407–417. [Google Scholar] [CrossRef]
- Aggarwal, K.; Joshi, S.; Jindal, P.; Patel, P.; Das Kurmi, B. Solid Lipid Nanoparticles: An Innovative Drug Delivery System for Enhanced Bioavailability and Targeted Therapy. AAPS PharmSciTech 2025, 26, 186. [Google Scholar] [CrossRef]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of curcumin-loaded zein nanoparticles for transport across the blood-brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Sampron, N.; Matheu, A. Current advances in temozolomide encapsulation for the enhancement of glioblastoma treatment. Theranostics 2023, 13, 2734–2756. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, X.; Guan, S.; Yan, Y.; Lin, H.; Hua, Z.-C. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget 2015, 6, 19469. [Google Scholar] [CrossRef]
- Ciftci, F.; Özarslan, A.C.; Kantarci, I.C.; Yelkenci, A.; Tavukcuoglu, O.; Ghorbanpour, M. Advances in drug targeting, drug delivery, and nanotechnology applications: Therapeutic significance in cancer treatment. Pharmaceutics 2025, 17, 121. [Google Scholar] [CrossRef]
- McLoughlin, C.D.; Nevins, S.; Stein, J.B.; Khakbiz, M.; Lee, K.B. Overcoming the Blood-Brain Barrier: Multifunctional Nanomaterial-Based Strategies for Targeted Drug Delivery in Neurological Disorders. Small Sci. 2024, 4, 2400232. [Google Scholar] [CrossRef]
- Li, D.; Fan, Y.; Shen, M.; Bányai, I.; Shi, X. Design of dual drug-loaded dendrimer/carbon dot nanohybrids for fluorescence imaging and enhanced chemotherapy of cancer cells. J. Mater. Chem. B 2019, 7, 277–285. [Google Scholar] [CrossRef]
- Maiti, P.; Scott, J.; Sengupta, D.; Al-Gharaibeh, A.; Dunbar, G.L. Curcumin and Solid Lipid Curcumin Particles Induce Autophagy, but Inhibit Mitophagy and the PI3K-Akt/mTOR Pathway in Cultured Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 399. [Google Scholar] [CrossRef]
- Dhungel, L.; Rowsey, M.E.; Harris, C.; Raucher, D. Synergistic Effects of Temozolomide and Doxorubicin in the Treatment of Glioblastoma Multiforme: Enhancing Efficacy through Combination Therapy. Molecules 2024, 29, 840. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Cerqueira, R.; Domingues, C.; Veiga, F.; Jarak, I.; Figueiras, A. Development and Characterization of Curcumin-Loaded TPGS/F127/P123 Polymeric Micelles as a Potential Therapy for Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 7577. [Google Scholar] [CrossRef]
- Raucher, D.; Dragojevic, S.; Ryu, J. Macromolecular Drug Carriers for Targeted Glioblastoma Therapy: Preclinical Studies, Challenges, and Future Perspectives. Front. Oncol. 2018, 8, 624. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Li, H.; Hu, T.Y. Recent advances in the bench-to-bedside translation of cancer nanomedicines. Acta Pharm. Sin. B 2025, 15, 97–122. [Google Scholar] [CrossRef]
- Dragoj, M.; Stojkovska, J.; Stanković, T.; Dinić, J.; Podolski-Renić, A.; Obradović, B.; Pešić, M. Development and Validation of a Long-Term 3D Glioblastoma Cell Culture in Alginate Microfibers as a Novel Bio-Mimicking Model System for Preclinical Drug Testing. Brain Sci. 2021, 11, 1025. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef]
- Aborig, M.; Alsefaou, M.; Osei, E.; Wettig, S. Engineered dual-functional gold nanoparticles enhance radiosensitization in prostate cancer cells: Synergistic action of curcumin and gold. Nanoscale Adv. 2025, 7, 5964–5977. [Google Scholar] [CrossRef]
- Ghazi, R.; Ibrahim, T.K.; Nasir, J.A.; Gai, S.; Ali, G.; Boukhris, I.; Rehman, Z. Iron oxide based magnetic nanoparticles for hyperthermia, MRI and drug delivery applications: A review. RSC Adv. 2025, 15, 11587–11616. [Google Scholar] [CrossRef]
- Alharbi, S.K.; Alsehli, B.R.; AlSuhaimi, A.O.; Thumayri, K.A.; AlMohaimadi, K.M.; Mehdar, Y.T.H.; Almalki, M.A.; Hussein, B.H.M. Mesoporous Silica Nanoparticles Functionalized with Bisphenol A for Dispersive Solid-Phase Extraction of 3-Chloroaniline from Water Matrices: Material Synthesis and Sorption Optimization. Nanomater. 2025, 15, 1751. [Google Scholar] [CrossRef]
- Song, J.; Lu, C.; Leszek, J.; Zhang, J. Design and Development of Nanomaterial-Based Drug Carriers to Overcome the Blood-Brain Barrier by Using Different Transport Mechanisms. Int. J. Mol. Sci. 2021, 22, 10118. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L.; Yin, X. Pathophysiological aspects of transferrin-A potential nano-based drug delivery signaling molecule in therapeutic target for varied diseases. Front. Pharmacol. 2024, 15, 1342181. [Google Scholar] [CrossRef]
- Ghorai, S.M.; Deep, A.; Magoo, D.; Gupta, C.; Gupta, N. Cell-Penetrating and Targeted Peptides Delivery Systems as Potential Pharmaceutical Carriers for Enhanced Delivery across the Blood-Brain Barrier (BBB). Pharmaceutics 2023, 15, 1999. [Google Scholar] [CrossRef]
- Paul, A.; Collins, M.G.; Lee, H.Y. Gene Therapy: The Next-Generation Therapeutics and Their Delivery Approaches for Neurological Disorders. Front. Genome Ed. 2022, 4, 899209. [Google Scholar] [CrossRef]
- Wahengbam, G.S.; Nirmal, S.; Nandwana, J.; Kar, S.; Kumari, V.; Mishra, R.; Singh, A. Polymeric Nanoparticles Revolutionizing Brain Cancer Therapy: A Comprehensive Review of Strategies and Advances. Crit. Rev. Ther. Drug Carr. Syst. 2025, 42, 73–106. [Google Scholar] [CrossRef]
- Khayatan, D.; Razavi, S.M.; Arab, Z.N.; Nasoori, H.; Fouladi, A.; Pasha, A.V.K.; Butler, A.E.; Karav, S.; Momtaz, S.; Abdolghaffari, A.H.; et al. Targeting mTOR with curcumin: Therapeutic implications for complex diseases. Inflammopharmacology 2025, 33, 1583–1616. [Google Scholar] [CrossRef]
- Tian, S.; Wu, L.; Zheng, H.; Zhong, X.; Yu, X.; Wu, W. Identification of autophagy-related genes in neuropathic pain through bioinformatic analysis. Hereditas 2023, 160, 8. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Xiao, J.; Liu, Y. Nanomedicine-based combination therapies for overcoming temozolomide resistance in glioblastomas. Cancer Biol. Med. 2023, 20, 325–343. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, Y.; Liu, Y.; Chen, Y.; Zhao, P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346. [Google Scholar] [CrossRef]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef]
- Afshari, A.R.; Sanati, M.; Aminyavari, S.; Keshavarzi, Z.; Ahmadi, S.S.; Oroojalian, F.; Karav, S.; Sahebkar, A. A novel approach to glioblastoma multiforme treatment using modulation of key pathways by naturally occurring small molecules. Inflammopharmacology 2025, 33, 1237–1254. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Wen, C.; Zhou, C.; Zhang, W.; Hu, X.; Wang, L.; You, C.; Shao, J. Curcumin sensitizes glioblastoma to temozolomide by simultaneously generating ROS and disrupting AKT/mTOR signaling. Oncol. Rep. 2014, 32, 1610–1616. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, C.W.; Lee, W.S.; Kim, H.J.; Jeong, H.J.; Park, M.J.; Jang, W.I.; Kim, E.H. Interaction of curcumin with glioblastoma cells via high and low linear energy transfer radiation therapy inducing radiosensitization effects. J. Radiat. Res. 2022, 63, 342–353. [Google Scholar] [CrossRef]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1040259. [Google Scholar] [CrossRef]
- Arora, A.; Jain, N.; Pandey, M.; Kaul, S.; Verma, R.; Gorain, B. Smart Nanometals: An Approach to Transform Brain Cancer Diagnosis and Therapy. Mol. Pharm. 2025, 22, 4512–4543. [Google Scholar] [CrossRef]
- Hedayati, N.; Safari, M.H.; Milasi, Y.E.; Kahkesh, S.; Farahani, N.; Khoshnazar, S.M.; Dorostgou, Z.; Alaei, E.; Alimohammadi, M.; Rahimzadeh, P.; et al. Modulation of the PI3K/Akt signaling pathway by resveratrol in cancer: Molecular mechanisms and therapeutic opportunity. Discov. Oncol. 2025, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Kumar, G.; Vibhavari, R.J.A.; Nandakumar, K.; Thorat, N.D.; Chamallamudi, M.R.; Kumar, N. Temozolomide Resistance: A Multifarious Review on Mechanisms Beyond O-6-Methylguanine-DNA Methyltransferase. CNS Neurol. Disord. Drug Targets 2023, 22, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.; Kar, S.K.; Gothalwal, R.; Gandhi, P. Curcumin in Combination with Other Adjunct Therapies for Brain Tumor Treatment: Existing Knowledge and Blueprint for Future Research. Int. J. Mol. Cell Med. 2021, 10, 163–181. [Google Scholar] [CrossRef]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing Curcumin Oral Bioavailability Through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Keshavarz Shahbaz, S.; Koushki, K.; Izadi, O.; Penson, P.E.; Sukhorukov, V.N.; Kesharwani, P.; Sahebkar, A. Advancements in curcumin-loaded PLGA nanoparticle delivery systems: Progressive strategies in cancer therapy. J. Drug Target. 2024, 32, 1207–1232. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Tsekeris, P.; Kyritsis, A.P.; Alexiou, G.A. Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers. Biomedicines 2022, 10, 312. [Google Scholar] [CrossRef]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Zangui, M.; Lotfi, M.; Ghayour-Mobarhan, M.; Ghorbani, A.; Jaliani, H.Z.; Sadeghnia, H.R.; Sahebkar, A. Therapeutic Potential of Curcumin in the Treatment of Glioblastoma Multiforme. Curr. Pharm. Des. 2019, 25, 333–342. [Google Scholar] [CrossRef]
- Mahdi Ghahari, S.M.; Ajami, A.; Sadeghizadeh, M.; Esmaeili Rastaghi, A.R.; Mahdavi, M. Nanocurcumin as an adjuvant in killed Toxoplasma gondii vaccine formulation: An experience in BALB/c mice. Exp. Parasitol. 2022, 243, 108404. [Google Scholar] [CrossRef]
- Parveen, S.; Konde, D.V.; Paikray, S.K.; Tripathy, N.S.; Sahoo, L.; Samal, H.B.; Dilnawaz, F. Nanoimmunotherapy: The smart trooper for cancer therapy. Explor. Target. Antitumor Ther. 2025, 6, 1002308. [Google Scholar] [CrossRef]
- Chimento, A.; D’Amico, M.; De Luca, A.; Conforti, F.L.; Pezzi, V.; De Amicis, F. Resveratrol, Epigallocatechin Gallate and Curcumin for Cancer Therapy: Challenges from Their Pro-Apoptotic Properties. Life 2023, 13, 261. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis. Oxid. Med. Cell Longev. 2020, 2020, 3656419. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Silvestre, F.; Santos, C.; Silva, V.; Ombredane, A.; Pinheiro, W.; Andrade, L.; Garcia, M.; Pacheco, T.; Joanitti, G.; Luz, G.; et al. Pharmacokinetics of Curcumin Delivered by Nanoparticles and the Relationship with Antitumor Efficacy: A Systematic Review. Pharm. 2023, 16, 943. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef]
- Prabhakar, S. Translational research challenges: Finding the right animal models. J. Investig. Med. 2012, 60, 1141–1146. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Patel, M.; Bajwa, N.; Prasad, R.; Vora, L.K. Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape. Small 2025, 21, e2502315. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules 2018, 23, 1848. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Cuciniello, R.; Petillo, G.D.; Piccioni, M.; Filosa, S.; Crispi, S. An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments. Int. J. Mol. Sci. 2023, 24, 12587. [Google Scholar] [CrossRef]
- Bristow, R.G.; Alexander, B.; Baumann, M.; Bratman, S.V.; Brown, J.M.; Camphausen, K.; Choyke, P.; Citrin, D.; Contessa, J.N.; Dicker, A.; et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: A guideline by the American Society for Radiation Oncology. Lancet Oncol. 2018, 19, e240–e251. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H. Systematic analysis of potential targets of the curcumin analog pentagamavunon-1 (PGV-1) in overcoming resistance of glioblastoma cells to bevacizumab. Saudi Pharm. J. 2021, 29, 1289–1302. [Google Scholar] [CrossRef]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells 2019, 8, 889. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef]
- Afrashteh Nour, M.; Rahmati-Yamchi, M.; Shimia, M.; Yousefi, B.; Majidinia, M. Emerging Insights into the PI3K/AKT/mTOR Signaling Pathway and Non-Coding RNA-mediated Drug Resistance in Glioblastoma. Curr. Mol. Med. 2025, 25, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Rao, L.; Kumar, H.; Bansal, N.; Deep, A.; Parashar, J.; Yadav, M.; Mittal, V.; Kaushik, D. Applications of Nanomedicine in Brain Tumor Therapy: Nanocarrierbased Drug Delivery Platforms, Challenges, and Perspectives. Recent. Pat. Nanotechnol. 2025, 19, 99–119. [Google Scholar] [CrossRef]

| Therapeutic Strategy | Preclinical Findings | Role of Nanocurcumin | Refs. |
|---|---|---|---|
| Curcumin Monotherapy in GBM Models | Induces apoptosis, cell cycle arrest, and inhibits proliferation in U87, U251, T98G cell lines; suppresses NF-κB and PI3K/AKT/mTOR pathways; increases Bax, cleaved caspases; reduces Bcl-2. In vivo, it reduces tumor size and angiogenesis. | Enhances curcumin stability and BBB penetration; improves overall bioavailability. | [30] |
| Combination with Temozolomide (TMZ) | Synergistically enhances TMZ effects by inhibiting MGMT expression and suppressing AKT/STAT3 pathways; increases apoptosis and reduces tumor growth in xenograft models. | Increases delivery efficiency; co-loaded PLGA nanoparticles of curcumin and TMZ show superior tumor inhibition and survival benefits compared to monotherapy. | [109] |
| Enhancing Radiotherapy | Radiosensitizes GBM cells by increasing ROS levels, suppressing DNA repair, and inhibiting NF-κB/STAT3 activation; enhances radiation-induced apoptosis. | Enables sustained delivery and localized tumor accumulation; increases radiotherapy response, decreases recurrence, and improves survival in mouse models. | [110] |
| Combination with Immunotherapy | Suppresses Tregs, IL-10, TGF-β; downregulates PD-L1; promotes CD8+ T-cell infiltration; enhances immune checkpoint blockade efficacy. | Facilitates co-delivery with anti-PD-1/PD-L1 antibodies; boosts immune activation and reduces tumor burden in GBM preclinical models. | [111] |
| Combination with Natural Compounds | Synergizes with agents like piperine, resveratrol, quercetin, EGCG; regulates autophagy, oxidative stress, and mitochondrial dysfunction; piperine improves curcumin uptake. | Co-encapsulation improves curcumin absorption and therapeutic efficacy through enhanced stability and targeted delivery. | [112] |
| Targeting Glioma Stem Cells (GSCs) | Inhibits self-renewal, promotes differentiation, and sensitizes GSCs to TMZ and radiation; shows cytotoxicity in resistant cell populations. | Dual drug nanocarriers with curcumin improve uptake and cytotoxicity in GSCs demonstrate enhanced anti-tumor effects in resistant GBM subpopulations. | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Rahman, M.A.; Yadab, M.K.; Ali, M.M. Therapeutic Advances of Curcumin and Nanocurcumin in Glioblastoma: Molecular Targets, Bioavailability, and Drug Delivery. Nutrients 2026, 18, 194. https://doi.org/10.3390/nu18020194
Rahman MA, Yadab MK, Ali MM. Therapeutic Advances of Curcumin and Nanocurcumin in Glioblastoma: Molecular Targets, Bioavailability, and Drug Delivery. Nutrients. 2026; 18(2):194. https://doi.org/10.3390/nu18020194
Chicago/Turabian StyleRahman, Md Ataur, Mahesh Kumar Yadab, and Meser M. Ali. 2026. "Therapeutic Advances of Curcumin and Nanocurcumin in Glioblastoma: Molecular Targets, Bioavailability, and Drug Delivery" Nutrients 18, no. 2: 194. https://doi.org/10.3390/nu18020194
APA StyleRahman, M. A., Yadab, M. K., & Ali, M. M. (2026). Therapeutic Advances of Curcumin and Nanocurcumin in Glioblastoma: Molecular Targets, Bioavailability, and Drug Delivery. Nutrients, 18(2), 194. https://doi.org/10.3390/nu18020194






