Fatigue and Related Sleep Disturbances in Hemodialysis Patients: Prevalence, Associated Factors, and the Influence of Nutritional Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population and Sample
2.3. Variables
- Sociodemographic variables: sex and age.
- Clinical variables: Clinical variables included etiology of CKD, body mass index (BMI), Charlson Comorbidity Index (CCI), and time on HD (months). Residual kidney function was defined as the remaining renal capacity to maintain urine production and to contribute to the elimination of solutes and excess fluid in patients undergoing hemodialysis. Classically, residual kidney function refers to the preservation of renal function associated with ongoing urine production (commonly defined as >100 mL/day) after dialysis initiation, regardless of the highly variable amount of uremic toxins excreted in urine at that stage [14]. In the present study, residual urine output was assessed based on patient-reported ongoing urine production at the time of evaluation (yes/no).
- Analytical parameters: hemoglobin, total cholesterol (HDL and LDL), albumin, total proteins, transferrin, transferrin saturation index, and 25-OH vitamin D.
- Primary study variables
- Fatigue
- Sleep disturbances
- Nutritional status
2.4. Data Collection and Measurement Instruments
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Sample Description
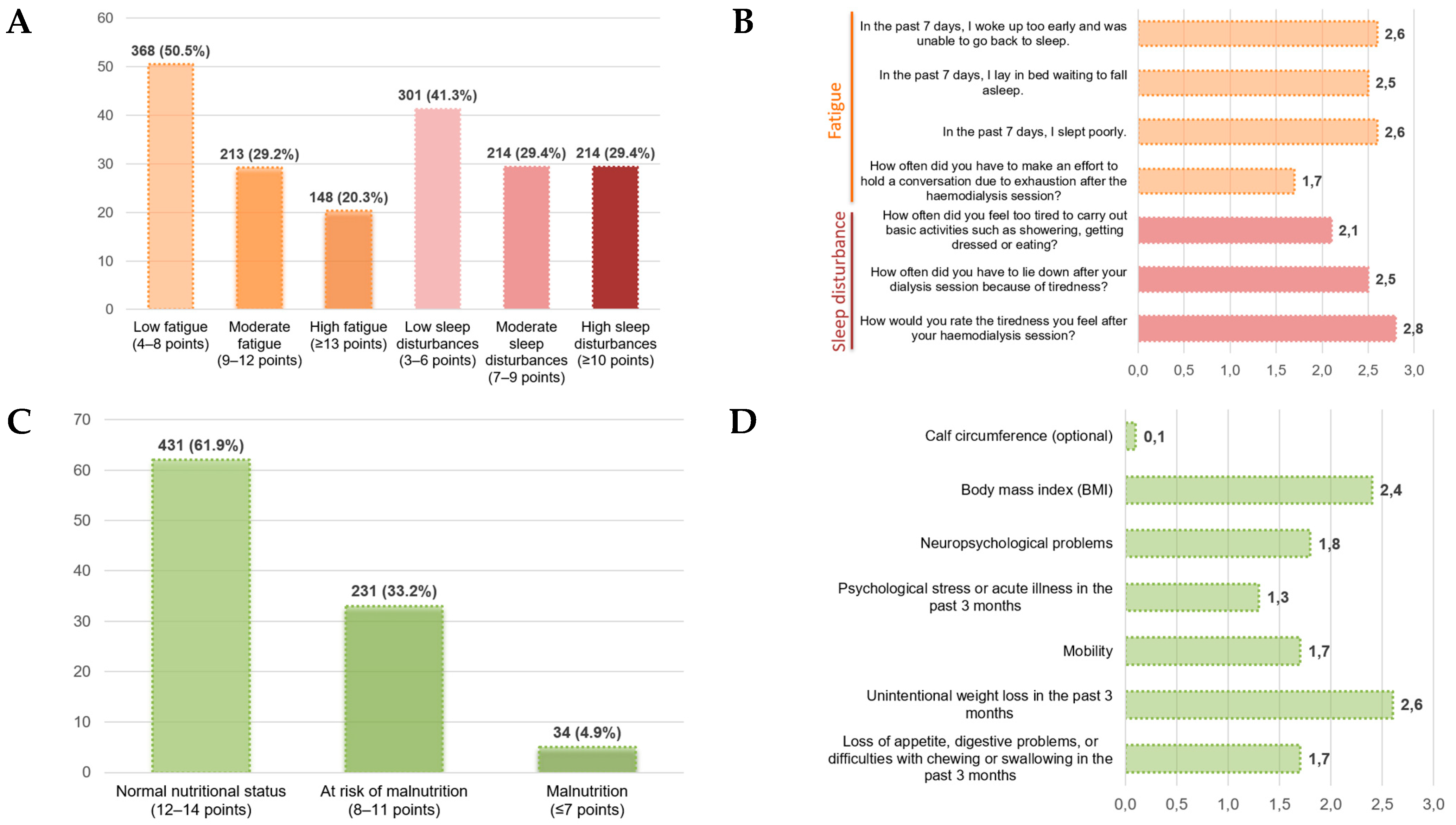
3.2. Results of the Main Variables: Fatigue, Sleep Disturbances, and Nutritional Status
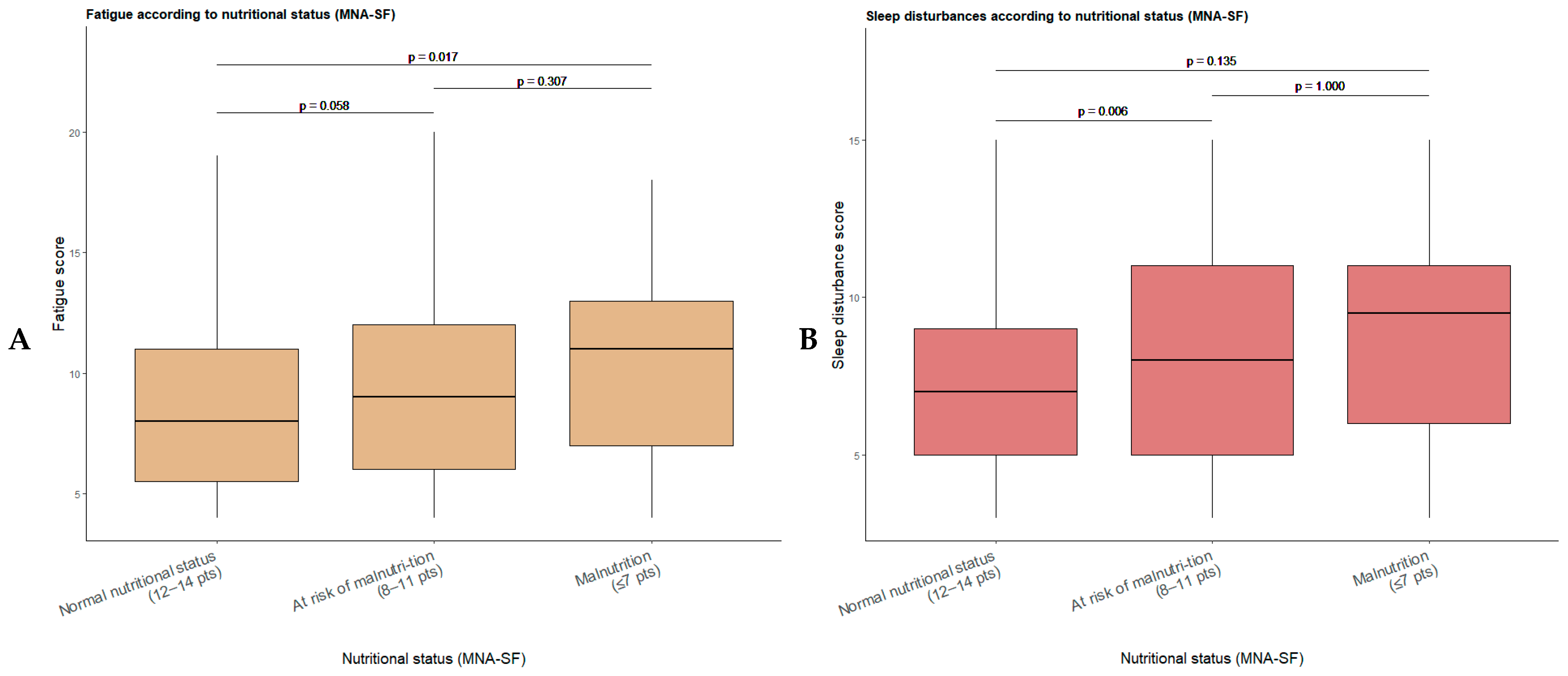
3.3. Relationship Between the Main Variables: Fatigue, Sleep Disturbances, and Nutritional Status
3.4. Relationship Between Fatigue, Sleep Disturbances, and the Remaining Clinical, Dialytic, and Analytical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CCI | Charlson Comorbidity Index |
| CKD | Chronic Kidney Disease |
| FRE | Fundación Renal Española |
| HD | Hemodialysis |
| Kt | Clearance–time product (dialysis dose) |
| MNA-SF | Mini Nutritional Assessment–Short Form |
| PEW | Protein–Energy Wasting |
| PROMs | Patient-Reported Outcome Measures |
| UF | Ultrafiltration |
References
- Dou, J.; Liu, H.; Ma, Y.; Wu, Y.Y.; Tao, X.B. Prevalence of post-dialysis fatigue: A systematic review and meta-analysis. BMJ Open 2023, 13, e064174. [Google Scholar] [CrossRef]
- You, Q.; Bai, D.X.; Wu, C.X.; Chen, H.; Hou, C.M.; Gao, J. Prevalence and Risk Factors of Postdialysis Fatigue in Patients Under Maintenance Hemodialysis: A Systematic Review and Meta-Analysis. Asian Nurs. Res. 2022, 16, 292–298. [Google Scholar] [CrossRef]
- Bossola, M.; Hedayati, S.S.; Brys, A.D.H.; Gregg, L.P. Fatigue in Patients Receiving Maintenance Hemodialysis: A Review. Am. J. Kidney Dis. 2023, 82, 464–480. [Google Scholar] [CrossRef]
- Gregg, L.P.; Bossola, M.; Ostrosky-Frid, M.; Hedayati, S.S. Fatigue in CKD: Epidemiology, Pathophysiology, and Treatment. Clin. J. Am. Soc. Nephrol. 2021, 16, 1445–1455. [Google Scholar] [CrossRef]
- Al Naamani, Z.; Gormley, K.; Noble, H.; Santin, O.; Al Maqbali, M. Fatigue, anxiety, depression and sleep quality in patients undergoing haemodialysis. BMC Nephrol. 2021, 22, 157. [Google Scholar] [CrossRef] [PubMed]
- Jhamb, M.; Liang, K.; Yabes, J.; Steel, J.L.; Dew, M.A.; Shah, N.; Unruh, M. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: Are sleep disorders a key to understanding fatigue? Am. J. Nephrol. 2013, 38, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tocino, M.L.; Mas-Fontao, S.; González-Parra, E.; Manso, P.; Burgos, M.; Carneiro, D.; Pereira, M.; Pereira, C.; Lopez-González, A.; Arenas, M.D.; et al. Comorbidity and Malnutrition as Key Determinants of Mortality in Elderly Haemodialysis Patients: A One-Year Observational Study. J. Cachexia Sarcopenia Muscle 2025, 16, e70062. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Singh, B.K.S.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Y.; Lv, W.; Fu, P.; Yuan, H. Prevalence and severity of sarcopenia in patients on maintenance hemodialysis: A cross-sectional study. BMC Nephrol. 2024, 25, 385. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Zheng, R.; Carter, C.E.; Mak, R.H. Cachexia/Protein energy wasting syndrome in CKD: Causation and treatment. Semin. Dial. 2019, 32, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (MNA-SF). J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Xia, A.; Meuleman, Y.; Dekker, F.W.; Hoogeveen, E.K. Risk Factors and Potential Treatments for Fatigue in Patients with Advanced CKD: A Narrative Review. Kidney Dial. 2025, 5, 8. [Google Scholar] [CrossRef]
- Liu, X.; Dai, C. Advances in Understanding and Management of Residual Renal Function in Patients with Chronic Kidney Disease. Kidney Dis. 2017, 2, 187–196. [Google Scholar] [CrossRef]
- Assimon, M.M.; Wenger, J.B.; Wang, L.; Flythe, J.E. Ultrafiltration Rate and Mortality in Maintenance Hemodialysis Patients. Am. J. Kidney Dis. 2016, 68, 911–922. [Google Scholar] [CrossRef]
- Lowrie, E.G.; Chertow, G.M.; Lew, N.L.; Lazarus, J.M.; Owen, W.F. The urea [clearance × dialysis time] product (Kt) as an outcome-based measure of hemodialysis dose. Kidney Int. 1999, 56, 729–737. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Fried, L.F.; Arnold, R.M.; Fine, M.J.; Levenson, D.J.; Peterson, R.A.; Switzer, G.E. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 2487–2494. [Google Scholar] [CrossRef]
- Jhamb, M.; Weisbord, S.D.; Steel, J.L.; Unruh, M. Fatigue in patients receiving maintenance dialysis: A review of definitions, measures, and contributing factors. Am. J. Kidney Dis. 2008, 52, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, W.; Sun, M.; Yang, C.; Liu, X.; Chen, C. Analysis of factors influencing the trajectory of fatigue in maintenance haemodialysis patients: A longitudinal study. Int. Urol. Nephrol. 2024, 56, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Valle Flores, J.A.; Rosado Álvarez, M.M.; Grijalva Grijalva, I.O.; Valarezo Suscal, E.P.; Escobar Valdivieso, G.S.; Zambrano Bonilla, J.C.; Alvarado Heras, J.C. Perfil bioquímico según patrones alimentarios en pacientes con enfermedad renal crónica sometidos a hemodiálisis. Nutr. Clin. Diet. Hosp. 2025, 45, 167–175. [Google Scholar] [CrossRef]
- Post, A.; Kremer, D.; Groothof, D.; van der Veen, Y.; de Blaauw, P.; van der Krogt, J.; Kema, I.P.; Westerhuis, R.; Heiner-Fokkema, M.R.; Bakker, S.J.L.; et al. Amino Acid Homeostasis and Fatigue in Chronic Hemodialysis Patients. Nutrients 2022, 14, 2810. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in CKD: A consensus statement. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Morvaridi, M.; Bavi Behbahani, H.; Alipour, M.; Zare Javid, A.; Keramatzadeh, S.; Shokri, S.; Tofighzadeh, P.; Fayazfar, F.; Soltaniyan Dehkordi, H.; Ghadimi, E.; et al. The association of Malnutrition-Inflammation Score with sleep quality and mental health in hemodialysis patients: A multicenter cross-sectional study. BMC Nephrol. 2025, 26, 305. [Google Scholar] [CrossRef]
- González-Ortiz, A.; Ramos-Acevedo, S.; Santiago-Ayala, V.; Gaytan, G.; Valencia-Flores, M.; Correa-Rotter, R.; Carrero, J.J.; Xu, H.; Espinosa-Cuevas, Á. Sleep Quality After Intradialytic Oral Nutrition: A New Benefit of This Anabolic Strategy? A Pilot Study. Front. Nutr. 2022, 9, 882367. [Google Scholar] [CrossRef]
- Sułkowski, L.; Matyja, A.; Matyja, M. Fatigue in Hemodialysis Patients: A Comparative Analysis with Healthy Controls. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Desvars, R.M.; Noguera, N.; Schupp, E. Relación entre tiempo en hemodiálisis y fatiga en pacientes en hemodiálisis crónica en el Hospital Regional de Concepción, Paraguay. Agosto 2024: Relationship between time on hemodialysis and fatigue in patients on chronic hemodialysis at the Regional Hospital of Concepción, Paraguay. Med. Signum. 2024, 3, 32–41. [Google Scholar]
- Bossola, M.; Di Stasio, E.; Antocicco, M.; Panico, L.; Pepe, G.; Tazza, L. Fatigue Is Associated with Increased Risk of Mortality in Patients on Chronic Hemodialysis. Nephron 2015, 130, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, M.; Naini, A.E.; Aghaghazvini, R.; Amra, B.; Gholamrezaei, A. Sleep quality in patients on maintenance hemodialysis and peritoneal dialysis. Int. J. Prev. Med. 2013, 4, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Emami Zeydi, A.; Jannati, Y.; Darvishi Khezri, H.; Gholipour Baradari, A.; Espahbodi, F.; Lesani, M.; Yaghoubi, T. Sleep quality and its correlation with serum C-reactive protein level in hemodialysis patients. Saudi J. Kidney Dis. Transplant. 2014, 25, 750–755. [Google Scholar] [CrossRef]
- Elgendy, A.; Abdelsalam, A.I.; Mansour, M.; Nassar, M.K. Can residual kidney function affect quality of life and cognitive function in hemodialysis patients? BMC Nephrol. 2022, 23, 263. [Google Scholar] [CrossRef]
- Kong, J.H.; Davies, M.R.P.; Mount, P.F. Relationship between residual kidney function and symptom burden in haemodialysis patients. Intern. Med. J. 2021, 51, 52–61. [Google Scholar] [CrossRef]
- Dopierała, M.; Schwermer, K.; Hoppe, K.; Kupczyk, M.; Pawlaczyk, K. Benefits of Preserving Residual Urine Output in Haemodialysis. Int. J. Nephrol. Renov. Dis. 2023, 16, 231–240. [Google Scholar] [CrossRef]
- Mangalgi, S.; Joshi, V.; Misra, M.; Chaudhary, K. Residual Kidney Function and the Impact of Dialysis Modality. Kidney Dial. 2025, 5, 43. [Google Scholar] [CrossRef]
- Lyons, O.D. Sleep disorders in chronic kidney disease. Nat. Rev. Nephrol. 2024, 20, 690–700. [Google Scholar] [CrossRef]
- Kjaergaard, K.D.; Jensen, J.D.; Peters, C.D.; Jespersen, B. Preserving residual renal function in dialysis patients: An update on evidence to assist clinical decision making. NDT Plus 2011, 4, 225–230. [Google Scholar] [CrossRef]
- Aoun, M.; Laruelle, E.; Duneau, G.; Duquennoy, S.; Legendre, B.; Baluta, S.; Maroun, T.; Lamri, A.; Gosselin, M.; Chemouny, J.; et al. Modifiable Factors Associated with Prolonged Dialysis Recovery Time and Fatigue. Kidney360 2024, 5, 1311–1321. [Google Scholar] [CrossRef]
- Watanabe, G.; Tanaka, K.; Saito, H.; Kimura, H.; Tani, Y.; Asai, J.; Suzuki, H.; Sato, K.; Nakayama, M.; Kazama, J.J. Post-dialysis fatigue predicts all-cause mortality in haemodialysis patients. Ther. Apher. Dial. 2025, 29, 12–22. [Google Scholar] [CrossRef]
- Schade van Westrum, S.; Hoogeveen, E.K.; Broekman, B.F.P.; Siegert, C.E.H.; Keurhorst, D.; Annema, C.; Hemmelder, M.H.; Bos, W.J.W.; Dekker, F.W.; Meuleman, Y.; et al. Fatigue across different chronic kidney disease populations. Clin. Kidney J. 2025, 18, sfaf118. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Okazaki, M.; Hishida, M.; Nishibori, N.; Kurasawa, S.; Kondo, T.; Kaneda, F.; Kaneda, H.; Maruyama, S. Ultrafiltration rate and mortality in extended-hours haemodialysis. Clin. Kidney J. 2025, 18, sfaf287. [Google Scholar] [CrossRef] [PubMed]
- Pojatić, Đ.; Nikić, D.; Tolj, I.; Pezerović, D.; Šantić, A.; Degmečić, D. Alexithymia, Phosphorus Levels, and Sleep Disorders in Hemodialysis Patients. J. Clin. Med. 2022, 11, 3218. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Manera, K. Life participation: When there is more to life than dialysis. Perit. Dial. Int. 2022, 42, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Sturgill, D.A.; Bal, N.; Nagavally, S.; Wolfgram, D.F. The Relationship Between Dialysis Metrics and Patient-Reported Cognition, Fatigue, and Physical Function. Kidney Dis. 2020, 6, 364–370. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Sociodemographic variables | |
| Age (years): mean ± SD | 67.7 ± 14.5 |
| ≤65 years, n (%) | 244 (33.5%) |
| ≥65 years, n (%) | 485 (66.5%) |
| Sex: male, n (%) | 489 (67.1%) |
| female, n (%) | 240 (32.9%) |
| Nationality: Spanish, n (%) | 614 (84.5%) |
| Migrant, n (%) | 113 (15.5%) |
| Clinical variables | |
| Etiology of CKD | |
| 188 (26.4%) |
| 141 (19.8%) |
| 111 (15.6%) |
| 55 (7.7%) |
| 113 (15.8%) |
| 57 (8.0%) |
| 48 (6.7%) |
| Body mass index (BMI), mean ± SD | 26.5 ± 5.3 |
| Charlson Comorbidity Index (CCI), mean ± SD | 7.8 ± 2.9 |
| Time on hemodialysis (months), median (P25–P75) | 57.0 (16–67.5) |
| Residual urine output present, n (%) | 140 (44.3%) |
| Not present | 176 (55.7) |
| Dialytic parameters | |
| Vascular access in use | |
| 409 (64.6%) |
| 224 (35.4%) |
| Session duration (hours), mean ± SD | 3.7 ± 0.4 |
| ≥4 h sessions, n (%) | 155 (21.4%) |
| Interdialytic weight gain (kg), mean ± SD | 2.2 ± 1.3 |
| Ultrafiltration rate (mL/kg/h), mean ± SD | 7.9 ± 3.4 |
| Ultrafiltration >10 mL/kg/h, n (%) | 185 (26%) |
| Sessions per week, mean ± SD | 2.9 ± 0.4 |
| Kt (L), mean ± SD | 51.8 ± 9.2 |
| Achieved Kt target, n (%) | 611 (84.2%) |
| Laboratory parameters | |
| Hemoglobin (g/dL), mean ± SD | 11.4 ± 1.3 |
| Total cholesterol (mg/dL), mean ± SD | 139.8 ± 39.6 |
| HDL cholesterol (mg/dL), mean ± SD | 45.5 ± 14.8 |
| LDL cholesterol (mg/dL), mean ± SD | 69.1 ± 31.0 |
| Albumin (g/dL), mean ± SD | 4.0 ± 0.4 |
| Total proteins (g/dL), mean ± SD | 6.5 ± 0.6 |
| Transferrin (mg/dL), mean ± SD | 69 ± 49.4 |
| Transferrin saturation (%), mean ± SD | 28 ± 12.3 |
| 25-OH vitamin D (ng/mL), mean ± SD | 567 ± 434 |
| Fatigue | Sleep Disturbances | |||||||
|---|---|---|---|---|---|---|---|---|
| Nutritional Status (MNA-SF) | Mean ± SD | Median (IQR) | Min–Max | p Global * | Mean ± SD | Median (IQR) | Min–Max | p Global * |
| Normal nutritional status (12–14 pts) (n = 431) | 8.75 ± 3.82 | 8 (5.5–11) | 4–20 | 0.003 | 7.31 ± 3.25 | 7 (5–9) | 3–15 | 0.003 |
| At risk of malnutrition (8–11 pts) (n = 231) | 9.51 ± 4.03 | 9 (6–12) | 4–20 | 8.25 ± 3.65 | 8 (5–11) | 3–15 | ||
| Malnutrition (≤7 pts) (n = 34) | 10.71 ± 4.10 | 11 (7–13) | 4–18 | 8.47 ± 3.47 | 9.5 (6–11) | 3–15 | ||
| Variable/Group | Statistic | N | Fatigue Subscale (Mean ± SD/ρ) | p-Value | Sleep Disturbance Subscale (Mean ± SD/ρ) | p-Value |
|---|---|---|---|---|---|---|
| Sociodemographic variables | ||||||
| Age (years) | Spearman ρ | 729 | ρ = −0.064 | 0.086 | ρ = −0.001 | 0.972 |
| Sex: men | Mann–Whitney U | 489 | 8.0 (6.0–11.0) | <0.001 | 7.0 (5.0–10.0) | 0.039 |
| women | 240 | 10.0 (6.8–13.0) | 8.0 (5.0–11.0) | |||
| Clinical variables | ||||||
| Body mass index (BMI) | Spearman ρ | 719 | ρ = 0.006 | 0.880 | ρ = 0.054 | 0.151 |
| Charlson Comorbidity Index | Spearman ρ | 723 | ρ = 0.003 | 0.941 | ρ = 0.041 | 0.270 |
| Time on hemodialysis (months) | Spearman ρ | 689 | ρ = 0.221 | <0.001 | ρ = 0.130 | <0.001 |
| Residual urine output: No | Mann–Whitney U | 176 | 9.0 (6.0–12.3) | 0.059 | 9.0 (6.0–11.0) | 0.002 |
| Yes | 140 | 8.0 (5.0–12.0) | 7.0 (4.0–10.0) | |||
| Dialytic parameters | ||||||
| Session duration (hours) | Spearman ρ | 725 | ρ = 0.073 | 0.049 | ρ = 0.047 | 0.202 |
| Weekly dialysis minutes | Spearman ρ | 722 | ρ = 0.093 | 0.012 | ρ = 0.057 | 0.128 |
| Kt (L) | Spearman ρ | 726 | ρ = −0.009 | 0.807 | ρ = 0.000 | 0.992 |
| Achieving Kt target: No | Mann–Whitney U | 115 | 8.0 (5.0–12.0) | 0.071 | 7.0 (4.0–9.0) | 0.114 |
| Yes | 611 | 9.0 (6.0–12.0) | 8.0 (5.0–10.0) | |||
| Interdialytic weight gain (kg) | Spearman ρ | 721 | ρ = 0.061 | 0.101 | ρ = 0.035 | 0.355 |
| Ultrafiltration rate (mL/kg/h) | Spearman ρ | 708 | ρ = 0.024 | 0.515 | ρ = −0.002 | 0.963 |
| Ultrafiltration rate <10 mL/kg/h | Mann–Whitney U | 523 | 8.0 (6.0–12.0) | 0.172 | 7.0 (5.0–10.0) | 0.398 |
| ≥10 mL/kg/h | 185 | 9.0 (6.0–12.0) | 8.0 (5.0–11.0) | |||
| Analytical parameters | ||||||
| Hemoglobin (g/dL) | Spearman ρ | 683 | ρ = 0.004 | 0.914 | ρ = −0.041 | 0.290 |
| Total cholesterol (mg/dL) | Spearman ρ | 680 | ρ = 0.033 | 0.385 | ρ = −0.046 | 0.234 |
| HDL cholesterol (mg/dL) | Spearman ρ | 580 | ρ = −0.059 | 0.156 | ρ = −0.042 | 0.313 |
| LDL cholesterol (mg/dL) | Spearman ρ | 575 | ρ = 0.011 | 0.798 | ρ = −0.072 | 0.082 |
| Albumin (g/dL) | Spearman ρ | 705 | ρ = 0.011 | 0.774 | ρ = −0.107 | 0.005 |
| Total proteins (g/dL) | Spearman ρ | 609 | ρ = −0.037 | 0.359 | ρ = −0.080 | 0.049 |
| Transferrin (mg/dL) | Spearman ρ | 700 | ρ = −0.093 | 0.014 | ρ = −0.091 | 0.016 |
| Transferrin saturation (%) | Spearman ρ | 705 | ρ = −0.009 | 0.804 | ρ = −0.007 | 0.856 |
| 25-OH vitamin D (ng/mL) | Spearman ρ | 663 | ρ = −0.007 | 0.855 | ρ = −0.072 | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Casaux-Huertas, A.; Sánchez-Tocino, M.L.; San Juan-Miguelsanz, M.; Audije-Gil, J.; Romero-Lugo, N.; Muñoz-Pilar, S.; Da Pena-Vielba, F.; Hernan-Gascueña, D.; Manso-Del Real, P.; Escribano-Loma, S.; et al. Fatigue and Related Sleep Disturbances in Hemodialysis Patients: Prevalence, Associated Factors, and the Influence of Nutritional Status. Nutrients 2026, 18, 124. https://doi.org/10.3390/nu18010124
Casaux-Huertas A, Sánchez-Tocino ML, San Juan-Miguelsanz M, Audije-Gil J, Romero-Lugo N, Muñoz-Pilar S, Da Pena-Vielba F, Hernan-Gascueña D, Manso-Del Real P, Escribano-Loma S, et al. Fatigue and Related Sleep Disturbances in Hemodialysis Patients: Prevalence, Associated Factors, and the Influence of Nutritional Status. Nutrients. 2026; 18(1):124. https://doi.org/10.3390/nu18010124
Chicago/Turabian StyleCasaux-Huertas, Ana, María Luz Sánchez-Tocino, Marta San Juan-Miguelsanz, Julia Audije-Gil, Neydu Romero-Lugo, Sonia Muñoz-Pilar, Fabiola Da Pena-Vielba, David Hernan-Gascueña, Paula Manso-Del Real, Soraya Escribano-Loma, and et al. 2026. "Fatigue and Related Sleep Disturbances in Hemodialysis Patients: Prevalence, Associated Factors, and the Influence of Nutritional Status" Nutrients 18, no. 1: 124. https://doi.org/10.3390/nu18010124
APA StyleCasaux-Huertas, A., Sánchez-Tocino, M. L., San Juan-Miguelsanz, M., Audije-Gil, J., Romero-Lugo, N., Muñoz-Pilar, S., Da Pena-Vielba, F., Hernan-Gascueña, D., Manso-Del Real, P., Escribano-Loma, S., Sánchez-Beato, C. C., Arenas-Jiménez, M. D., Research Unit, Fundación Renal Española, & FRAGILDIAL Working Group, Fundación Renal Española. (2026). Fatigue and Related Sleep Disturbances in Hemodialysis Patients: Prevalence, Associated Factors, and the Influence of Nutritional Status. Nutrients, 18(1), 124. https://doi.org/10.3390/nu18010124












