Specific Bacterial Taxa and Their Metabolite, DHPS, May Be Linked to Gut Dyshomeostasis in Patients with Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis
Highlights
- A microbial metabolite, 2,3-dihydroxypropane-1-sulfonate (DHPS), is linked to gut dysbiosis in the early stages of neurodegenerative diseases.
- DHPS-metabolizing bacteria levels were significantly increased in neurodegenerative disease patients.
- DHPS may serve as a potential biomarker for gut dysbiosis in the early stages of neurodegenerative disease.
- DHPS can be obtained via consuming leafy greens.
Abstract
:1. Introduction
1.1. Microbial and Metabolic Dysbiosis Associated with NDDs
1.1.1. Alzheimer’s Disease
1.1.2. Amyotrophic Lateral Sclerosis
1.1.3. Parkinson’s Disease
1.2. Gut Imbalances, Inflammation, Oxidative Stress, and Mitochondrial Dysfunction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Subjects Were in Early Diagnostic Stages
2.4. Sample Collection, Preservation, DNA Extraction
2.5. Metabolomics
2.5.1. Metabolite Extractions
2.5.2. UHPLC-HRMS
2.5.3. Metabolomics Data Processing
2.5.4. Statistical Analysis
2.6. 16s rRNA Sequencing
16S Data Processing and Analysis
3. Results
3.1. Global Metabolomics
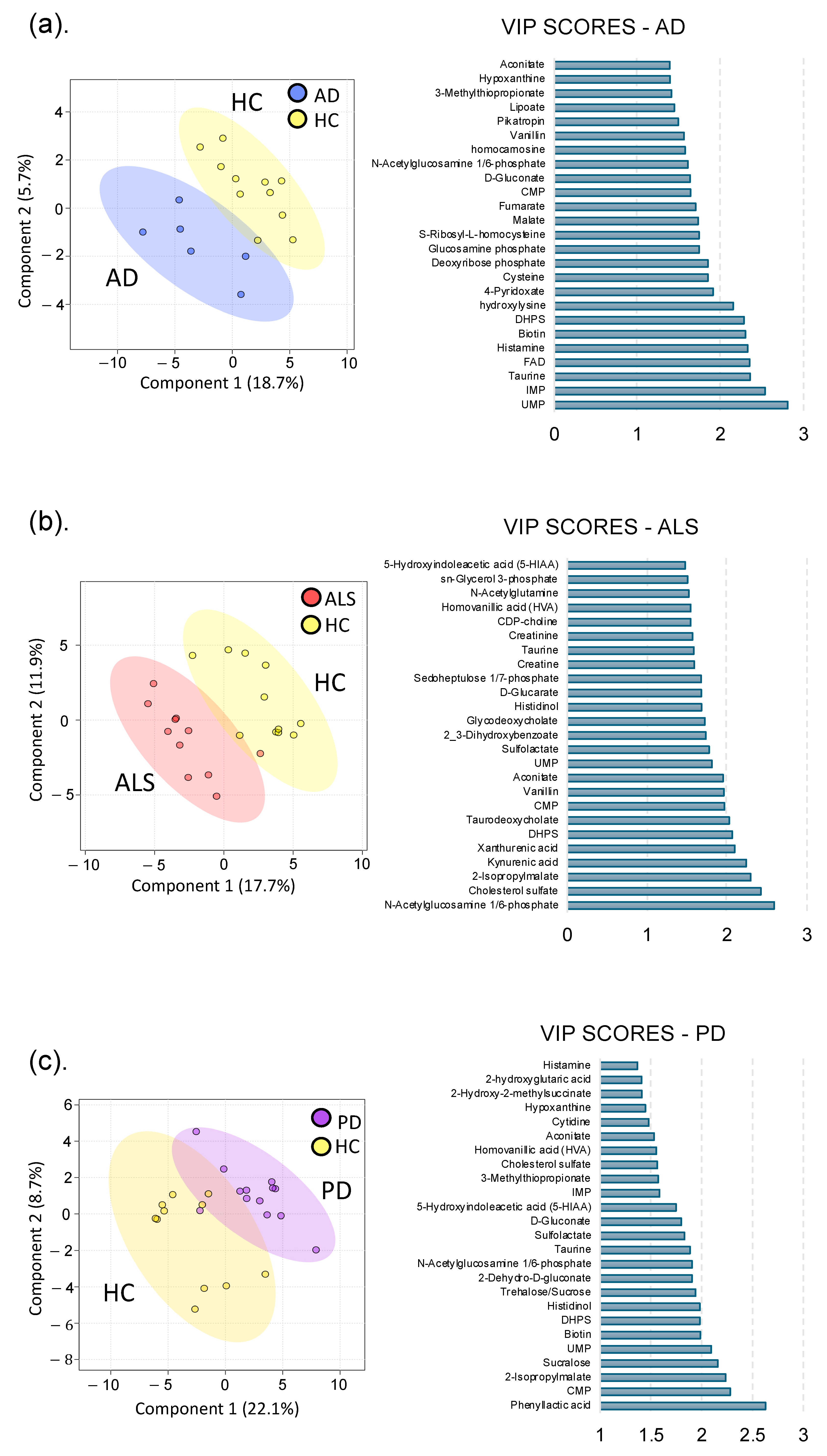
3.1.1. Alzheimer’s Disease
3.1.2. Amyotrophic Lateral Sclerosis
3.1.3. Parkinson’s Disease
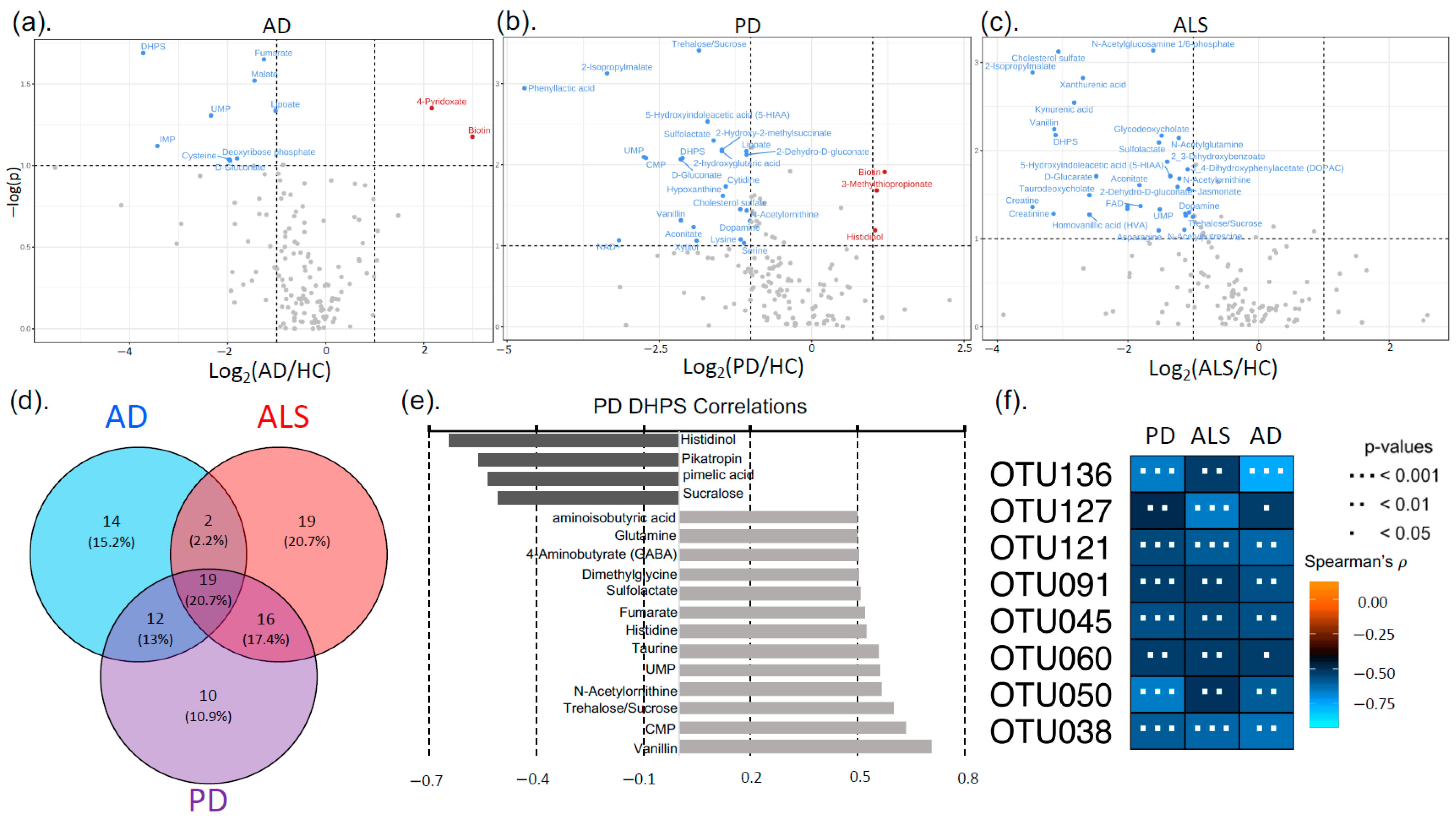
3.2. Neurodegenerative Disease Metabolic Markers
3.3. Sequencing (16s rRNA)
4. Discussion
4.1. Metabolome Alterations in NDDs
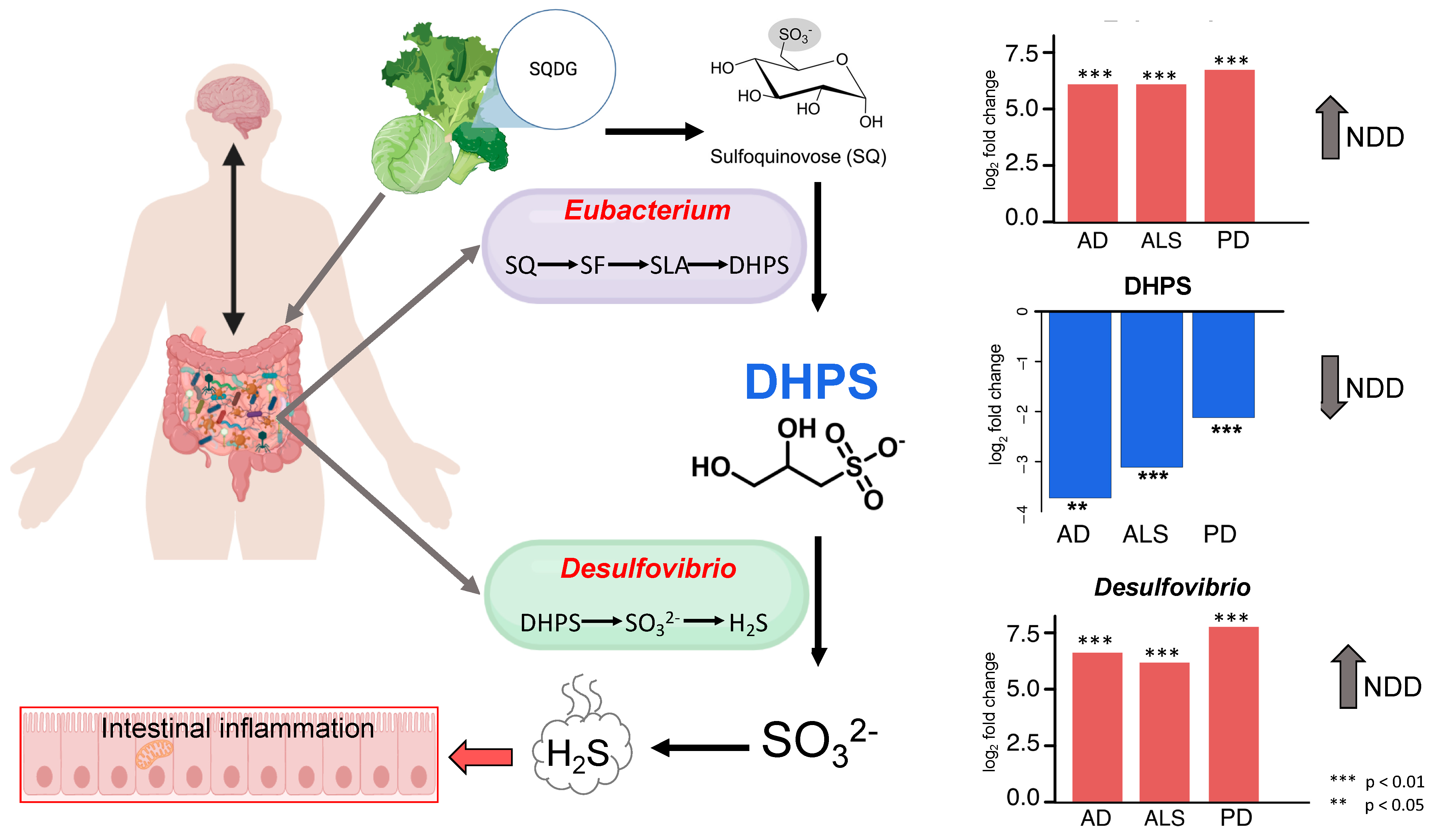
4.2. Cryptic Sulfur Metabolism in NDDs
4.3. DHPS Microbial Metabolism in NDDs
4.4. Melainabacteria Could Be Associated with DHPS-Mediated Cryptic Sulfur Metabolism in NDDs
4.5. Potential Physiological Role of DHPS
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Dorsey, E.R.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Park. Dis. 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Blanchard, M.; Bonar, K.; Drane, E.; Murton, M.; Ploug, U.; Ricchetti-Masterson, K.; Savic, N.; Worthington, E.; Heiman-Patterson, T. Epidemiology and economic burden of amyotrophic lateral sclerosis in the United States: A literature review. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 436–448. [Google Scholar] [CrossRef]
- Better, M.A. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar]
- Augustin, A.; Guennec, A.L.; Umamahesan, C.; Kendler-Rhodes, A.; Tucker, R.M.; Chekmeneva, E.; Takis, P.; Lewis, M.; Balasubramanian, K.; DeSouza, N.; et al. Faecal metabolite deficit, gut inflammation and diet in Parkinson’s disease: Integrative analysis indicates inflammatory response syndrome. Clin. Transl. Med. 2023, 13, e1152. [Google Scholar] [CrossRef]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; A Vasco, D.; Pietzner, M.; Stewart, I.; Wareham, N.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernandez, E.; Gebeyehu, G.G.; Flain, L.; Slater, R.; Frau, A.; Ijaz, U.Z.; Warner, T.; Probert, C. The faecal metabolome and mycobiome in Parkinson’s disease. Park. Relat. Disord. 2022, 95, 65–69. [Google Scholar] [CrossRef]
- Baldini, F.; Hertel, J.; Sandt, E.; Thinnes, C.C.; Neuberger-Castillo, L.; Pavelka, L.; Betsou, F.; Krüger, R.; Thiele, I. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Vascellari, S.; Melis, M.; Palmas, V.; Pisanu, S.; Serra, A.; Perra, D.; Santoru, M.L.; Oppo, V.; Cusano, R.; Uva, P.; et al. Clinical Phenotypes of Parkinson’s Disease Associate with Distinct Gut Microbiota and Metabolome Enterotypes. Biomolecules 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, F.; Cao, J.; Ding, W.; Yan, S.; Shi, W.; Wen, S.; Yao, L. Alterations of gut microbiota and metabolome with Parkinson’s disease. Microb. Pathog. 2021, 160, 105187. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Y.; Wang, Y.; Yan, S.; Li, J.; Wei, H. Research progress of regulating intestinal flora in the treatment of Alzheimer’s disease. J. Xinxiang Med. Univ. 2022, 39, 387–391. [Google Scholar]
- Ubeda, C.; Vázquez-Carretero, M.D.; Luque-Tirado, A.; Ríos-Reina, R.; Rubio-Sánchez, R.; Franco-Macías, E.; García-Miranda, P.; Calonge, M.L.; Peral, M.J. Fecal Volatile Organic Compounds and Microbiota Associated with the Progression of Cognitive Impairment in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 707. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Ba, L.; Tang, J.; Yang, Y.; Li, Z.; Liu, M.; Yang, C.; Ding, F.; Zhang, M. Gut microbiota links with cognitive impairment in amyotrophic lateral sclerosis: A multi-omics study. J. Biomed. Res. 2022, 37, 125–137. [Google Scholar] [CrossRef]
- Zeng, Q.; Shen, J.; Chen, K.; Zhou, J.; Liao, Q.; Lu, K.; Yuan, J.; Bi, F.-F. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 2020, 10, 12998. [Google Scholar] [CrossRef]
- Brenner, D.; Hiergeist, A.; Adis, C.; Mayer, B.; Gessner, A.; Ludolph, A.C.; Weishaupt, J.H. The fecal microbiome of ALS patients. Neurobiol. Aging 2018, 61, 132–137. [Google Scholar] [CrossRef]
- Talavera Andújar, B.; Aurich, D.; Aho, V.T.E.; Singh, R.R.; Cheng, T.; Zaslavsky, L.; Bolton, E.E.; Mollenhauer, B.; Wilmes, P.; Schymanski, E.L. Studying the Parkinson’s disease metabolome and exposome in biological samples through different analytical and cheminformatics approaches: A pilot study. Anal. Bioanal. Chem. 2022, 414, 7399–7419. [Google Scholar] [CrossRef]
- Nyangale, E.P.; Mottram, D.S.; Gibson, G.R. Gut Microbial Activity, Implications for Health and Disease: The Potential Role of Metabolite Analysis. J. Proteome Res. 2012, 11, 5573–5585. [Google Scholar] [CrossRef]
- Claudino Dos Santos, J.C.; Lima, M.P.P.; Brito, G.A.C.; de Barros Viana, G.S. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 2023, 84, 101812. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef] [PubMed]
- Matisz, C.E.; Gruber, A.J. Neuroinflammatory remodeling of the anterior cingulate cortex as a key driver of mood disorders in gastrointestinal disease and disorders. Neurosci. Biobehav. Rev. 2022, 133, 104497. [Google Scholar] [CrossRef]
- Rob, M.; Yousef, M.; Lakshmanan, A.P.; Mahboob, A.; Terranegra, A.; Chaari, A. Microbial signatures and therapeutic strategies in neurodegenerative diseases. Biomed. Pharmacother. 2025, 184, 117905. [Google Scholar] [CrossRef]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Katzman, R.; Saitoh, T. Advances in Alzheimer’s disease. FASEB J. 1991, 5, 278–286. [Google Scholar] [CrossRef]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Clark, C.M.; Xie, S.; Chittams, J.; Ewbank, D.; Peskind, E.; Galasko, D.; Morris, J.C.; McKeel, D.W.; Farlow, M.; Weitlauf, S.L.; et al. Cerebrospinal fluid tau and β-amyloid: How well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch. Neurol. 2003, 60, 1696–1702. [Google Scholar] [CrossRef]
- Cheng, X.; Tan, Y.; Li, H.; Huang, J.; Zhao, D.; Zhang, Z.; Yi, M.; Zhu, L.; Hui, S.; Yang, J.; et al. Fecal 16S rRNA sequencing and multi-compartment metabolomics revealed gut microbiota and metabolites interactions in APP/PS1 mice. Comput. Biol. Med. 2022, 151 Pt A, 106312. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, H.; Li, X.; Shi, W.; Guo, Y.; Du, X.; Zhang, L.; Su, L.; Qin, C. Comparative Metagenomics and Metabolomes Reveals Abnormal Metabolism Activity Is Associated with Gut Microbiota in Alzheimer’s Disease Mice. Int. J. Mol. Sci. 2022, 23, 11560. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, Q.; Jin, Q.; Du, Y.; Yan, J.; Gao, H.; Zheng, H. Urinary and faecal metabolic characteristics in APP/PS1 transgenic mouse model of Alzheimer’s disease with and without cognitive decline. Biochem. Biophys. Res. Commun. 2022, 604, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Liang, C.; Pereira, R.; Zhang, Y.; Rojas, O.L. Gut Microbiome in Alzheimer’s Disease: From Mice to Humans. Curr. Neuropharmacol. 2024, 22, 2314–2329. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients with Alzheimer’s Disease. Front. Cell Dev. Biol. 2020, 8, 634069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am. J. Clin. Nutr. 2021, 114, 429–440. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Peng, J.; Huang, X.; Xiao, L.; Huang, F.; Zuo, Z. Gut Microbiome Features of Chinese Patients Newly Diagnosed with Alzheimer’s Disease or Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 80, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Ding, D.; Zhu, H.; Wang, R.; Su, F.; Wu, W.; Xiao, Z.; Liang, X.; Zhao, Q.; Hong, Z.; et al. Disturbed microbial ecology in Alzheimer’s disease: Evidence from the gut microbiota and fecal metabolome. BMC Microbiol. 2021, 21, 226. [Google Scholar] [CrossRef]
- Lu, G.; Wen, Q.; Cui, B.; Li, Q.; Zhang, F. Washed microbiota transplantation stopped the deterioration of amyotrophic lateral sclerosis: The first case report and narrative review. J. Biomed. Res. 2022, 37, 69–76. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Angot, E.; Brundin, P. Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Park. Relat. Disord. 2009, 15 (Suppl. 3), S143–S147. [Google Scholar] [CrossRef]
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693 Pt B, 201–206. [Google Scholar] [CrossRef]
- Tan, A.H.; Chong, C.W.; Lim, S.Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.-T.; Wu, Y.-W.; Liou, J.-M.; Wu, M.-S.; Kuo, C.-H.; Lin, C.-H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients with Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Asha, N.; Sharma, K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Markovinovic, A.; Greig, J.; Martín-Guerrero, S.M.; Salam, S.; Paillusson, S. Endoplasmic reticulum-mitochondria signaling in neurons and neurodegenerative diseases. J. Cell Sci. 2022, 135, jcs248534. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.W.; Venkatachalam, K.V. Sulfur-Element containing metabolic pathways in human health and crosstalk with the microbiome. Biochem. Biophys. Rep. 2023, 35, 101529. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.; Alizadeh-Ghamsari, A.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef]
- Hanson, B.T.; Dimitri Kits, K.; Löffler, J.; Burrichter, A.G.; Fiedler, A.; Denger, K.; Frommeyer, B.; Herbold, C.W.; Rattei, T.; Karcher, N.; et al. Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut. ISME J. 2021, 15, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Braccia, D.J.; Jiang, X.; Pop, M.; Hall, A.B. The Capacity to Produce Hydrogen Sulfide (H(2)S) via Cysteine Degradation Is Ubiquitous in the Human Gut Microbiome. Front. Microbiol. 2021, 12, 705583. [Google Scholar] [CrossRef]
- Szabo, C.; Papapetropoulos, A. International union of basic and clinical pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol. Rev. 2017, 69, 497–564. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Zhu, Y.C.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.M.; Whiteman, M.; Salto-Tellez, M.; Moore, P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef]
- Libiad, M.; Vitvitsky, V.; Bostelaar, T.; Bak, D.W.; Lee, H.-J.; Sakamoto, N.; Fearon, E.; Lyssiotis, C.A.; Weerapana, E.; Banerjee, R. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J. Biol. Chem. 2019, 294, 12077–12090. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; van Mil, S.W.C.; Müller, M.; Kleerebezem, M.; van der Meer, R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef]
- Murros, K.E.; Huynh, V.A.; Takala, T.M.; Saris, P.E.J. Desulfovibrio Bacteria Are Associated with Parkinson’s Disease. Front. Cell Infect. Microbiol. 2021, 11, 652617. [Google Scholar] [CrossRef]
- Murros, K.E. Sulfate reducing gut bacteria and Parkinson’s disease. Eur. J. Neurol. 2021, 28, e21. [Google Scholar] [CrossRef]
- Munteanu, C.; Iordan, D.A.; Hoteteu, M.; Popescu, C.; Postoiu, R.; Onu, I.; Onose, G. Mechanistic Intimate Insights into the Role of Hydrogen Sulfide in Alzheimer’s Disease: A Recent Systematic Review. Int. J. Mol. Sci. 2023, 24, 15481. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.D.; Chaudhari, S.N.; Devlin, A.S. Host–microbiome orchestration of the sulfated metabolome. Nat. Chem. Biol. 2024, 20, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef]
- Brooks, B.R. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J. Neurol. Sci. 1994, 124, 96–107. [Google Scholar]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Besser, L.; Kukull, W.; Knopman, D.S.; Chui, H.; Galasko, D.; Weintraub, S.; Jicha, G.; Carlsson, C.; Burns, J.; Quinn, J.; et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis. Assoc. Disord. 2018, 32, 351–358. [Google Scholar] [CrossRef]
- Appalachian Regional Commission. Creating a Culture of Health in Appalachia: Disparities and Bright Spots; Appalachian Regional Commission: Watshington, DC, USA, 2024; p. 16. [Google Scholar]
- Rabinowitz, J.D.; Kimball, E. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal. Chem. 2007, 79, 6167–6173. [Google Scholar] [CrossRef] [PubMed]
- Byerley, L.O.; Gallivan, K.M.; Christopher, C.J.; Taylor, C.M.; Luo, M.; Dowd, S.E.; Davis, G.M.; Castro, H.F.; Campagna, S.R.; Ondrak, K.S. Gut Microbiome and Metabolome Variations in Self-Identified Muscle Builders Who Report Using Protein Supplements. Nutrients 2022, 14, 533. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Richard, A.J.; Salbaum, J.M.; Newman, S.; Carmouche, R.; Webb, S.; Bruce-Keller, A.J.; Stephens, J.M.; Campagna, S.R. Cross-Omics Analysis of Fenugreek Supplementation Reveals Beneficial Effects Are Caused by Gut Microbiome Changes Not Mammalian Host Physiology. Int. J. Mol. Sci. 2022, 23, 3654. [Google Scholar] [CrossRef]
- Lu, W.; Clasquin, M.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic Analysis via Reversed-Phase Ion-Pairing Liquid Chromatography Coupled to a Stand Alone Orbitrap Mass Spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef]
- Bazurto, J.V.; Dearth, S.P.; Tague, E.D.; Campagna, S.R.; Downs, D.M. Untargeted metabolomics confirms and extends the understanding of the impact of aminoimidazole carboxamide ribotide (AICAR) in the metabolic network of Salmonella enterica. Microb. Cell 2017, 5, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Tang, W.H.; Römpp, A.; Neumann, S.; Pizarro, A.D.; et al. mzML—A community standard for mass spectrometry data. Mol. Cell Proteom. 2011, 10, R110.000133. [Google Scholar] [CrossRef]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, 37, 14.11.1–14.11.23. [Google Scholar] [CrossRef]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Yourstone, S.; Mieczkowski, P.; Jones, C.D.; Dangl, J.L. Practical innovations for high-throughput amplicon sequencing. Nat. Methods 2013, 10, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.; Dillon, M.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Whiteman, M.; et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef] [PubMed]
- Lohr, K.M.; Frost, B.; Scherzer, C.; Feany, M.B. Biotin rescues mitochondrial dysfunction and neurotoxicity in a tauopathy model. Proc. Natl. Acad. Sci. USA 2020, 117, 33608–33618. [Google Scholar] [CrossRef]
- Luan, H.; Wang, X.; Cai, Z. Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass. Spectrom. Rev. 2019, 38, 22–33. [Google Scholar] [CrossRef]
- Kepka, A.; Ochocinska, A.; Borzym-Kluczyk, M.; Skorupa, E.; Stasiewicz-Jarocka, B.; Chojnowska, S.; Waszkiewicz, N. Preventive Role of L-Carnitine and Balanced Diet in Alzheimer’s Disease. Nutrients 2020, 12, 1987. [Google Scholar] [CrossRef]
- Rosenfeld, J.; Ellis, A. Nutrition and dietary supplements in motor neuron disease. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 573–589. [Google Scholar] [CrossRef]
- Bolaños, J.P.; Almeida, A. The pentose-phosphate pathway in neuronal survival against nitrosative stress. IUBMB Life 2010, 62, 14–18. [Google Scholar] [CrossRef]
- Dunn, L.; Allen, G.F.; Mamais, A.; Ling, H.; Li, A.; Duberley, K.E.; Hargreaves, I.P.; Pope, S.; Holton, J.L.; Lees, A.; et al. Dysregulation of glucose metabolism is an early event in sporadic Parkinson’s disease. Neurobiol. Aging 2014, 35, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, Y.; Lin, L.; Teng, L.; Yin, J.; Lu, Q.; Chen, J.; Zheng, Y.; Li, Y.; Xu, R.; et al. Two radical-dependent mechanisms for anaerobic degradation of the globally abundant organosulfur compound dihydroxypropanesulfonate. Proc. Natl. Acad. Sci. USA 2020, 117, 15599–15608. [Google Scholar] [CrossRef]
- Peggion, C.; Calì, T.; Brini, M. Mitochondria Dysfunction and Neuroinflammation in Neurodegeneration: Who Comes First? Antioxidants 2024, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Csordás, G.; Renken, C.; Várnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnóczky, G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006, 174, 915–921. [Google Scholar] [CrossRef]
- Lau, D.H.W.; Hartopp, N.; Welsh, N.J.; Mueller, S.; Glennon, E.B.; Mórotz, G.M.; Annibali, A.; Gomez-Suaga, P.; Stoica, R.; Paillusson, S.; et al. Disruption of ER-mitochondria signalling in fronto-temporal dementia and related amyotrophic lateral sclerosis. Cell Death Dis. 2018, 9, 327. [Google Scholar] [CrossRef]
- Paillusson, S.; Stoica, R.; Gomez-Suaga, P.; Lau, D.H.W.; Mueller, S.; Miller, T.; Miller, C.C.J. There’s Something Wrong with my MAM; the ER-Mitochondria Axis and Neurodegenerative Diseases. Trends Neurosci. 2016, 39, 146–157. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef]
- Sakurai, T.; Uruno, T.; Sugiura, Y.; Tatsuguchi, T.; Yamamura, K.; Ushijima, M.; Hattori, Y.; Kukimoto-Niino, M.; Mishima-Tsumagari, C.; Watanabe, M.; et al. Cholesterol sulfate is a DOCK2 inhibitor that mediates tissue-specific immune evasion in the eye. Sci. Signal. 2018, 11, eaao4874. [Google Scholar] [CrossRef]
- Wang, F.; Beck-García, K.; Zorzin, C.; Schamel, W.W.; Davis, M.M. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 2016, 17, 844–850. [Google Scholar] [CrossRef]
- Lobel, L.; Cao, Y.G.; Fenn, K.; Glickman, J.N.; Garrett, W.S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 2020, 369, 1518–1524. [Google Scholar] [CrossRef]
- Stewart Campbell, A.; Needham, B.D.; Meyer, C.R.; Tan, J.; Conrad, M.; Preston, G.M.; Bolognani, F.; Rao, S.G.; Heussler, H.; Griffith, R.; et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: An open-label phase 1b/2a trial. Nat. Med. 2022, 28, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Ma, W.; Wang, D.D.; Cao, Y.; Mallick, H.; Gerbaba, T.K.; Lloyd-Price, J.; Abu-Ali, G.; Hall, A.B.; Sikavi, D.; et al. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology 2020, 158, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Frommeyer, B.; Fiedler, A.W.; Oehler, S.R.; Hanson, B.T.; Loy, A.; Franchini, P.; Spiteller, D.; Schleheck, D. Environmental and Intestinal Phylum Firmicutes Bacteria Metabolize the Plant Sugar Sulfoquinovose via a 6-Deoxy-6-sulfofructose Transaldolase Pathway. iScience 2020, 23, 101510. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, X.; Wu, X.; Zhang, C.; Liu, J.; Hou, S. Eubacterium rectale contributes to colorectal cancer initiation via promoting colitis. Gut Pathog. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kang, W.; Hwang, Y.S.; Lee, S.H.; Park, K.W.; Kim, M.S.; Lee, H.; Yoon, H.J.; Park, Y.K.; Chalita, M.; et al. Oral and gut dysbiosis leads to functional alterations in Parkinson’s disease. npj Park. Dis. 2022, 8, 87. [Google Scholar] [CrossRef]
- Murros, K.E. Hydrogen Sulfide Produced by Gut Bacteria May Induce Parkinson’s Disease. Cells 2022, 11, 978. [Google Scholar] [CrossRef]
- Kwon, D.; Zhang, K.; Paul, K.C.; Folle, A.D.; Del Rosario, I.; Jacobs, J.P.; Keener, A.M.; Bronstein, J.M.; Ritz, B. Diet and the gut microbiome in patients with Parkinson’s disease. npj Park. Dis. 2024, 10, 89. [Google Scholar] [CrossRef]
- Di Rienzi, S.C.; Sharon, I.; Wrighton, K.C.; Koren, O.; Hug, L.A.; Thomas, B.C.; Goodrich, J.K.; Bell, J.T.; Spector, T.D.; Banfield, J.F.; et al. The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria. eLife 2013, 2, e01102. [Google Scholar] [CrossRef]
- Silva, D.F.; Candeias, E.; Esteves, A.R.; Magalhães, J.D.; Ferreira, I.L.; Nunes-Costa, D.; Rego, A.C.; Empadinhas, N.; Cardoso, S.M. Microbial BMAA elicits mitochondrial dysfunction, innate immunity activation, and Alzheimer’s disease features in cortical neurons. J. Neuroinflamm. 2020, 17, 332. [Google Scholar] [CrossRef]
- Soo, R.M.; Skennerton, C.T.; Sekiguchi, Y.; Imelfort, M.; Paech, S.J.; Dennis, P.G.; Steen, J.A.; Parks, D.H.; Tyson, G.W.; Hugenholtz, P. An expanded genomic representation of the phylum cyanobacteria. Genome Biol. Evol. 2014, 6, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Costa, D.; Magalhães, J.D.; G-Fernandes, M.; Cardoso, S.M.; Empadinhas, N. Microbial BMAA and the Pathway for Parkinson’s Disease Neurodegeneration. Front. Aging Neurosci. 2020, 12, 26. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Non-Photosynthetic Melainabacteria (Cyanobacteria) in Human Gut: Characteristics and Association with Health. Life 2022, 12, 476. [Google Scholar] [CrossRef]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef] [PubMed]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Zaparte, A.; Christopher, C.; Arnold, C.; Richey, L.; Castille, A.; Mistretta, K.; Taylor, C.M.; Lin, H.; Nelson, S.; Kirwan, J.P.; et al. Effects of E-Cigarettes on the Lung and Systemic Metabolome in People with HIV. Metabolites 2024, 14, 434. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, J.; Fonseca, A.G.; Moshensky, A.; Kothari, T.; Sayed, I.M.; Ibeawuchi, S.-R.; Pranadinata, R.F.; Ear, J.; Sahoo, D.; et al. E-cigarettes compromise the gut barrier and trigger inflammation. iScience 2021, 24, 102035. [Google Scholar] [CrossRef]
- Reekes, T.H.; Ledbetter, C.R.; Alexander, J.S.; Stokes, K.Y.; Pardue, S.; Bhuiyan, M.A.N.; Patterson, J.C.; Lofton, K.T.; Kevil, C.G.; Disbrow, E.A. Elevated plasma sulfides are associated with cognitive dysfunction and brain atrophy in human Alzheimer’s disease and related dementias. Redox Biol. 2023, 62, 102633. [Google Scholar] [CrossRef]
- Davoli, A.; Greco, V.; Spalloni, A.; Guatteo, E.; Neri, C.; Rizzo, G.R.; Cordella, A.; Romigi, A.; Cortese, C.; Bernardini, S.; et al. Evidence of hydrogen sulfide involvement in amyotrophic lateral sclerosis. Ann. Neurol. 2015, 77, 697–709. [Google Scholar] [CrossRef]

| ALS | AD | PD | HC | |
|---|---|---|---|---|
| Number | 11 | 5 | 13 | 11 |
| Age in years mean (range) | 72.5 (71–74) | 64.5 (43–77) | 65.5 (51–80) | 66.1 (45–87) |
| Female | 4 | 3 | 9 | 8 |
| Male | 8 | 2 | 4 | 6 |
| BMI mean (range) | 25.4 (17.7–28.8) | 27.7 (19.2–37.8) | 28.3 (22.4–39.5) | 30.1 (20–58.4) |
| Taking medications for NDD | 69% | 100% | 7% | 0 |
| Months from symptom onset: average (range) | 27.2 (6–82) | 35.7 (30–117) | 31.6 (15–57) | |
| Months from diagnosis: average (range) | 16.9 (1–64) | 18.3 (9–68) | 3 (0–20) | |
| MoCA score (average) | 19.8 | 26.5 | ||
| Clinical Dementia Rating Scale raw (average) | 2.75 | 0 | ||
| Clinical Dementia Rating Scale global (average) | 0.42 | 0 | ||
| ALS bulbar | 23% | |||
| ALS spinal | 77% | |||
| UPDRS-part I (average) | 6.9 | |||
| UPDRS-part II (average) | 6.7 | |||
| UPDRS-part III (average) | 33.9 | |||
| UPDRS-part IV (average) | 0 | |||
| UPDRS gastrointestinal symptoms (average) | 0.23 | |||
| Hoehn and Yahr | 2.0 |
| Primer | Sequence |
|---|---|
| 338F_f1_bc1 | TCCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNTGANNNNTCACTCCTACGGGAGGCAGCA |
| 338F_f2_bc1 | CCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNTTGANNNNTCACTCCTACGGGAGGCAGCA |
| 338F_f3_bc1 | TCCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNCTTGANNNNTCACTCCTACGGGAGGCAGCA |
| 338F_f4_bc1 | TCCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNACTTGANNNNTCACTCCTACGGGAGGCAGCA |
| 338F_f5_bc1 | TCCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNGACTTGANNNNTCACTCCTACGGGAGGCAGCA |
| 338F_f6_bc1 | TCCCTCGCGCCATCAGAGATGTGTATAAGAGACAGNNNNTGACTTGANNNNTCACTCCTACGGGAGGCAGCA |
| JMPM_806R_C | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNCTAGGACTACHVGGGTWTCTAAT |
| JMPM_806R_2T | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNTCTAGGACTACHVGGGTWTCTAAT |
| JMPM_806R_2A | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNATCTAGGACTACHVGGGTWTCTAAT |
| JMPM_806R_G | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNGGACTACHVGGGTWTCTAAT |
| JMPM_806R_A | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNAGGACTACHVGGGTWTCTAAT |
| JMPM_806R_T | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNTAGGACTACHVGGGTWTCTAAT |
| Adaptor_prim1 | AATGATACGGCGACCACCGAGATCTACACGCCTCCCTCGCGCCATCAGAGATGTG |
| PCR Primer, Index X | CAAGCAGAAGACGGCATACGAGATXXXXXXGTGACTGGAGTTCAGACGTGTGCTC |
| NextF_Read1_seq | GCCTCCCTCGCGCCATCAGAGATGTGTATAAGAGACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopher, C.J.; Morgan, K.H.; Tolleson, C.M.; Trudell, R.; Fernandez-Romero, R.; Rice, L.; Abiodun, B.A.; Vickery, Z.; Jones, K.A.; Woodall, B.M.; et al. Specific Bacterial Taxa and Their Metabolite, DHPS, May Be Linked to Gut Dyshomeostasis in Patients with Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Nutrients 2025, 17, 1597. https://doi.org/10.3390/nu17091597
Christopher CJ, Morgan KH, Tolleson CM, Trudell R, Fernandez-Romero R, Rice L, Abiodun BA, Vickery Z, Jones KA, Woodall BM, et al. Specific Bacterial Taxa and Their Metabolite, DHPS, May Be Linked to Gut Dyshomeostasis in Patients with Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Nutrients. 2025; 17(9):1597. https://doi.org/10.3390/nu17091597
Chicago/Turabian StyleChristopher, Courtney Jayde, Katherine Hope Morgan, Christopher Mahone Tolleson, Randall Trudell, Roberto Fernandez-Romero, Lexis Rice, Blessing A. Abiodun, Zane Vickery, Katarina A. Jones, Brittni Morgan Woodall, and et al. 2025. "Specific Bacterial Taxa and Their Metabolite, DHPS, May Be Linked to Gut Dyshomeostasis in Patients with Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis" Nutrients 17, no. 9: 1597. https://doi.org/10.3390/nu17091597
APA StyleChristopher, C. J., Morgan, K. H., Tolleson, C. M., Trudell, R., Fernandez-Romero, R., Rice, L., Abiodun, B. A., Vickery, Z., Jones, K. A., Woodall, B. M., Nagy, C., Mieczkowski, P. A., Bowen, G., Campagna, S. R., & Ellis, J. C. (2025). Specific Bacterial Taxa and Their Metabolite, DHPS, May Be Linked to Gut Dyshomeostasis in Patients with Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Nutrients, 17(9), 1597. https://doi.org/10.3390/nu17091597






