Urinary Caffeine Levels in Chinese Children: Insights from Diet, Gender, and Regional Variations
Highlights
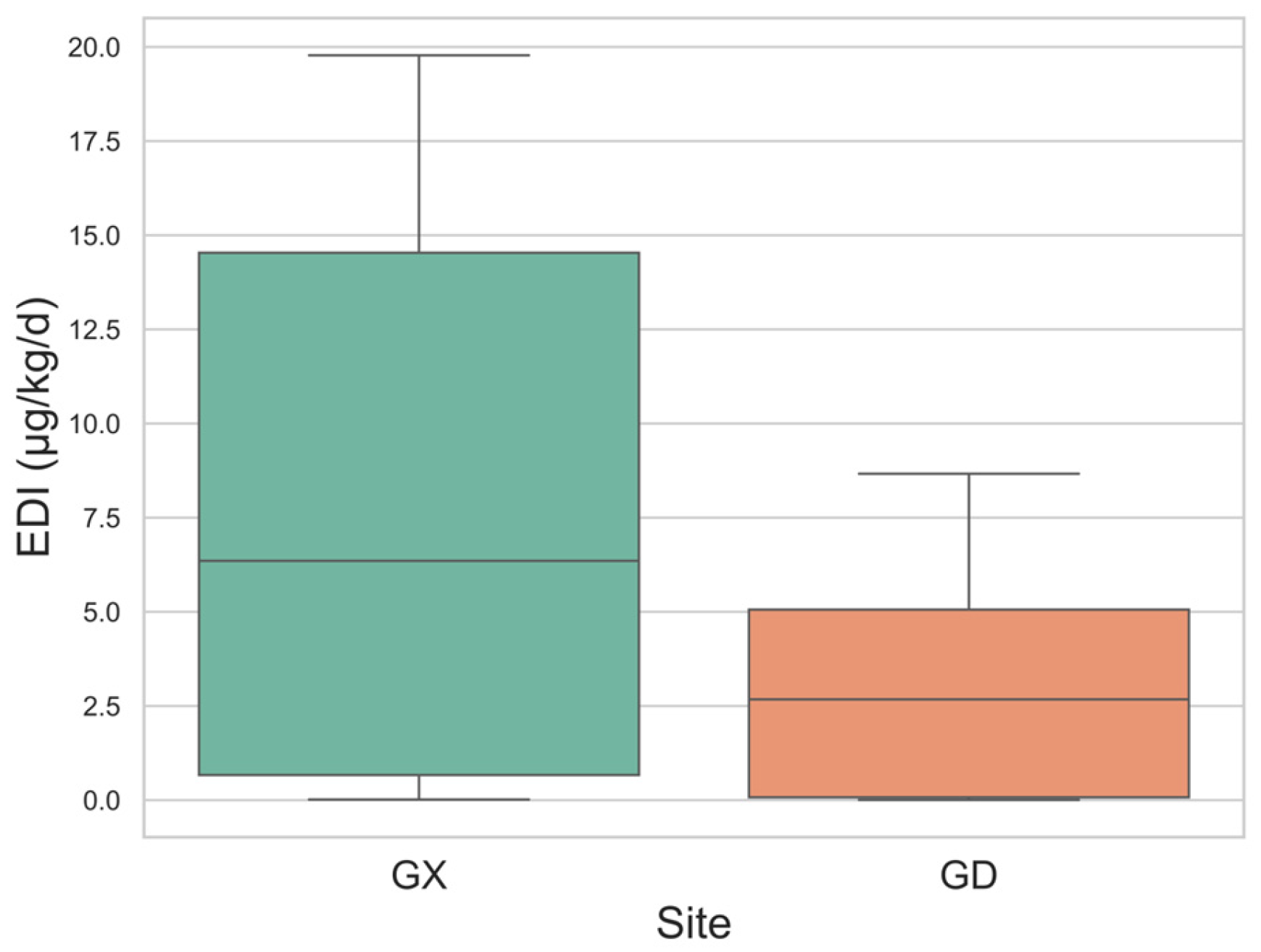
- Overall, urinary caffeine levels were higher in Guangdong; however, these levels remained lower than those reported in most studies, with all Estimated Daily Intake (EDI) values falling within the normal intake range.
- Increased dairy consumption was significantly associated with a higher detection rate of urinary caffeine in children.
- Large-scale sampling should be conducted to assess urinary caffeine and its metabolite levels, as well as dietary habits, among Chinese children across different regions. This will provide a more comprehensive understanding of caffeine exposure patterns and their potential health impacts.
Abstract
1. Introduction
2. Materials and Methods
2.1. Urine Collection/Pretreatment, Chemicals and Materials
2.2. Quality Control
2.3. Health Risk Assessment
2.4. Statistical Analysis
3. Results and Discussion
3.1. Urinary Caffeine Concentration and Creatinine-Adjusted Caffeine Concentration
3.2. Urine Caffeine in Children
3.3. Regional Difference
3.4. Age Difference
3.5. Eating Habits
3.6. Gender Difference
3.7. EDI Estimation of Caffeine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Liu, J.; Ying, G.G.; Lee, J.C.K.; Leung, T.F.; Covaci, A.; Deng, W.J. Endocrine disrupting chemicals in children’s and their parents’ urine: Is the exposure related to the Chinese and Western lifestyle? Int. J. Hyg. Environ. Health 2024, 259, 114383. [Google Scholar] [CrossRef] [PubMed]
- Luna, J. Caffeine Consumption Among Adolescents: Implementing Regulations on Highly Caffeinated Beverages in the United States; Southern University Law Center: Baton Rouge, LA, USA, 2023. [Google Scholar]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Soós, R.; Gyebrovszki, Á.; Tóth, Á.; Jeges, S.; Wilhelm, M. Effects of caffeine and caffeinated beverages in children, adolescents and young adults: Short review. Int. J. Environ. Res. Public Health 2021, 18, 12389. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L. Review: Trends, safety, and recommendations for caffeine use in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 36–45. [Google Scholar] [CrossRef]
- Huhtinen, H.; Lindfors, P.; Rimpelä, A. Adolescents’ use of energy drinks and caffeine-induced health complaints in Finland. Eur. J. Public Health 2013, 23, ckt123-050. [Google Scholar] [CrossRef]
- Heatherley, S.V.; Hancock, K.M.F.; Rogers, P.J. Psychostimulant and other effects of caffeine in 9- to 11-year-old children. J. Child Psychol. Psychiatry 2006, 47, 135–142. [Google Scholar] [CrossRef]
- Suresh, S.; Temple, J.L. Relationships among soda and energy drink consumption, substance use, mental health and risk-taking behavior in adolescents. Children 2024, 11, 1448. [Google Scholar] [CrossRef]
- Jee, H.J.; Lee, S.G.; Bormate, K.J.; Jung, Y.S. Effect of caffeine consumption on the risk for neurological and psychiatric disorders: Sex differences in humans. Nutrients 2020, 12, 3080. [Google Scholar] [CrossRef]
- Chinese Institute of Food Science and Technology. Scientific consensus on caffeine. J. Chin. Inst. Food Sci. Technol. 2024, 24, 498–504. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar]
- Quadra, G.R.; Paranaíba, J.R.; Vilas-Boas, J.; Roland, F.; Amado, A.M.; Barros, N.; Dias, R.J.P.; Cardoso, S.J. A global trend of caffeine consumption over time and related environmental impacts. Environ. Pollut. 2020, 256, 113343. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, B.; Lapolla, R.; Rutigliano, I.; Pettoello Mantovani, M.; Campanozzi, A. Nearly half of the adolescents in an Italian school-based study exceeded the recommended upper limits for daily caffeine consumption. Acta Paediatr. 2018, 107, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fong, D.Y.T.; Wang, Y.Z.; Lin, Z.; Shang, X.C.; Gong, W.J. Nonrestorative sleep and its associated factors in Chinese adolescents and the moderation effects of coffee or tea consumption. BMC Public Health 2024, 24, 2398. [Google Scholar] [CrossRef] [PubMed]
- Tong, O.; Cao, Y.; Song, Y.; Song, J.; Xiao, X.; Yong, L.; Wei, S. Caffeine intake from foods and beverages and trends among Chinese children and adolescents: 2004–2018. Food Chem. Toxicol. 2024, 193, 115025. [Google Scholar] [CrossRef]
- Meigs, J.M.; Bartolomeo, V.R.; Wolfson, A.R. Methodological review of caffeine assessment strategies with a focus on adolescents. Sleep Med. Rev. 2022, 62, 101587. [Google Scholar] [CrossRef]
- Bracken, M.B.; Triche, E.; Grosso, L.; Hellenbrand, K.; Belanger, K.; Leaderer, B.P. Heterogeneity in assessing self-reports of caffeine exposure: Implications for studies of health effects. Epidemiology 2002, 13, 165. [Google Scholar] [CrossRef]
- Petrovic, D.; Estoppey Younes, S.; Pruijm, M.; Ponte, B.; Ackermann, D.; Ehret, G.; Ansermot, N.; Mohaupt, M.; Paccaud, F.; Vogt, B.; et al. Relation of 24-hour urinary caffeine and caffeine metabolite excretions with self-reported consumption of coffee and other caffeinated beverages in the general population. Nutr. Metab. 2016, 13, 81. [Google Scholar] [CrossRef]
- Rios-Leyvraz, M.; Bochud, M.; Tabin, R.; Genin, B.; Russo, M.; Rossier, M.F.; Eap, C.B.; Bovet, P.; Chiolero, A. Monitoring caffeine intake in children with a questionnaire and urine collection: A cross-sectional study in a convenience sample in Switzerland. Eur. J. Nutr. 2020, 59, 3537–3543. [Google Scholar] [CrossRef]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. In Methylxanthines; Fredholm, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 33–91. [Google Scholar]
- Beckford, K.; Grimes, C.A.; Margerison, C.; Riddell, L.J.; Skeaff, S.A.; West, M.L.; Nowson, C.A. A systematic review and meta-analysis of 24-h urinary output of children and adolescents: Impact on the assessment of iodine status using urinary biomarkers. Eur. J. Nutr. 2020, 59, 3113–3131. [Google Scholar] [CrossRef]
- Guessous, I.; Pruijm, M.; Ponte, B.; Ackermann, D.; Ehret, G.; Ansermot, N.; Vuistiner, P.; Staessen, J.; Gu, Y.; Paccaud, F.; et al. Associations of ambulatory blood pressure with urinary caffeine and caffeine metabolite excretions. Hypertension 2015, 65, 691–696. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ. Health Perspect. 2016, 124, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Schultz, H.B.; Ward, M.B.; Grant, C.L.; Paech, G.M.; Banks, S.; Evans, A.M. The effect of high-dose, short-term caffeine intake on the renal clearance of calcium, sodium and creatinine in healthy adults. Br. J. Clin. Pharmacol. 2021, 87, 4461–4466. [Google Scholar] [CrossRef] [PubMed]
- Mage, D.T.; Allen, R.H.; Kodali, A. Creatinine corrections for estimating children’s and adults’ pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Wemple, R.; Lamb, D.; McKeever, K. Caffeine vs caffeine-free sports drinks: Effects on urine production at rest and during prolonged exercise. Int. J. Sports Med. 1997, 18, 40–46. [Google Scholar] [CrossRef]
- Muckelbauer, R.; Sarganas, G.; Grüneis, A.; Müller-Nordhorn, J. Association between water consumption and body weight outcomes: A systematic review. Am. J. Clin. Nutr. 2013, 98, 282–299. [Google Scholar] [CrossRef]
- Rybak, M.E.; Sternberg, M.R.; Pao, C.I.; Ahluwalia, N.; Pfeiffer, C.M. Urine excretion of caffeine and select caffeine metabolites is common in the US population and associated with caffeine intake. J. Nutr. 2015, 145, 766–774. [Google Scholar] [CrossRef]
- Vanderlee, L.; Reid, J.L.; White, C.M.; Acton, R.B.; Kirkpatrick, S.I.; Pao, C.I.; Rybak, M.E.; Hammond, D. Evaluation of a 24-hour caffeine intake assessment compared with urinary biomarkers of caffeine intake among young adults in Canada. J. Acad. Nutr. Diet. 2018, 118, 2245–2253.e1. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Herrick, K. Caffeine intake from food and beverage sources and trends among children and adolescents in the United States: Review of national quantitative studies from 1999 to 2011. Adv. Nutr. 2015, 6, 102–111. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef]
- Dong, Y.; Yuan, C.; Dang, J.; Song, X.; Cheng, G.; Chen, Y.; Wang, H.; Mi, J.; Xi, B.; Song, Y. Control of childhood obesity and implications for policy in China. Lancet Public Health 2024, 9, e1125–e1135. [Google Scholar] [CrossRef]
- Xu, Y.; Bi, X.; Gao, T.; Yang, T.; Xu, P.; Gan, Q.; Xu, J.; Cao, W.; Wang, H.; Pan, H.; et al. Effect of school-based nutrition and health education for rural Chinese children. Nutrients 2022, 14, 3997. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Xu, P.; Yang, T.; Cao, W.; Xu, J.; Li, L.; Pan, H.; Zhao, W.; Zhang, Q. Sugar-sweetened beverage consumption status and its association with childhood obesity among Chinese children aged 6–17 years. Nutrients 2021, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, L.; Zhang, J.; Yang, Z.; Yang, L.; Huang, J.; Fang, H.; Guo, Q.; Xu, X.; Ju, L.; et al. China Nutrition and Health Surveys (1982−2017). China CDC Wkly. 2021, 3, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Xiao, X.; Sui, H.; Yang, D.; Yong, L.; Song, Y. Trends of caffeine intake from food and beverage among Chinese adults: 2004–2018. Food Chem. Toxicol. 2023, 173, 113629. [Google Scholar] [CrossRef]
- Alabbad, M.H.; AlMussalam, M.Z.; AlMusalmi, A.M.; Alealiwi, M.M.; Alresasy, A.I.; Alyaseen, H.N.; Badar, A. Determinants of energy drinks consumption among the students of a Saudi University. J. Fam. Community Med. 2019, 26, 36. [Google Scholar]
- Wang, K.; Xiang, Q.; Hu, L.; Wang, L.; Zhang, Y. Frequency of egg intake associated with mortality in Chinese adults: An 8-year nationwide cohort study. Int. J. Environ. Res. Public Health 2022, 19, 14777. [Google Scholar] [CrossRef]
- Pan, X.F.; Yang, J.J.; Lipworth, L.P.; Shu, X.O.; Cai, H.; Steinwandel, M.D.; Blot, W.J.; Zheng, W.; Yu, D. Cholesterol and egg intakes with cardiometabolic and all-cause mortality among Chinese and low-income Black and White Americans. Nutrients 2021, 13, 2094. [Google Scholar] [CrossRef]
- Choi, H.K.; Curhan, G. Coffee, tea, and caffeine consumption and serum uric acid level: The third national health and nutrition examination survey. Arthritis Care Res. 2007, 57, 816–821. [Google Scholar] [CrossRef]
- Murphy, M.M.; Douglass, J.S.; Johnson, R.K.; Spence, L.A. Drinking flavored or plain milk is positively associated with nutrient intake and is not associated with adverse effects on weight status in US children and adolescents. J. Am. Diet. Assoc. 2008, 108, 631–639. [Google Scholar] [CrossRef]
- Li, X.E.; Lopetcharat, K.; Drake, M.A. Parents’ and children’s acceptance of skim chocolate milks sweetened by monk fruit and stevia leaf extracts. J. Food Sci. 2015, 80, S1083–S1092. [Google Scholar] [CrossRef]
- Li, X.E.; Drake, M. Sensory perception, nutritional role, and challenges of flavored milk for children and adults. J. Food Sci. 2015, 80, R665–R670. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Langille, D.; Asbridge, M. An emerging adolescent health risk: Caffeinated energy drink consumption patterns among high school students. Prev. Med. 2014, 62, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Hoyte, C.O.; Albert, D.; Heard, K.J. The use of energy drinks, dietary supplements, and prescription medications by United States college students to enhance athletic performance. J. Community Health 2013, 38, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Ziegler, A.M. Gender differences in subjective and physiological responses to caffeine and the role of steroid hormones. J. Caffeine Res. 2011, 1, 41–48. [Google Scholar] [CrossRef]
- Ali, A.; O’Donnell, J.; Starck, C.; Rutherfurd-Markwick, K. The effect of caffeine ingestion during evening exercise on subsequent sleep quality in females. Int. J. Sports Med. 2015, 36, 433–439. [Google Scholar] [CrossRef]
- Vilhjalmsson, R.; Kristjansdottir, G. Gender differences in physical activity in older children and adolescents: The central role of organized sport. Soc. Sci. Med. 2003, 56, 363–374. [Google Scholar] [CrossRef]
- Zaigler, M.; Rietbrock, S.; Szymanski, J.; Dericks-Tan, J.S.E.; Staib, A.H.; Fuhr, U. Variation of CYP1A2-dependent caffeine metabolism during menstrual cycle in healthy women. Clin. Pharmacol. 2000, 38, 235–244. [Google Scholar] [CrossRef]
- Ruxton, C.H.S. The suitability of caffeinated drinks for children: A systematic review of randomised controlled trials, observational studies and expert panel guidelines. J. Hum. Nutr. Diet. 2014, 27, 342–357. [Google Scholar] [CrossRef]

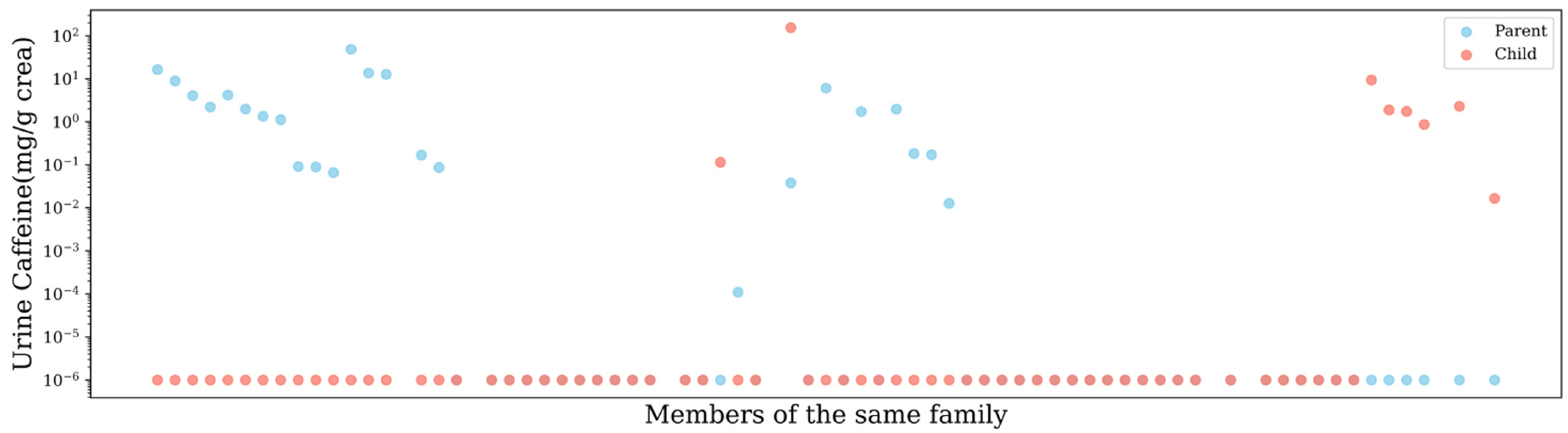
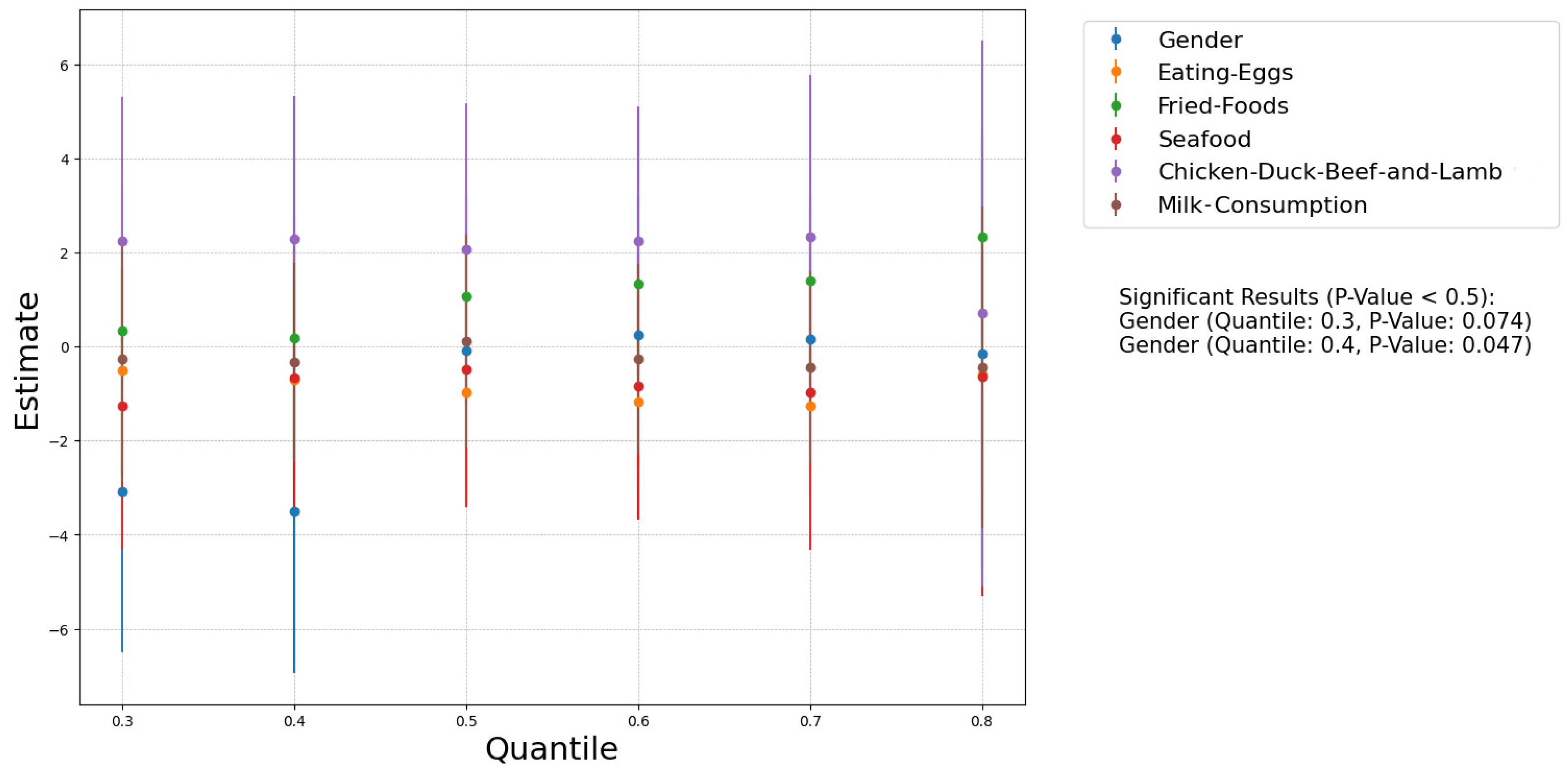
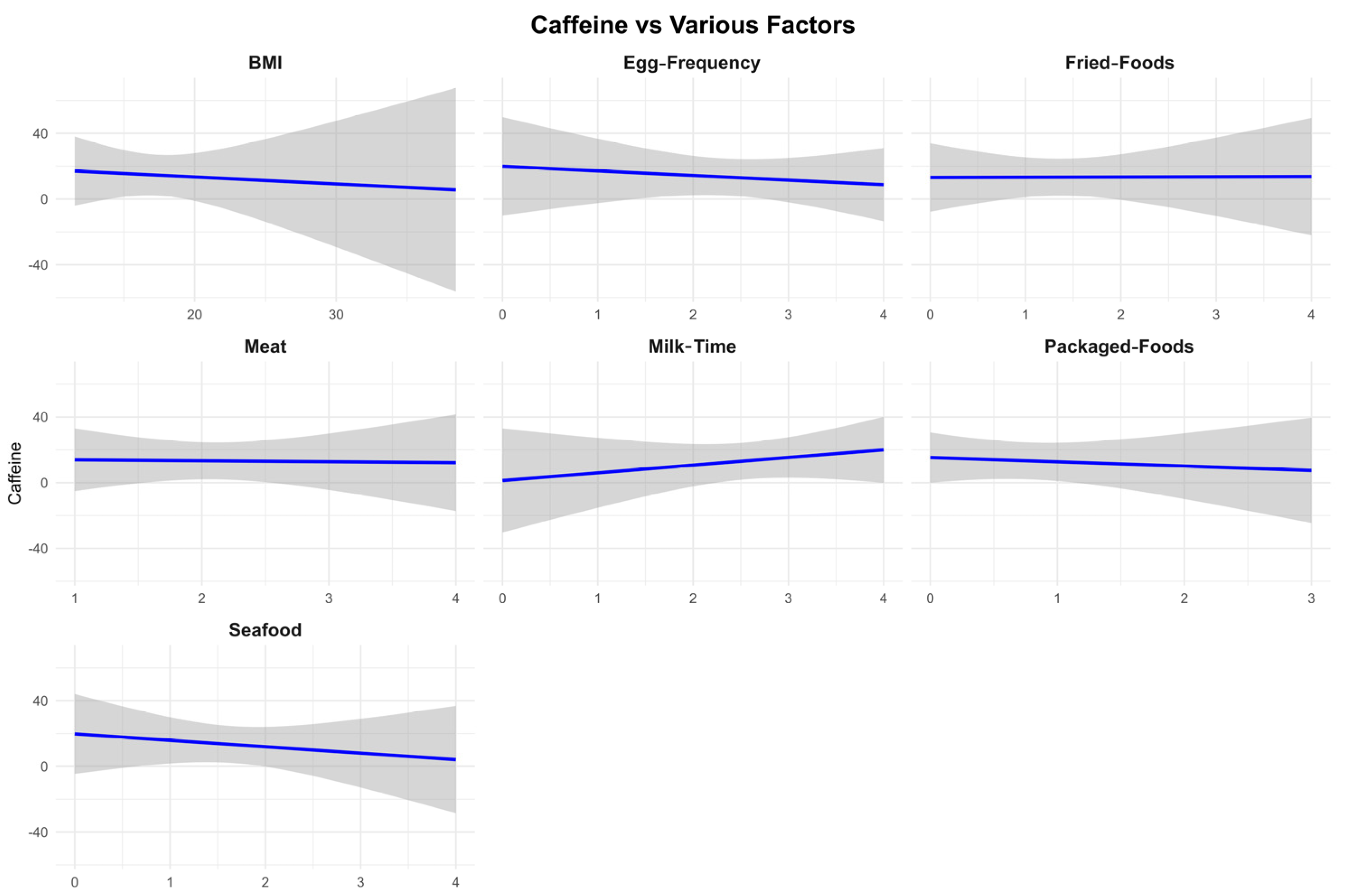
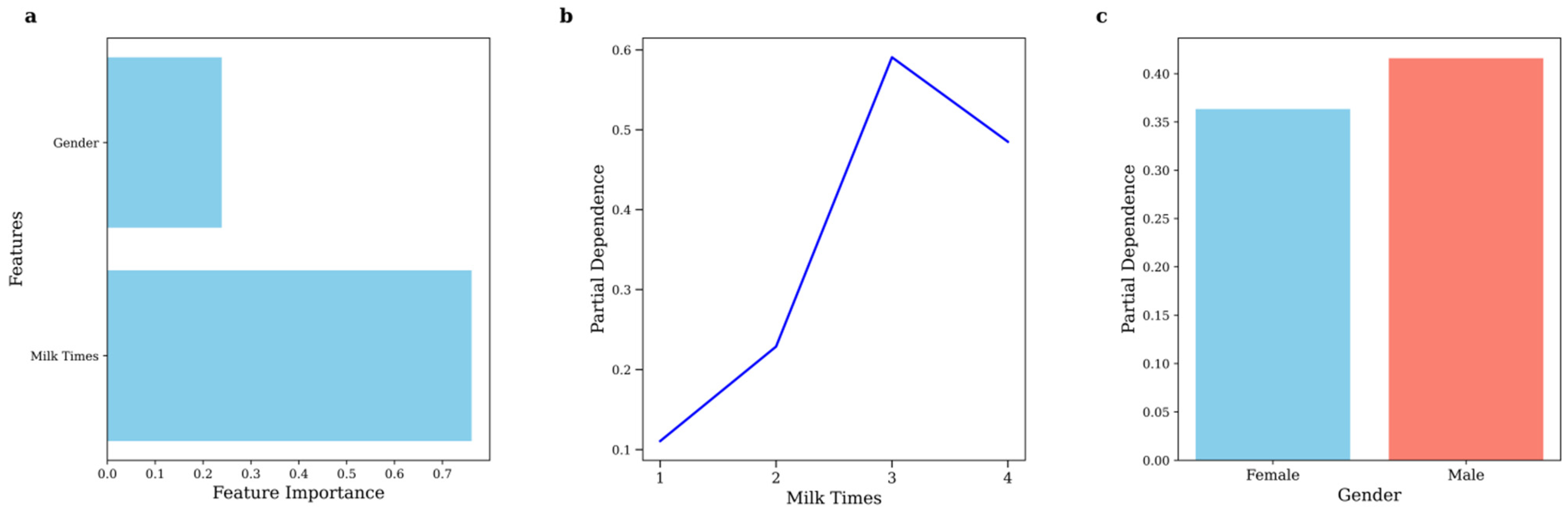
| Sample Size | Mean | Median | 25th Percentile | 75th Percentile | Range | |
|---|---|---|---|---|---|---|
| Guangdong-Children (µg/gcrea) | 40 | 48.26 | 5.201 | 0.053 | 11.72 | 0.00615–152.6 |
| Guangxi-Children (μg/gcrea) | 97 | 26.35 | 1.581 | 0.228 | 4.013 | 0.0164–462.2 |
| Guangxi-Parent (μg/gcrea) | 116 | 8.304 | 4.304 | 0.533 | 8.737 | 0.0655–48.74 |
| Children in total (μg/gcrea) | 137 | 26.35 | 3.400 | 0.108 | 9.423 | 0.00615–462.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, W.-J.; Lee, J.C.-K. Urinary Caffeine Levels in Chinese Children: Insights from Diet, Gender, and Regional Variations. Nutrients 2025, 17, 1594. https://doi.org/10.3390/nu17091594
Deng W-J, Lee JC-K. Urinary Caffeine Levels in Chinese Children: Insights from Diet, Gender, and Regional Variations. Nutrients. 2025; 17(9):1594. https://doi.org/10.3390/nu17091594
Chicago/Turabian StyleDeng, Wen-Jing, and John Chi-Kin Lee. 2025. "Urinary Caffeine Levels in Chinese Children: Insights from Diet, Gender, and Regional Variations" Nutrients 17, no. 9: 1594. https://doi.org/10.3390/nu17091594
APA StyleDeng, W.-J., & Lee, J. C.-K. (2025). Urinary Caffeine Levels in Chinese Children: Insights from Diet, Gender, and Regional Variations. Nutrients, 17(9), 1594. https://doi.org/10.3390/nu17091594








