Hair as an Indicator of Prolonged Paraben Exposure and Its Relation to Weight Gain in a Sample of Spanish Children: A Proof-of-Concept Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Determination of Parabens in Biological Samples
2.3.1. Determination of Parabens in Hair Samples
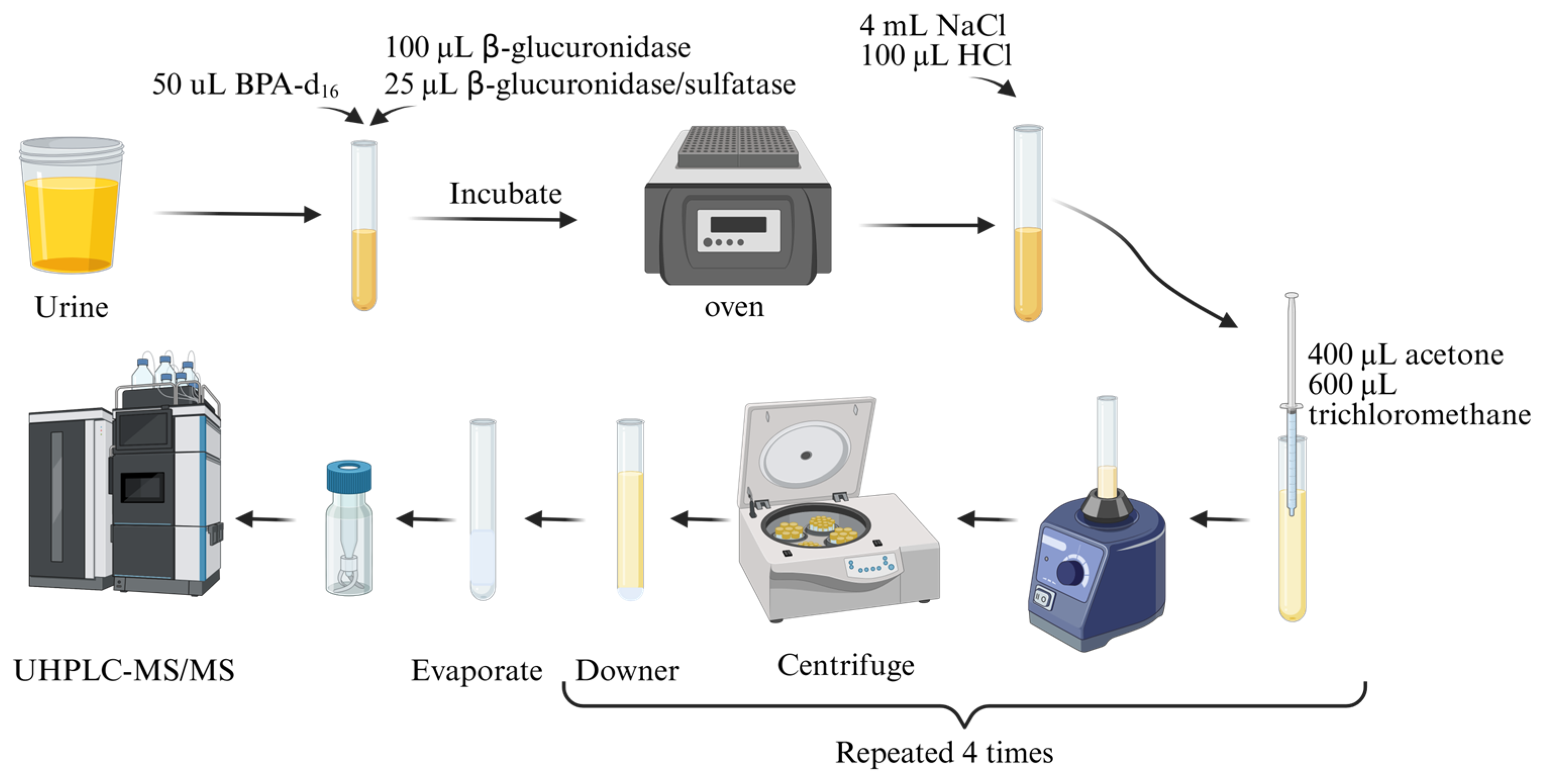
2.3.2. Determination of Parabens in Urine Samples
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| ButPB | Butylparaben |
| CEI | Centre for Biomedical Research of Granada |
| CI | Confidence interval |
| EDCs | Endocrine-disrupting chemicals |
| EthPB | Ethylparaben |
| HCl | Hydrochloric acid |
| IQR | Interquartile range |
| i-ButPB | Isobutylparaben |
| i-PropPB | Isopropylparaben |
| LOD | Limit of detection |
| MetPB | Methylparaben |
| NaCl | Sodium chloride |
| OR | Odds ratio |
| PropPB | Propylparaben |
| SD | Standard deviation |
| UHPLC-MS/MS | Ultra-high performance liquid chromatography-tandem mass spectrometry |
References
- Di Pietro, G.; Forcucci, F.; Chiarelli, F. Endocrine disruptor chemicals and children’s health. Int. J. Mol. Sci. 2023, 24, 2671. [Google Scholar] [CrossRef] [PubMed]
- Strømmen, K.; Lyche, J.L.; Moltu, S.J.; Müller, M.H.B.; Blakstad, E.W.; Almaas, A.N.; Sakhi, A.K.; Thomsen, C.; Nakstad, B.; Rønnestad, A.E.; et al. High urinary concentrations of parabens and bisphenol A in very low birth weight infants. Chemosphere 2021, 271, 129570. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.; Martins, F.; Silva, M.G.; Correia, E.; Videira, R.; Peixoto, F. Use of parabens (methyl and butyl) during the gestation period: Mitochondrial bioenergetics of the testes and antioxidant capacity alterations in testes and other vital organs of the f1 generation. Antioxidants 2020, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Kek, T.; Geršak, K.; Virant-Klun, I. Exposure to endocrine disrupting chemicals (bisphenols, parabens, and triclosan) and their associations with preterm birth in humans. Reprod. Toxicol. 2024, 125, 108580. [Google Scholar] [CrossRef]
- Byford, J.R.; Shaw, L.E.; Drew, M.G.B.; Pope, G.S.; Sauer, M.J.; Darbre, P.D. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 80, 49–60. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing causal links between chemical exposures and obesity. Biochem. Pharmacol. 2022, 199, 115015. [Google Scholar] [CrossRef]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Nigro, C.; Oriente, F.; Formisano, P.; Miele, C.; Beguinot, F. Low-dose bisphenol-A promotes epigenetic changes at pparγ promoter in adipose precursor cells. Nutrients 2020, 12, 3498. [Google Scholar] [CrossRef]
- Martínez, M.; Blanco, J.; Rovira, J.; Kumar, V.; Domingo, J.L.; Schuhmacher, M. Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line. Food Chem. Toxicol. 2020, 140, 111298. [Google Scholar] [CrossRef]
- Hu, J.; Raikhel, V.; Gopalakrishnan, K.; Fernandez-Hernandez, H.; Lambertini, L.; Manservisi, F.; Falcioni, L.; Bua, L.; Belpoggi, F.; Teitelbaum, S.L.; et al. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome 2016, 4, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, P.N.H.; Trasande, L.; Legler, J. Systematic review and meta-analysis of early-life exposure to bisphenol A and obesity-related outcomes in rodents. Environ. Health Perspect. 2017, 125, 106001. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ling, Y.; Jiang, J.; Wang, D.; Wang, J.; Li, J.; Wang, X.; Wang, H. Differential mechanisms regarding triclosan vs. bisphenol A and fluorene-9-bisphenol induced zebrafish lipid-metabolism disorders by RNA-Seq. Chemosphere 2020, 251, 126318. [Google Scholar] [CrossRef]
- Qin, J.; Ru, S.; Wang, W.; Hao, L.; Ru, Y.; Wang, J.; Zhang, X. Long-term bisphenol S exposure aggravates non-alcoholic fatty liver by regulating lipid metabolism and inducing endoplasmic reticulum stress response with activation of unfolded protein response in male zebrafish. Environ. Pollut. 2020, 263 Pt B, 114535. [Google Scholar] [CrossRef]
- Leppert, B.; Strunz, S.; Seiwert, B.; Schlittenbauer, L.; Schlichting, R.; Pfeiffer, C.; Röder, S.; Bauer, M.; Borte, M.; Stangl, G.I.; et al. Maternal paraben exposure triggers childhood overweight development. Nat. Commun. 2020, 11, 561–573. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Beserra, B.T.S.; Silva, N.G.; Lima, C.L.; Rocha, P.R.S.; Coelho, M.S.; Neves, F.A.R.; Amato, A.A. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: A systematic review and meta-analysis. BMJ Open 2020, 10, e033509. [Google Scholar] [CrossRef]
- Alves, A.; Kucharska, A.; Erratico, C.; Xu, F.; Den-Hond, E.; Koppen, G.; Vanermen, G.; Covaci, A.; Voorspoels, S. Human biomonitoring of emerging pollutants through non-invasive matrices: State of the art and future potential. Anal. Bioanal. Chem. 2014, 406, 4063–4088. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.; Binson, G.; Albouy, M.; Sauvaget, A.; Pierre-Eugène, P.; Migeot, V.; Dupuis, A.; Venisse, N. Analytical method for the biomonitoring of bisphenols and parabens by liquid chromatography coupled to tandem mass spectrometry in human hair. Ecotoxicol. Environ. Saf. 2022, 243, 113986. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, R.; Martín, J.; Zafra-Gómez, A.; Alonso, E.; Vílchez, J.L.; Navalón, A. Biomonitoring of 21 endocrine disrupting chemicals in human hair samples using ultra-high performance liquid chromatography-tandem mass spectrometry. Chemosphere 2017, 168, 676–684. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Moscoso-Ruiz, I.; Gálvez-Ontiveros, Y.; Giles-Mancilla, M.C.; Gómez-Regalado, M.; Rivas, A.; Zafra-Gómez, A. Improved method for the determination of endocrine-disrupting chemicals in urine of school-age children using microliquid-liquid extraction and UHPLC-MS/MS. Anal. Bioanal. Chem. 2022, 414, 6681–6694. [Google Scholar] [CrossRef]
- Peake, M.; Whiting, M. Measurement of serum creatinine—Current status and future goals. Clin. Biochem. Rev. 2006, 27, 173–184. [Google Scholar]
- Weber, J.A.; Van-Zanten, A.P. Interferences in current methods for measurements of creatinine. Clin. Chem. 1991, 37, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, H.; Braun, J.M.; Zheng, T.; Zhang, B.; Xia, W.; Zhang, W.; Li, J.; Zhou, Y.; Li, H.; et al. Associations of trimester-specific exposure to bisphenols with size at birth: A Chinese prenatal cohort study. Environ. Health Perspect. 2019, 127, 107001. [Google Scholar] [CrossRef]
- Moscoso-Ruiz, I.; Gálvez-Ontiveros, Y.; Samaniego-Sánchez, C.; Almazán-Fernández de Bobadilla, V.; Monteagudo, C.; Zafra-Gómez, A.; Rivas, A. Presence of parabens in different children biological matrices and its relationship with body mass index. Nutrients 2023, 15, 1154. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Ontiveros, Y.; Moscoso-Ruiz, I.; Almazán-Fernández de Bobadilla, V.; Monteagudo, C.; Giménez-Martínez, R.; Rodrigo, L.; Zafra-Gómez, A.; Rivas, A. Levels of Bisphenol A and its analogs in nails, saliva, and urine of children: A case control study. Front. Nutr. 2023, 10, 1226820. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef]
- Charisiadis, P.; Andrianou, X.D.; Van Der Meer, T.P.; Den-Dunnen, W.F.A.; Swaab, D.F.; Wolffenbuttel, B.H.R.; Makris, K.C.; Van Vliet-Ostaptchouk, J.V. Possible obesogenic effects of bisphenols accumulation in the human brain. Sci. Rep. 2018, 8, 8186. [Google Scholar] [CrossRef]
- Karzi, V.; Tzatzarakis, M.N.; Vakonaki, E.; Alegakis, T.; Katsikantami, I.; Sifakis, S.; Rizos, A.; Tsatsakis, A.M. Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults. J. Appl. Toxicol. 2018, 38, 1144–1152. [Google Scholar] [CrossRef]
- Philippat, C.; Botton, J.; Calafat, A.M.; Ye, X.; Charles, M.A.; Slama, R. Prenatal exposure to phenols and growth in boys. Epidemiology 2014, 25, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, Q.; Sakthivel, S.; Pavithran, P.V.; Vasukutty, J.R.; Kannan, K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ. Res. 2015, 137, 120–128. [Google Scholar] [CrossRef]
- Berger, K.; Hyland, C.; Ames, J.L.; Mora, A.M.; Huen, K.; Eskenazi, B.; Holland, N.; Herley, K.G. Prenatal exposure to mixtures of phthalates, parabens, and other phenols and obesity in five-year-olds in the CHAMACOS Cohort. Int. J. Environ. Res. Public. Health. 2021, 18, 1796. [Google Scholar] [CrossRef]
- Dugandzic, R.; Konstantelos, N.; Yu, Y.; Lavigne, E.; Srugo, S.; Lang, J.J.; Larsen, K.; Pollock, T.; Villeneuve, P.; Thomson, E.M.; et al. Associations between paediatric obesity, chemical mixtures and environmental factors, in a national cross-sectional study of Canadian children. Pediatr. Obes. 2024, 19, e13117. [Google Scholar] [CrossRef]
- Ouidir, M.; Cissé, A.H.; Botton, J.; Lyon-Caen, S.; Thomsen, C.; Sakhi, A.K.; Sabaredzovic, A.; Bayat, S.; Slama, R.; Heude, B.; et al. Fetal and infancy exposure to phenols, parabens, and phthalates and anthropometric measurements up to 36 months, in the longitudinal SEPAGES cohort. Environ. Health Perspect. 2024, 132, 057002. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, C.; Robles-Aguilera, V.; Salcedo-Bellido, I.; Gálvez-Ontiveros, Y.; Samaniego-Sánchez, C.; Aguilera, M.; Zafra-Gómez, A.; Martínez-Burgos, M.A.; Rivas, A. Dietary exposure to parabens and body mass index in an adolescent Spanish population. Environ Res. 2021, 201, 111548. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Coppola, L.; Masella, R.; Tammaro, A.; La Rocca, C. Sex and gender differences on the impact of metabolism-disrupting chemicals on obesity: A systematic review. Nutrients 2024, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Exposure assessment to parabens, bisphenol A and perfluoroalkyl compounds in children, women and men by hair analysis. Sci. Total Environ. 2019, 695, 133864. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.; Albouy, M.; Jourdain, B.; Binson, G.; Sauvaget, A.; Pierre-Eugène, P.; Wu, L.; Migeot, V.; Dupuis, A.; Venisse, N. Assessment of endocrine disruptor exposure in hospital professionals using hair and urine analyses: An awareness campaign. Ther. Drug Monit. 2024, 46, 102–110. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1129 (accessed on 27 February 2025).
- Fäys, F.; Hardy, E.M.; Palazzi, P.; Haan, S.; Beausoleil, C.; Appenzeller, B.M.R. Biomonitoring of fast-elimination endocrine disruptors—Results from a 6-month follow up on human volunteers with repeated urine and hair collection. Sci. Total Environ. 2021, 778, 146330. [Google Scholar] [CrossRef]
- Hernández, A.F.; Lozano-Paniagua, D.; González-Alzaga, B.; Kavvalakis, M.P.; Tzatzarakis, M.N.; López-Flores, I.; Aguilar-Garduño, C.; Caparros-Gonzalez, R.A.; Tsatsakis, A.M.; Lacasaña, M. Biomonitoring of common organophosphate metabolites in hair and urine of children from an agricultural community. Environ. Int. 2019, 131, 104997. [Google Scholar] [CrossRef] [PubMed]
- Hardy, E.M.; Dereumeaux, C.; Guldner, L.; Briand, O.; Vandentorren, S.; Oleko, A.; Zaros, C.; Appenzeller, B.M.R. Hair versus urine for the biomonitoring of pesticide exposure: Results from a pilot cohort study on pregnant women. Environ. Int. 2021, 152, 106481. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Biomonitoring: Facts and Figures. 2025. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2015-3209-42967-60040 (accessed on 27 February 2025).
- Claessens, J.; Pirard, C.; Charlier, C. Determination of contamination levels for multiple endocrine disruptors in hair from a non-occupationally exposed population living in Liege (Belgium). Sci. Total. Environ. 2022, 815, 152734. [Google Scholar] [CrossRef]
- Artacho-Cordón, F.; Fernández, M.F.; Frederiksen, H.; Iribarne-Durán, L.M.; Jiménez-Díaz, I.; Vela-Soria, F.; Andersson, A.M.; Martin-Olmedo, P.; Peinado, F.M.; Olea, N.; et al. Environmental phenols and parabens in adipose tissue from hospitalized adults in Southern Spain. Environ. Int. 2018, 119, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Ontiveros, Y.; Moscoso-Ruiz, I.; Rodrigo, L.; Aguilera, M.; Rivas, A.; Zafra-Gómez, A. Presence of parabens and bisphenols in food commonly consumed in Spain. Foods 2021, 10, 92. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Cantarero-Malagón, S.; Hidalgo, F.; Navalón, A.; Zafra-Gómez, A. Determination of endocrine disrupting chemicals in human nails using an alkaline digestion prior to ultra-high performance liquid chromatography-tandem mass spectrometry. Talanta 2020, 208, 120429. [Google Scholar] [CrossRef]

| Controls (n = 166) | Cases (n = 104) | p-Value | ||
|---|---|---|---|---|
| Gender (%) | Male | 49.4 | 48.1 | 0.833 a |
| Female | 50.6 | 51.9 | ||
| Age, years | Mean | 7.2 | 8.9 | <0.001 b |
| SD | 2.5 | 2.5 | ||
| Energy intake, Kcal/day | Mean | 2092.2 | 2057.4 | 0.647 c |
| SD | 514.5 | 568.1 | ||
| Smoking among family members (%) | No | 87.9 | 56.7 | <0.001 a |
| Yes | 12.1 | 43.3 | ||
| Physical activity (out-of-school) (%) | No | 25.9 | 46.2 | 0.001 a |
| Yes | 74.1 | 53.8 | ||
| Body fat percentage | Median | 20.3 | 33.6 | <0.001 b |
| IQR | 17.2–22.7 | 29.58–38.5 |
| Boys (n = 132) | Girls (n = 138) | ||||||
|---|---|---|---|---|---|---|---|
| Controls (n = 82) | Cases (n = 50) | p-Value | Controls (n = 84) | Cases (n = 54) | p-Value | ||
| Age, years | Mean | 7.0 | 8.9 | <0.001 a | 7.4 | 8.9 | 0.001 a |
| SD | 2.5 | 2.5 | 2.5 | 2.6 | |||
| Energy intake, Kcal/day | Mean | 2087.9 | 2226.1 | 0.212 b | 2096.7 | 1901.4 | 0.059 b |
| SD | 473.1 | 654.0 | 557.5 | 426.7 | |||
| Physical activity (out-of-school) (%) | No | 19.5 | 46.0 | 0.001 c | 32.1 | 46.3 | 0.094 c |
| Yes | 80.5 | 54.0 | 67.9 | 53.7 | |||
| Smoking among family members (%) | No | 92.6 | 50.0 | <0.001 c | 83.3 | 63.0 | 0.007 c |
| Yes | 7.4 | 50.0 | 16.7 | 37.0 | |||
| Body fat percentage | Median | 18.1 | 33.6 | <0.001 a | 21.7 | 33.7 | <0.001 a |
| IQR | 15.6–20.6 | 29.0–38.1 | 19.8–23.5 | 30.4–39.0 | |||
| Controls | Cases | ||
|---|---|---|---|
| Median (IQR) | Median (IQR) | p-Value | |
| Methylparaben | 1411 (873.1–3460.3) | 1687.8 (866.8–4668.0) | 0.447 |
| Ethylparaben | 66.1 (30.9–260.5) | 82.4 (37.1–299.8) | 0.490 |
| Propylparaben | 52.8 (5.8–259.1) | 77.9 (19.6–266.6) | 0.341 |
| Isopropylparaben | 2.6 (0.2–8.3) | 2.6 (0.2–11.6) | 0.764 |
| Total Parabens | 1803.3 (1050.4–4016.8) | 1971.7 (999.1–6137.4) | 0.377 |
| Boys (n = 132) | Girls (n = 138) | |||||
|---|---|---|---|---|---|---|
| Controls (n = 82) | Cases (n = 50) | Controls (n = 84) | Cases (n = 54) | |||
| Median (IQR) | Median (IQR) | p | Median (IQR) | Median (IQR) | p | |
| Methylparaben | 1411 (748.1–3891.0) | 1707.4 (883.0–4106.5) | 0.466 | 1422.3 (887.0–3279.6) | 1462.6 (831.0–5856.0) | 0.834 |
| Ethylparaben | 61.9 (29.6–355.8) | 91.1 (36.6–267.7) | 0.755 | 70.4 (32.6–245.5) | 81.8 (37.6–368.0) | 0.481 |
| Propylparaben | 33.6 (1.0–277.9) | 89.7 (23.6–273.0) | 0.119 | 84.1 (13.4–252.6) | 60.8 (18.5–221.0) | 0.722 |
| Isopropylparaben | 2.1 (0.2–7.2) | 0.2 (0.2–12.1) | 0.866 | 3.4 (0.2–10.5) | 3.3 (0.2–10.8) | 0.877 |
| Total parabens | 1672.5 (865.9–4371.0) | 1971.7 (1245.0–5239.0) | 0.327 | 1919.5 (1134.0–3996.0) | 2081.3 (980.0–7012.0) | 0.804 |
| MetPB | EthPB | PropPB | i-PropPB | Tot. PBs | ||
|---|---|---|---|---|---|---|
| Overall Sample | Spearman Coef. | 0.007 | 0.144 | 0.016 | 0.496 | 0.100 |
| p-Value | 0.938 | 0.102 | 0.855 | <0.001 | 0.256 | |
| Boys | Spearman coef. | −0.144 | 0.078 | −0.113 | 0.652 | −0.104 |
| p-Value | 0.263 | 0.534 | 0.376 | <0.001 | 0.408 | |
| Girls | Spearman coef. | 0.166 | 0.177 | 0.178 | 0.326 | 0.308 |
| p-Value | 0.193 | 0.159 | 0.160 | 0.008 | 0.013 |
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Methylparaben (High exposure) | 1.36 | 0.83–2.23 | 0.218 | 1.14 a | 0.43–3.07 | 0.791 |
| Ethylparaben (High exposure) | 1.21 | 0.74–1.98 | 0.442 | 1.11 b | 0.66–1.87 | 0.683 |
| Propylparaben (High exposure) | 1.36 | 0.83–2.23 | 0.218 | 1.53 c | 0.84–2.80 | 0.164 |
| Isopropylparaben (High exposure) | 1.00 | 0.61–1.63 | 1.000 | 0.91 d | 0.34–2.45 | 0.852 |
| Total parabens (High exposure) | 1.21 | 0.74–1.98 | 0.442 | 1.13 d | 0.42–3.05 | 0.807 |
| Boys (n = 132) | Girls (n = 138) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Methylparaben (High exposure) | 1.78 | 0.86–3.66 | 0.118 | 1.86 a | 0.76–4.59 | 0.176 | 1.07 | 0.54–2.13 | 0.832 | 0.59 f | 0.16–2.18 | 0.433 |
| Ethylparaben (High exposure) | 1.36 | 0.67–2.75 | 0.394 | 1.60 b | 0.32–7.89 | 0.564 | 1.08 | 0.55–2.15 | 0.819 | 1.17 a | 0.50–2.73 | 0.711 |
| Propylparaben (High exposure) | 2.42 | 1.17–4.99 | 0.016 | 4.67 c | 1.08–20.11 | 0.039 | 0.79 | 0.40–1.57 | 0.500 | 1.05 g | 0.29–3.75 | 0.943 |
| Isopropylparaben (High exposure) | 0.87 | 0.43–1.76 | 0.691 | 1.55 d | 0.30–7.87 | 0.600 | 1.14 | 0.57–2.26 | 0.715 | 0.88 e | 0.36–2.17 | 0.794 |
| Total parabens (High exposure) | 1.52 | 0.75–3.10 | 0.245 | 1.36 e | 0.52–3.56 | 0.527 | 0.98 | 0.49–1.94 | 0.952 | 0.79 g | 0.21–2.89 | 0.715 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gálvez-Ontiveros, Y.; González-Palacios, P.; Ramírez, V.; Monteagudo, C.; Samaniego-Sánchez, C.; Rivas, A.; Zafra-Gómez, A. Hair as an Indicator of Prolonged Paraben Exposure and Its Relation to Weight Gain in a Sample of Spanish Children: A Proof-of-Concept Study. Nutrients 2025, 17, 1593. https://doi.org/10.3390/nu17091593
Gálvez-Ontiveros Y, González-Palacios P, Ramírez V, Monteagudo C, Samaniego-Sánchez C, Rivas A, Zafra-Gómez A. Hair as an Indicator of Prolonged Paraben Exposure and Its Relation to Weight Gain in a Sample of Spanish Children: A Proof-of-Concept Study. Nutrients. 2025; 17(9):1593. https://doi.org/10.3390/nu17091593
Chicago/Turabian StyleGálvez-Ontiveros, Yolanda, Patricia González-Palacios, Viviana Ramírez, Celia Monteagudo, Cristina Samaniego-Sánchez, Ana Rivas, and Alberto Zafra-Gómez. 2025. "Hair as an Indicator of Prolonged Paraben Exposure and Its Relation to Weight Gain in a Sample of Spanish Children: A Proof-of-Concept Study" Nutrients 17, no. 9: 1593. https://doi.org/10.3390/nu17091593
APA StyleGálvez-Ontiveros, Y., González-Palacios, P., Ramírez, V., Monteagudo, C., Samaniego-Sánchez, C., Rivas, A., & Zafra-Gómez, A. (2025). Hair as an Indicator of Prolonged Paraben Exposure and Its Relation to Weight Gain in a Sample of Spanish Children: A Proof-of-Concept Study. Nutrients, 17(9), 1593. https://doi.org/10.3390/nu17091593







