Practical Application of Evidence-Based Dietary Therapy in Inflammatory Bowel Disease: The DELECTABLE Program
Abstract
1. Introduction
2. Materials and Methods
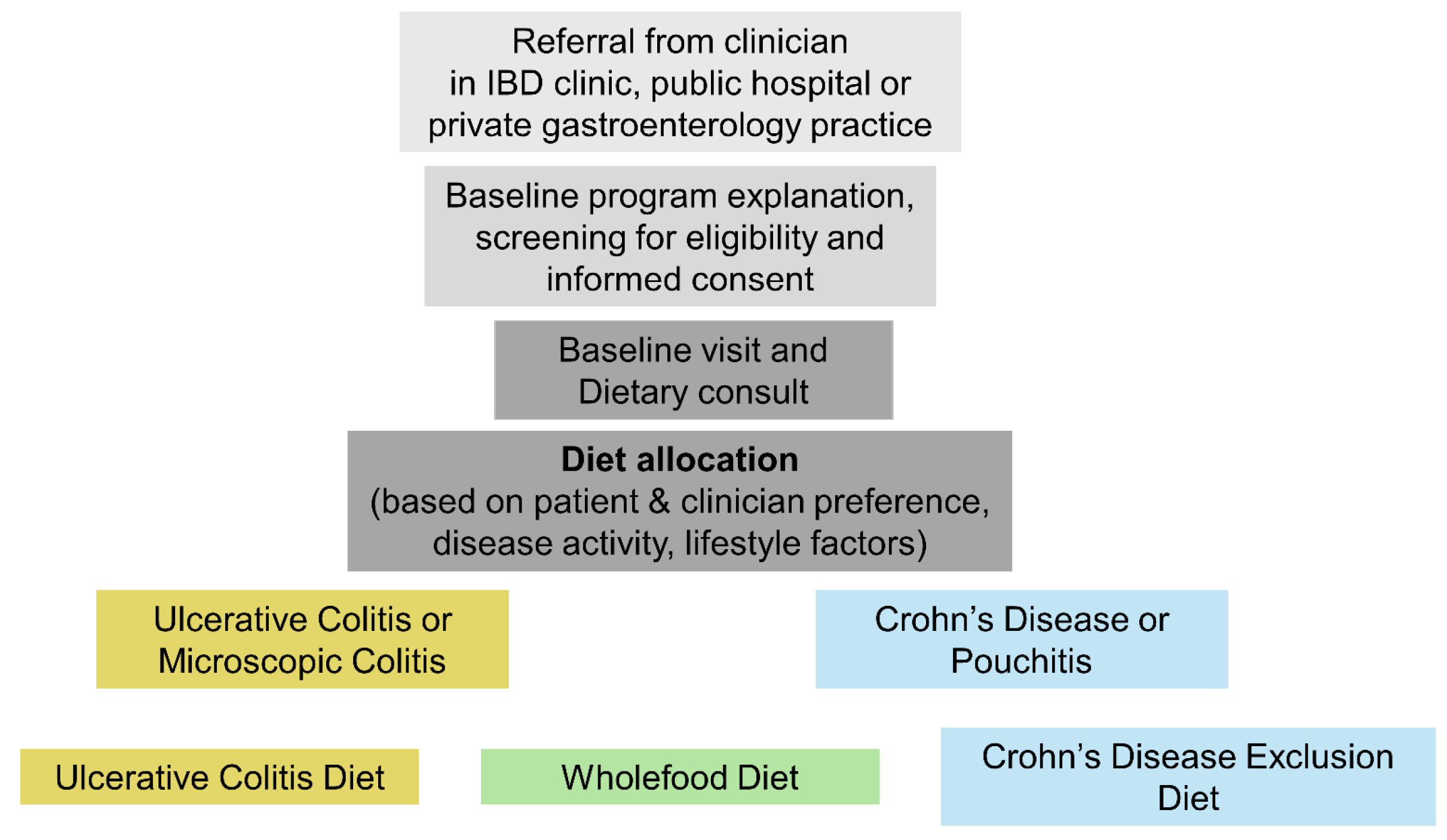
2.1. Study Design and Setting
2.2. Dietary Interventions
2.2.1. Crohn’s Disease Exclusion Diet
2.2.2. Wholefood Diet
2.2.3. Ulcerative Colitis Diet
2.3. Inclusion and Exclusion Criteria
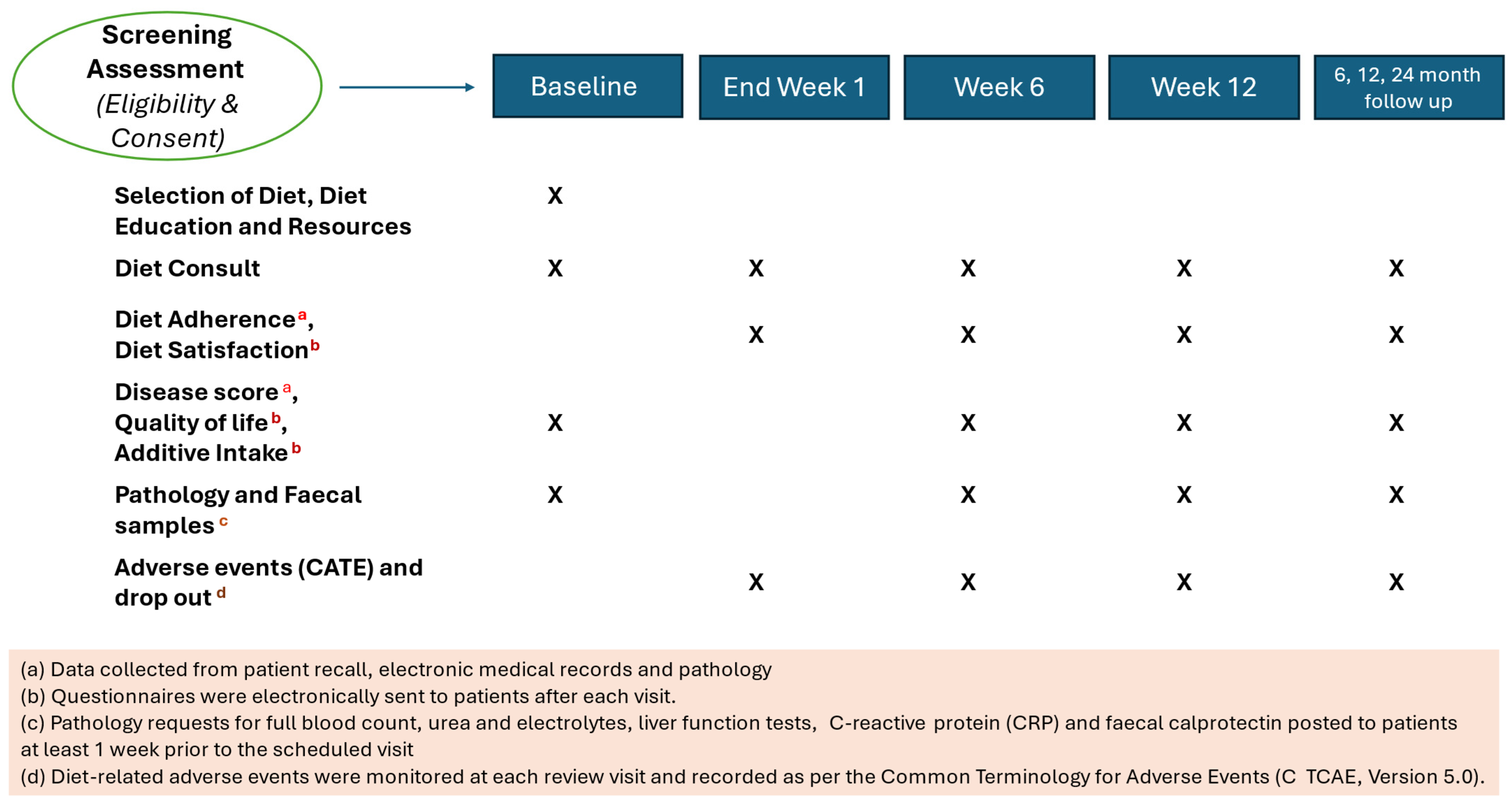
2.4. Outcome Measures
2.4.1. Adherence
2.4.2. Diet Satisfaction
2.4.3. Clinical Efficacy
2.4.4. Quality of Life
2.5. Statistical Analysis
3. Results
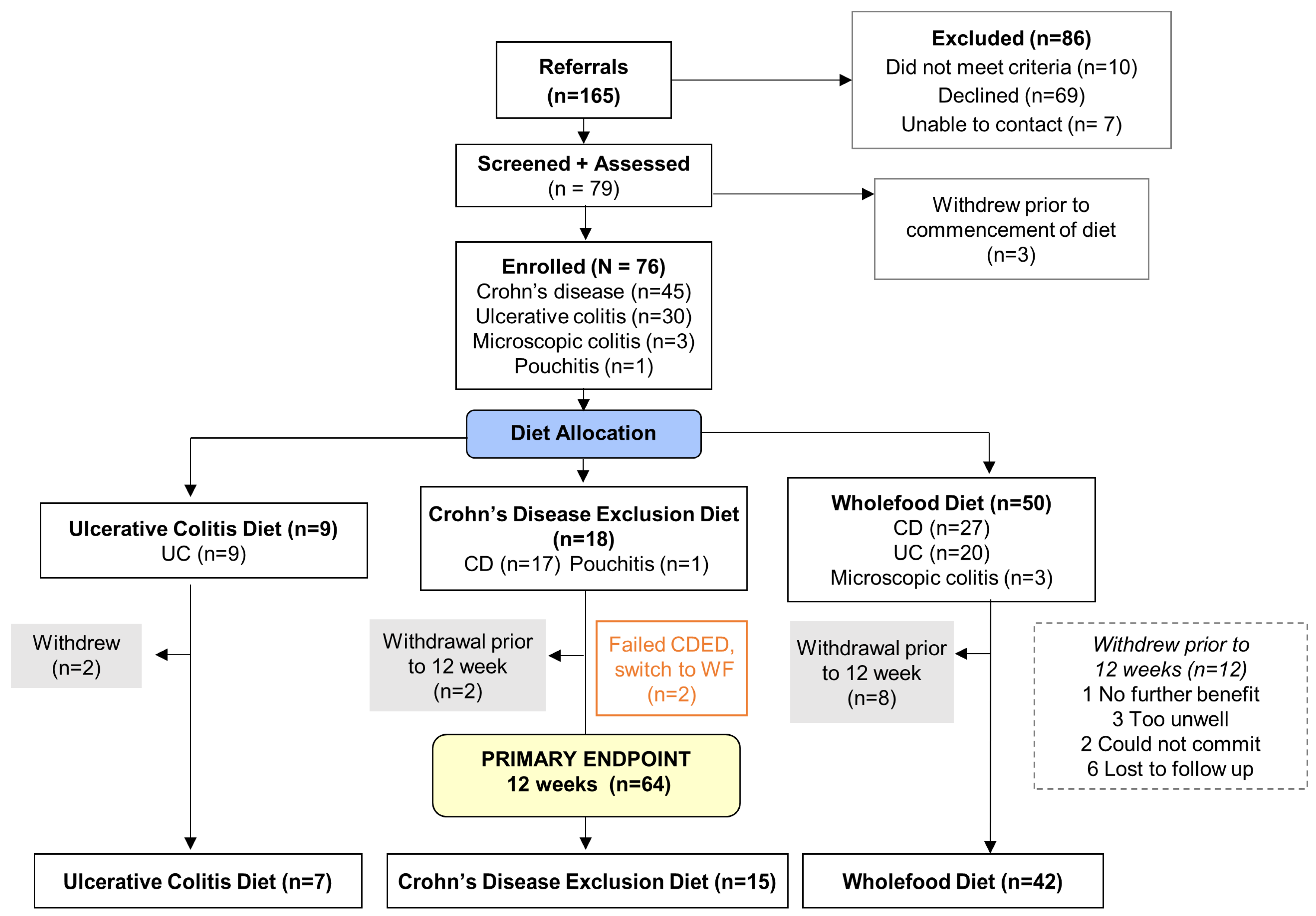
3.1. Patient Enrollment and Allocation
3.2. Demographics and Baseline Characteristics
3.3. Short-Term (Week 1 to 12) Diet Adherence
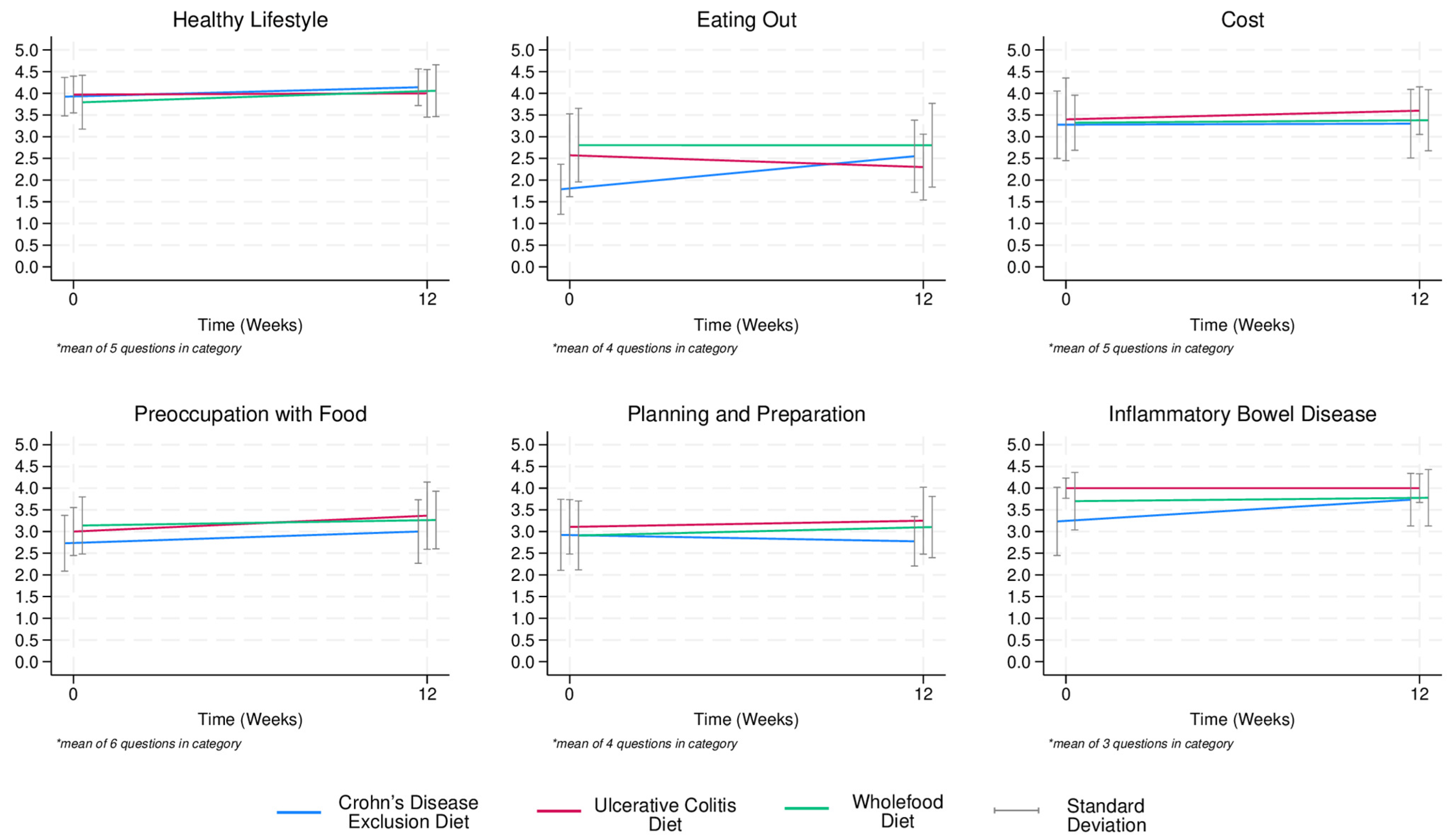
3.4. Short-Term (Week 1 to 12) Diet Satisfaction
3.5. Short-Term (Week 1 to 12) Clinical Efficacy
3.6. Short-Term (Week 1 to 12) Quality of Life
3.7. Long-Term (Week 1 to 12 Months) Diet Adherence and Satisfaction
4. Discussion
4.1. Feasibility and Adherence
4.2. Diet Satisfaction
4.3. Clinical Efficacy
4.4. Strengths
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDAI | Crohn’s Disease Activity Index |
| CDED | Crohn’s Disease Exclusion Diet |
| CRP | C-reactive Protein |
| EEN | Exclusive Enteral Nutrition |
| IBD | Inflammatory Bowel Disease |
| RCT | Randomised Control Trial |
| UCD | Ulcerative Colitis Diet |
| WFD | Wholefood Diet |
References
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse Effects of Biologics Used for Treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Chen, A.; Tiao, D.; Selinger, C.; Leong, R. Medication Adherence in Inflammatory Bowel Disease. Intest. Res. 2017, 15, 434–445. [Google Scholar] [CrossRef]
- Grover, Z.; Muir, R.; Lewindon, P. Exclusive Enteral Nutrition Induces Early Clinical, Mucosal and Transmural Remission in Paediatric Crohn’s Disease. Inflamm. Bowel Dis. 2012, 18, 246–253. [Google Scholar] [CrossRef]
- Wall, C.L.; Day, A.S.; Gearry, R.S. Use of Exclusive Enteral Nutrition in Adults with Crohn’s Disease: A Review. World J. Gastroenterol. 2013, 19, 7652–7660. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Melton, S.L.; Fitzpatrick, J.A.; Taylor, K.M.; Halmos, E.P.; Gibson, P.R. Lessons from an Audit of Exclusive Enteral Nutrition in Adult Inpatients and Outpatients with ACTIVE Crohn’s disease: A Single-Centre Experience. Front. Gastroenterol. 2023, 14, 6–12. [Google Scholar] [CrossRef]
- Day, A.; Wood, J.; Melton, S.; Bryant, R.V. An Optimal Care Pathway for Use in Adult Patients with Active Crohn’s Disease. JGH Open 2020, 4, 260–266. [Google Scholar] [CrossRef]
- Ashton, J.J.; Gavin, J.; Beattie, R.M. Exclusive Enteral Nutrition in Crohn’s Disease: Evidence and Practicalities. Clin. Nutr. 2019, 38, 80–89. [Google Scholar] [CrossRef]
- de Vries, J.H.; Dijkhuizen, M.; Tap, P.; Witteman, B.J. Patient’s Dietary Beliefs and Behaviours in Inflammatory Bowel Disease. Dig. Dis. 2019, 37, 131–139. [Google Scholar] [CrossRef]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.Q.; Strauss, B.J.; Moore, G.T. Patients with Inflammatory Bowel Disease and Their Treating Clinicians Have Different Views Regarding Diet. J. Hum. Nutr. Diet. 2017, 30, 66–72. [Google Scholar] [CrossRef]
- Day, A.S.; Yao, C.K.; Costello, S.P.; Andrews, J.M.; Bryant, R.V. Food Avoidance, Restrictive Eating Behaviour and Association with Quality of Life in Adults with Inflammatory Bowel Disease: A Systematic Scoping Review. Appetite 2021, 167, 105650. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.A.; Melton, S.L.; Yao, C.K.; Gibson, P.R.; Halmos, E.P. Dietary Management of Adults with IBD—The Emerging Role of Dietary Therapy. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 652–669. [Google Scholar] [CrossRef]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving Role of Diet in the Pathogenesis and Treatment of Inflammatory BOWEL diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary Emulsifiers Directly Alter Human Microbiota Composition and Gene Expression ex vivo Potentiating Intestinal Inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, J.; Park, S.-H.; Do, K.H.; Yang, H.; Moon, Y. Pro-Inflammatory NF-κB and Early Growth Response Gene 1 Regulate Epithelial Barrier Disruption by Food Additive Carrageenan in Human Intestinal Epithelial Cells. Toxicol. Lett. 2012, 211, 289–295. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial Overgrowth and Inflammation of Small Intestine After Carboxymethylcellulose Ingestion in Genetically Susceptible Mice. Inflamm. Bowel Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef]
- Jimenez Loayza, J.; Berendsen, E.; Teh, J.; Hoedt, E.; Zhang, J.; Liu, Q.; Hamilton, A.; Wilson-O’Brien, A.; Trakman, G.; Lin, W. P837 The Common Food Additives Sodium Sulfite and Polysorbate 80 Have a Profound Inhibitory Effect on the Commensal, Anti-Inflammatory Bacterium Faecalibacterium Prausnitzii: The Enigma Study. J. Crohns Colitis 2019, 13, S542–S543. [Google Scholar] [CrossRef]
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M.; Whelan, K. Emulsifiers Impact Colonic Length in Mice and Emulsifier Restriction is Feasible in People with Crohn’s Disease. Nutrients 2020, 12, 2827. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Amamou, A.; Savoye, G.; Ghosh, S. Inflammatory Bowel Diseases and Food Additives: To Add Fuel on the Flames! Nutrients 2019, 11, 1111. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; O Lindsay, J.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef]
- Levine, A.; Rhodes, J.M.; Lindsay, J.O.; Abreu, M.T.; Kamm, M.A.; Gibson, P.R.; Gasche, C.; Silverberg, M.S.; Mahadevan, U.; Boneh, R.S.; et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440.e8–450.e8. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; A Cohen, N.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s Disease Exclusion Diet for Induction and Maintenance of Remission in Adults with Mild-to-Moderate Crohn’s Disease (CDED-AD): An Open-Label, Pilot, Randomised Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef]
- Szczubełek, M.; Pomorska, K.; Korólczyk-Kowalczyk, M.; Lewandowski, K.; Kaniewska, M.; Rydzewska, G. Effectiveness of Crohn’s Disease Exclusion Diet for Induction of Remission in Crohn’s Disease Adult Patients. Nutrients 2021, 13, 4112. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.E.; Day, A.S.; Dimitroff, C.; Trakman, G.L.; Silva, H.; Bryant, R.V.; Purcell, L.; Yao, C.K.; Landorf, E.; A Fitzpatrick, J. Practical Application of the Crohn’s Disease Exclusion Diet as Therapy in an Adult Australian Population. J. Gastroenterol. Hepatol. 2023, 39, 446–456. [Google Scholar] [CrossRef]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults With Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9. [Google Scholar] [CrossRef]
- Nitescu, M.; Istratescu, D.; Preda, C.M.; Manuc, T.E.; Louis, E.; Manuc, M.; Stroie, T.; Catrinoiu, M.; Tieranu, C.G.; Badea, L.E.; et al. Role of an Exclusion Diet (Reduced Disaccharides, Saturated Fats, Emulsifiers, Red and Ultraprocessed Meats) in Maintaining the Remission of Chronic Inflammatory Bowel Diseases in Adults. Medicina 2023, 59, 329. [Google Scholar] [CrossRef]
- Vasseur, P.; Dugelay, E.; Benamouzig, R.; Savoye, G.; Lan, A.; Srour, B.; Hercberg, S.; Touvier, M.; Hugot, J.-P.; Julia, C.; et al. Dietary Patterns, Ultra-processed Food, and the Risk of Inflammatory Bowel Diseases in the NutriNet-Santé Cohort. Inflamm. Bowel Dis. 2020, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Trakman, G.L.; Lin, W.Y.Y.; Hamilton, A.L.; Wilson-O’brien, A.L.; Stanley, A.; Ching, J.Y.; Yu, J.; Mak, J.W.Y.; Sun, Y.; Niu, J.; et al. Processed Food as a Risk Factor for the Development and Perpetuation of Crohn’s Disease—The ENIGMA Study. Nutrients 2022, 14, 3627. [Google Scholar] [CrossRef]
- Wellens, J.; Debrauwere, M.; Hoekx, S.; Vanderstappen, J.; De Maegd, E.; Vissers, E.; Ferrante, M.; Verstockt, B.; Vermeire, S.; Sabino, J. P0622 Total Emulsifier Intake, but not Ultra-Processed Food Consumption Is Higher in Patients with Inflammatory Bowel Disease Compared to Healthy Controls. J. Crohns Colitis 2025, 19, i1228–i1230. [Google Scholar] [CrossRef]
- Marsh, A.; Chachay, V.; Banks, M.; Okano, S.; Hartel, G.; Radford-Smith, G. A Pilot Randomized Controlled Trial Investigating the Effects of an Anti-Inflammatory Dietary Pattern on Disease Activity, Symptoms and Microbiota Profile in Adults with Inflammatory Bowel Disease. Eur. J. Clin. Nutr. 2024, 78, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.K.; Sonestedt, E.; Ohlsson, B.; Manjer, J.; Sjöberg, K. The Association between the Intake of Specific Dietary Components and Lifestyle Factors and Microscopic Colitis. Eur. J. Clin. Nutr. 2016, 70, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, Z.S.; Yao, C.K.; Sparrow, M.P.; Gibson, P.R. Review Article: The Impact of Diet on Ileoanal Pouch Function and on the Pathogenesis of Pouchitis. Aliment. Pharmacol. Ther. 2020, 52, 1323–1340. [Google Scholar] [CrossRef]
- Day, A.S.; Yao, C.K.; Costello, S.P.; Ruszkiewicz, A.; Andrews, J.M.; Gibson, P.R.; Bryant, R.V. Therapeutic Potential of the 4 Strategies to SUlfide-REduction (4-SURE) Diet in Adults with Mild to Moderately Active Ulcerative Colitis: An Open-Label Feasibility Study. J. Nutr. 2022, 152, 1690–1701. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10−/− Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Teigen, L.M.; Geng, Z.; Sadowsky, M.J.; Vaughn, B.P.; Hamilton, M.J.; Khoruts, A. Dietary Factors in Sulfur Metabolism and Pathogenesis of Ulcerative Colitis. Nutrients 2019, 11, 931. [Google Scholar] [CrossRef]
- Pitcher, M.C.; Cummings, J.H. Hydrogen Sulphide: A Bacterial Toxin in Ulcerative Colitis? Gut 1996, 39, 1–4. [Google Scholar] [CrossRef]
- Devkota, S.; Chang, E.B. Interactions between Diet, Bile Acid Metabolism, Gut Microbiota, and Inflammatory Bowel Diseases. Dig. Dis. Sci. 2015, 33, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.; Levinson, C.; Colombel, J.-F.; Manning, L.; E Sands, B.; Kayal, M. Dietary Interventions and Supplementation in Patients with an Ileal Pouch–Anal Anastomosis: A Systematic Review. Inflamm. Bowel Dis. 2024, 31, 246–258. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Nickerson, K.P.; McDonald, C. Crohn’s Disease-Associated Adherent-Invasive Escherichia coli Adhesion Is Enhanced by Exposure to the Ubiquitous Dietary Polysaccharide Maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The Artificial Sweetener Acesulfame Potassium Affects the Gut Microbiome and Body Weight Gain in CD-1 Mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Qin, X. Food Additives: Possible Cause for Recent Remarkable Increase of Inflammatory Bowel Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 564. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Bancil, A.S.; Lindsay, J.O.; Chassaing, B. Ultra-Processed Foods and Food Additives in Gut Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 406–427. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Dietary Fibre–Microbiota Interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef]
- Nutrient Reference Values for Australia and New Zealand; National Health and Medical Research Council: Canberra, Australia, 2016.
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Unni, E.J.; Olson, J.L.; Farris, K.B. Revision and validation of Medication Adherence Reasons Scale (MAR-Scale). Curr. Med. Res. Opin. 2014, 30, 211–221. [Google Scholar] [CrossRef]
- Trakman, G.L.; Lin, W.; Wilson-O’brien, A.L.; Stanley, A.; Hamilton, A.L.; Tang, W.; Or, L.; Ching, J.; Morrison, M.; Yu, J.; et al. Development and Validation of Surveys to Estimate Food Additive Intake. Nutrients 2020, 12, 812. [Google Scholar] [CrossRef]
- James, B.L.; Loken, E.; Roe, L.S.; Myrissa, K.; Lawton, C.L.; Dye, L.; Rolls, B.J. Validation of the Diet Satisfaction Questionnaire: A new measure of satisfaction with diets for weight management. Obes. Sci. Pract. 2018, 4, 506–514. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the Noninvasive Components of the Mayo Score to Assess Clinical Response in Ulcerative Colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Alcalá, M.-J.; Prieto, L.; Miró, J.-R.A.; Malagelada, J.-R. Assessment of the Influence of Disease Activity on the Quality of Life of Patients with Inflammatory Bowel Disease Using a Short Questionnaire. Am. J. Gastroenterol. 2004, 99, 457–461. [Google Scholar] [CrossRef]
- Wall, C.L.; Bensley, R.; Glyn, T.; Haines, M.; Rowbotham, D.; Bissett, I.; Eglinton, T.; Gearry, R.B. Preoperative Crohn’s Disease Exclusion Diet and Exclusive Enteral Nutrition in Adults with Crohn’s Disease: A Feasibility Randomised Controlled Trial. Nutrients 2024, 16, 2105. [Google Scholar] [CrossRef]
- Frutkoff, Y.A.; Plotkin, L.; Shavit, Z.; Focht, G.; Livovsky, J.; Lev-Zion, R.; Ledder, O.; Assa, A.; Yogev, D.; Orlanski-Meyer, E.; et al. OP02 Tasty & Healthy Flexible Diet Induces clinical and biological Remission in Children and Young Adults with Mild-Moderate Crohn’s Disease similar to EEN: Results from the “TASTI-MM” randomized, physician-blinded, controlled trial. J. Crohns Colitis 2025, 19, i4. [Google Scholar] [CrossRef]
- Katsoudas, N.; Tavakoli, P.; Wu, N.; Shapiro, A.; Leach, S.T.; Williams, A.-J.; Paramsothy, R.; Ghaly, S.; Connor, S.J.; Samocha-Bonet, D.; et al. Dietary Emulsifier Exposure in People with Inflammatory Bowel Disease Compared with Healthy Controls: Is There a Cause for Concern? Inflamm. Bowel Dis. 2024, 30, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.J.; Crimarco, A.; Perelman, D.; Durand, L.R.; Petlura, C.; Aronica, L.; Robinson, J.L.; Kim, S.H.; Gardner, C.D. Adherence to Ketogenic and Mediterranean Study Diets in a Crossover Trial: The Keto–Med Randomized Trial. Nutrients 2021, 13, 967. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, E.; Vermeire, S.; Ferrante, M.; Sabino, J.; Lambrechts, T.; Avedano, L.; Haaf, I.; De Rocchis, M.S.; Broggi, A.; Sajak-Szczerba, M.; et al. What are the Unmet Needs and Most Relevant Treatment Outcomes According to Patients with Inflammatory Bowel Disease? A Qualitative Patient Preference Study. J. Crohns Colitis 2023, 17, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Bancil, A.; Rossi, M.; Sandall, A.; Cox, S.; Dalrymple, K.; Kelaiditis, C.; Buckley, A.; Burke, S.; Xu, Y.; Smith, L.; et al. Dop097 Emulsifier Restriction Is an Effective Therapy for Active Crohn’s Disease: The Addapt Trial—A Multi-Centre, Randomised, Double-Blind, Placebo-Controlled, Re-Supplementation Trial in 154 Patients. J. Crohns Colitis 2025, 19, i262. [Google Scholar] [CrossRef]
- Knowles, S.R.; Graff, L.A.; Wilding, H.; Hewitt, C.; Keefer, L.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses—Part I. Inflamm. Bowel Dis. 2018, 24, 742–751. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Groven, S.; Lewis, J.D.; Levy, S.S.; Diamant, C.; Singh, E.; Konijeti, G.G. An Autoimmune Protocol Diet Improves Patient-Reported Quality of Life in Inflammatory Bowel Disease. Crohn’s Colitis 360 2019, 1, otz019. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Buckley, A.; Xu, Y.; Burke, S.; Bancil, A.; Sandall, A.; Cox, S.; Rucco, V.; Kelaiditis, C.; Gibson, R.; Chassaing, B.; et al. P0757 Emulsifier Restriction Does not Impair Nutrient Intake and Improves Food-Related Quality of Life in Active Crohn’s Disease: Dietary Analysis of the ADDapt Randomised Controlled Trial. J. Crohns Colitis 2025, 19, i1460–i1462. [Google Scholar] [CrossRef]
| Whole Cohort (n = 64) | CDED (n = 15) | WF Diet (n = 42) | UC Diet (n = 7) | p Value | |
|---|---|---|---|---|---|
| Age, Mean ± SD | 36.2 ± 12.7 | 39.4 ± 12.8 | 35.5 ± 12.9 | 34.0 ± 3.8 | 0.549 |
| Sex Male, n (%) Female, n (%) | 34 (53.1%) 30 (46.9%) | 10 (66.7%) 5 (33.3%) | 21 (50.0%) 21 (50.0%) | 3 (42.9%) 4 (57.1%) | 0.457 |
| Country of birth, n (%) Australia Outside Australia | 54 (84.4%) 10 (15.6) | 13 (86.7%) 2 (13.3%) | 35 (78.6%) 7 (16.7%) | 6 (85.7%) 1 (14.3%) | 0.949 |
| BMI (kg/m2) Median (IQR) | 24.2 (5.7) | 24.3 (6.2) | 25.2 (4.9) | 22.9 (3.9) | 0.484 |
| Disease type, n (%) Crohn’s disease Ulcerative colitis Pouchitis Microscopic colitis | 37 (57.8%) 25 (39.1%) - 2 (3.1%) | 15 (100%) + - - - | 22 (52.4%) 18 (42.9%) - 2 (4.8%) | - 7 (100%) - | <0.001 + |
| Age at diagnosis Median (IQR) | 27.0 (7.0) | 34.0 (19.0) | 24.0 (12.0) | 30.5(12.0) | 0.070 |
| Years since diagnosis Median (IQR) | 4.0 (7.0) | 3.0 (5.0) | 4.0 (10.0) | 3.0 (5.0) | 0.327 |
| IBD drug therapy, n (%) | |||||
| No treatment | 14 (21.9%) | 5 (33.3%) | 9 (21.4%) | 0 (0%) | |
| Single therapy | 31 (48.4%) | 6 (40.0%) | 23 (54.8%) | 2 (28.6%) | 0.027 € |
| Combination therapy | 5 (29.7%) | 4 (26.7%) | 10(23.8%) | 5 (71.4%) | |
| Therapy type, n (%) Topical treatment Systemic ASA Systemic steroid Immunosuppressant Biologic | 11 (15.1%) 18 (24.7%) 6 (8.2%) 13 (17.8%) 25(34.2%) | 2(15.4%) 2 (15.4%) 2 (15.4%) 2 (15.4%) 5 (38.5%) | 6 (13.0%) 12 (27.9%) 3(7.0%) 10 (23.3%) 15 (34.9%) | 3(21.4%) 4(28.6%) 1 (7.1%) 1 (7.1%) 5 (35.7%) | 0.616 |
| Timepoint | CDED (n = 9) Median [IQR] | p Value | WF Diet (n = 26) Median [IQR] | p Value | UC Diet (n = 7) Median [IQR] | p Value | |
|---|---|---|---|---|---|---|---|
| Total Additive intake | Baseline | 4032.4 [7239.6] | 0.441 | 3229.3 [4847.3] | 0.009 | 12,625.9 [1811.6] | 0.018 |
| Week 12 | 2491.1 [6619.8] | 1580.7 [3647.2] | 2796.5 [4618.6] | ||||
| Aspartame | Baseline | 245.6 [737.9] | 0.374 | 264.3 [315.9] | 0.096 | 866.3 [2522.1] | 0.043 |
| Week 12 | 222.9 [344.7] | 128.5 [310.8] | 142.7 [282.4] | ||||
| Carboxymethylcellulose | Baseline | 1117.0 [500.0] | 0.484 | 1279.1 [1759.8] | 0.002 | 2853.3 [2534.2] | 0.018 |
| Week 12 | 157.8 [1934.6] | 162.6 [551.4] | 394.3 [2221.0] | ||||
| Carrageenan | Baseline | 1272.8 [2066.8] | 0.441 | 1052.8 [1285.4] | 0.013 | 2153.3 [4484.6] | 0.028 |
| Week 12 | 766.1 [1983.0] | 387.1 [1486.4] | 737.5 [763.3] | ||||
| Polysorbate-80 | Baseline | 747.4 [1842.1] | 0.374 | 466.9 [763.5] | 0.041 | 2426.7 [2752.0] | 0.028 |
| Week 12 | 556.6 [898.6] | 299.6 [754.6] | 307.4 [827.9] | ||||
| Saccharin | Baseline | 123.0 [238.0] | 0.374 | 85.5 [80.8] | 0.052 | 232.2 [698.2] | 0.018 |
| Week 12 | 80.3 [200.8] | 55.6 [99.4] | 97.6 [193.3] | ||||
| Sucralose | Baseline | 110.0 [368.4] | 0.374 | 136.5 [167.9] | 0.018 | 743.7 [2029.5] | 0.028 |
| Week 12 | 142.6 [192.2] | 74.6 [139.3] | 69.9 [651.9] | ||||
| Sulphites | Baseline | 40.2 [116.4] | 0.767 | 36.4 [150.8] | 0.007 | 120.6 [421.4] | 0.018 |
| Week 12 | 39.6 [54.6] | 14.7 [33.7] | 15.2 [74.7] | ||||
| Titanium dioxide | Baseline | 114.1 [212.2] | 0.889 | 32.7 [68.9] | 0.443 | 149.5 [430.1] | 0.018 |
| Week 12 | 85.4 [174.2] | 44.0 [107.7] | 33.0 [52.6] |
| CDED | WFD | UCD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Week 6 | Week 12 | p Value | Baseline | Week 6 | Week 12 | p Value | Baseline | Week 6 | Week 12 |
| CRP (mg/L), Median [IQR] | 7.5 [15.5] | 5.0 [6.0] | 4.6 [7.3] c | 0.043 | 3.6 [5.6] | 2.0 [5.0] | 1.9 [5.8] | 0.545 | 0.0 [2.5] | 1.0 [2.0] | 1.0 [2.7] |
| CRP (mg/L), n (%) | a, 1.00 | a, 0.69 | |||||||||
| <5 (normal) | 6 (40%) | 4 (40%) | 6 (46.2%) | b, 1.00 | 6 (85.7%) | 6 (85.7%) | 5 (83.3%) | b, 1.00 | 6 (85.7%) | 6 (85.7%) | 5 (83.3%) |
| ≥5 (elevated) | 9 (60%) | 6 (60%) | 7 (53.8%) | c, 1.00 | 1 (14.3%) | 1 (14.3%) | 1 (16.7%) | c, 0.344 | 1 (14.3%) | 1 (14.3%) | 1 (16.7%) |
| Calprotectin, Median [IQR] | 195.0 [560.0] | 123.0 [98.0] a | 62.0 [228.0] c | 0.062 | 17.6 [237.4] | 11.3 [237.4] | 17.8 [88.2] | 0.958 | 112.0 [−] | 46.0 [−] | 174.0 [−] |
| Calprotectin, n (%) | a, n/a | a, 1.00 | |||||||||
| <50 (normal) | 1 (6.7%) | 1 (11.1%) | 5 (45.5%) | b, 1.000 | 22 (52.4%) | 14 (58.3%) | 16 (61.5%) | b, 0.69 | 3 (42.9%) | 2 (66.7%) | 5 (71.4%) |
| ≥50 (elevated) | 12 (92.3%) | 8 (58.9%) | 6 (54.5%) | c, 1.000 | 7 (40.5%) | 10 (41.7%) | 10 (38.5%) | c, 1.00 | 4 (57.1%) | 1 (33.3%) | 2 (28.6%) |
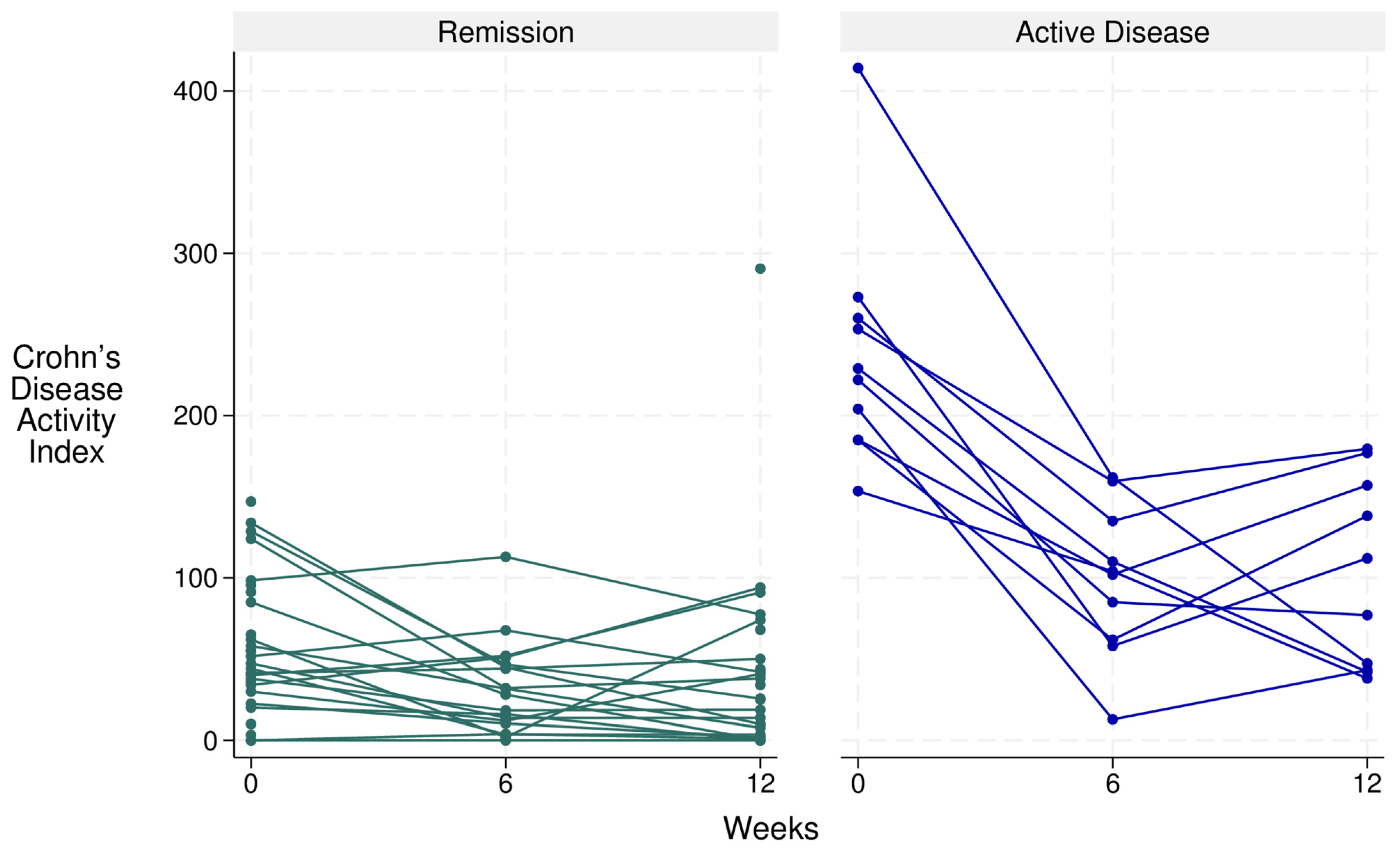
| CDAI, Median [IQR] | 153.0 [172.0] | 61.8 [103.0] a | 43.1 [84.0] c | 0.023 | 49.5 [92.0] | 38.0 [40.0] a | 31.8 [77.0] c | 0.038 | - | - | - |
| CDAI, n (%) | a, 0.031 | a, 0.037 | n/a | n/a | n/a | ||||||
| Remission (0–149) | 8 (53.3%) | 12 (92.3%) | 13 (86.7%) | b, 1.00 | 20 (87%) | 15 (93.8%) | 20(90.9%) | b, 1.00 | n/a | n/a | n/a |
| Active disease (>149) | 7 (46.7%) | 1 (7.7%) | 2 (13.3%) | c, 0.063 | 3 (13%) | 1 (6.3%) | 2 (4.8%) | c, 1.00 | n/a | n/a | n/a |
| Partial Mayo, Median [IQR] | n/a | n/a | n/a | n/a | 2.0 [4.0] | 0.0 [4.0] a | 0.0 [1.0] c | 0.02 | 3.0 [5.0] | 3.0 [3.0] | 3.0 [3.0] |
| Partial Mayo, n (%) | n/a | n/a | n/a | n/a | a, >0.001 | ||||||
| Remission (0–1) | n/a | n/a | n/a | n/a | 5 (26.3%) | 15 (72.2%) | 15 (78.9%) | b, 0.625 | 3 (42.9%) | 3 (42.9%) | 3 (42.9%) |
| Active disease (>1) | n/a | n/a | n/a | n/a | 14 (73.7%) | 5 (27.8%) | 4 (21.1%) | c, 0.002 | 4 (57.1%) | 5 (57.1%) | 6 (57.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trakman, G.L.; Russell, E.E.; Hamilton, A.L.; Wilson-O’Brien, A.; Thompson, E.; Simmance, N.; Niewiadomski, O.; Kamm, M.A. Practical Application of Evidence-Based Dietary Therapy in Inflammatory Bowel Disease: The DELECTABLE Program. Nutrients 2025, 17, 1592. https://doi.org/10.3390/nu17091592
Trakman GL, Russell EE, Hamilton AL, Wilson-O’Brien A, Thompson E, Simmance N, Niewiadomski O, Kamm MA. Practical Application of Evidence-Based Dietary Therapy in Inflammatory Bowel Disease: The DELECTABLE Program. Nutrients. 2025; 17(9):1592. https://doi.org/10.3390/nu17091592
Chicago/Turabian StyleTrakman, Gina L., Erin E. Russell, Amy L. Hamilton, Amy Wilson-O’Brien, Emily Thompson, Natalie Simmance, Ola Niewiadomski, and Michael A. Kamm. 2025. "Practical Application of Evidence-Based Dietary Therapy in Inflammatory Bowel Disease: The DELECTABLE Program" Nutrients 17, no. 9: 1592. https://doi.org/10.3390/nu17091592
APA StyleTrakman, G. L., Russell, E. E., Hamilton, A. L., Wilson-O’Brien, A., Thompson, E., Simmance, N., Niewiadomski, O., & Kamm, M. A. (2025). Practical Application of Evidence-Based Dietary Therapy in Inflammatory Bowel Disease: The DELECTABLE Program. Nutrients, 17(9), 1592. https://doi.org/10.3390/nu17091592





