Hyperphosphatemia in Kidney Failure: Pathophysiology, Challenges, and Critical Role of Phosphorus Management
Abstract
1. Introduction
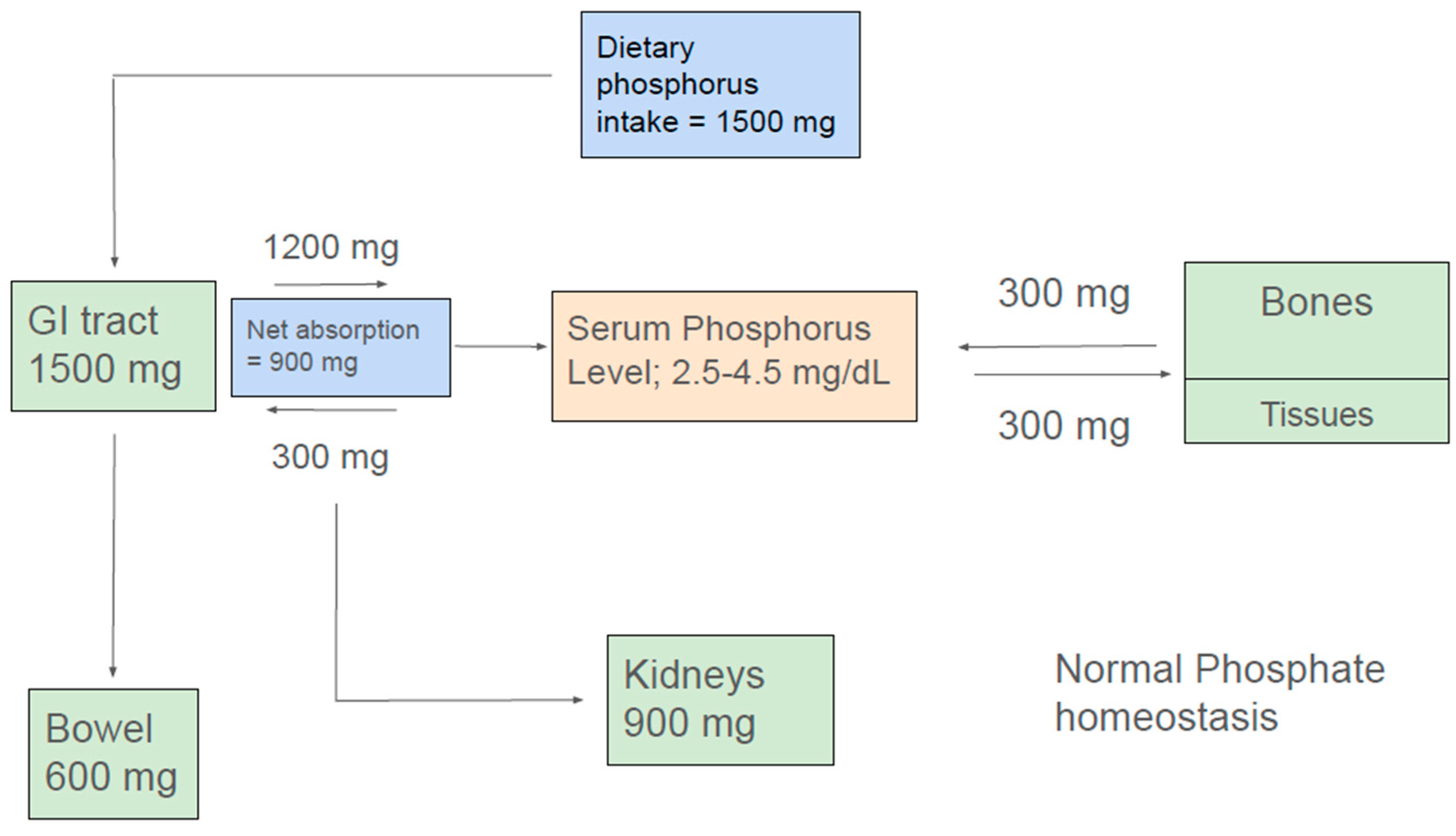
2. Phosphate Homeostasis
2.1. Phosphate Balance in Health
2.2. Intestinal Phosphorus Handling
2.3. Renal Phosphate Handling
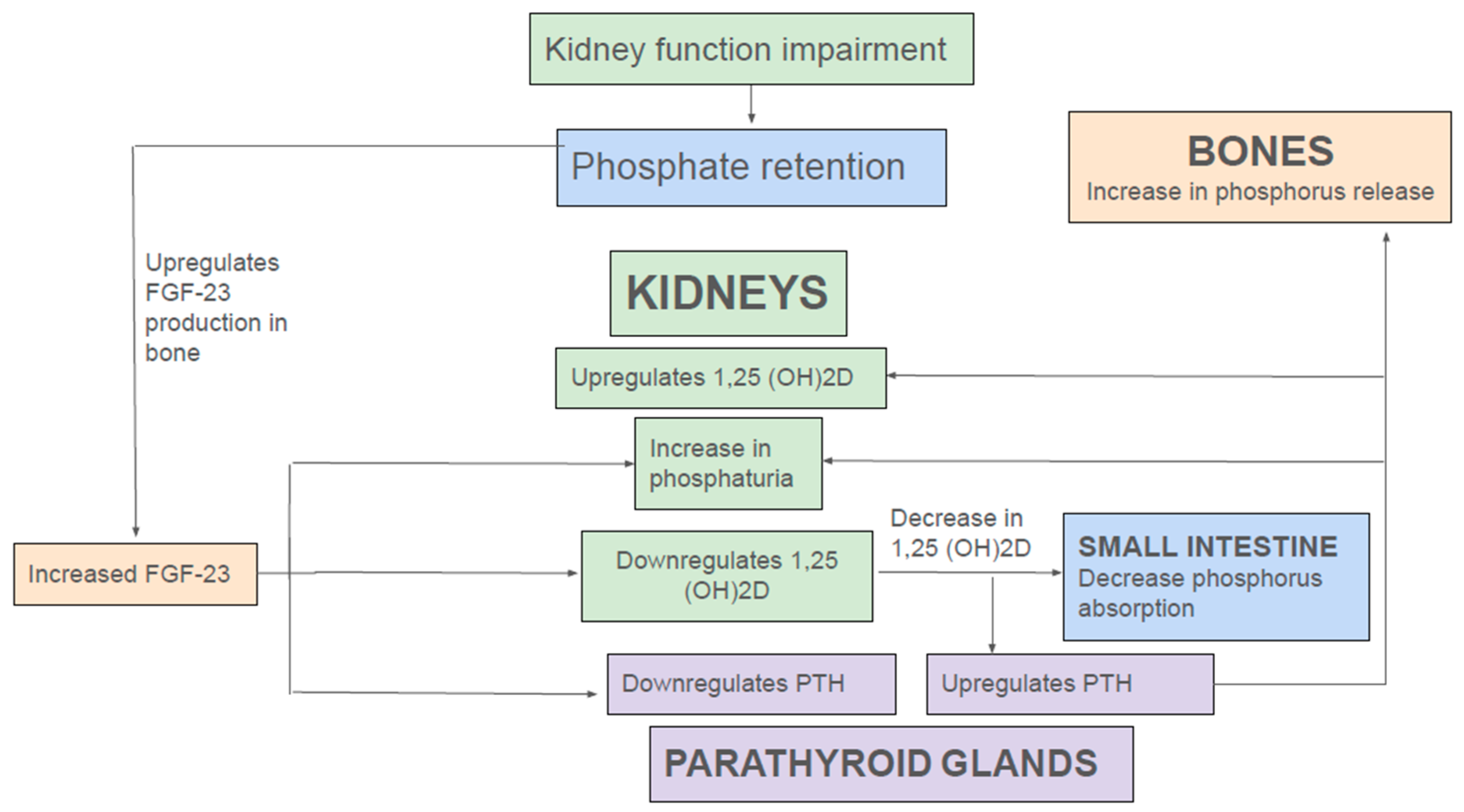
2.4. Endocrine Regulation of Phosphate Homeostasis in Health and Kidney Disease
3. Complications and Outcomes Associated with Hyperphosphatemia
3.1. Hyperphosphatemia: Cardiovascular Risks and Mortality
3.2. Hyperphosphatemia and Risk of Mortality and Progression of Renal Disease
3.3. Hyperphosphatemia and Metabolic Bone Disease
4. Management of Hyperphosphatemia
4.1. Removal of Phosphate by Dialysis
4.2. Dietary Management of Hyperphosphatemia
5. Forms of Phosphorus in the Diet: Organic and Inorganic
6. Intervention
6.1. Reducing Intestinal Phosphate Absorption
6.1.1. Metal-Based Phosphate Binders
6.1.2. Iron-Based Binders: Ferric Citrate and Sucroferric Oxyhydroxide
6.1.3. Drugs Inhibiting Intestinal Phosphate Transport
7. Controversies and Challenges in the Management of Hyperphosphatemia
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APD | automated peritoneal dialysis |
| ATP | adenosine triphosphate |
| BBM | brush border membrane |
| CAPD | continuous ambulatory peritoneal dialysis |
| CARE | cholesterol and recurrent events |
| CCPD | continuous cyclic peritoneal dialysis |
| CKD | chronic kidney disease |
| CRIC | chronic renal insufficiency cohort |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| DOPPS | dialysis outcomes and practice patterns study |
| FDA | Food and Drug administration |
| FGF-23 | fibroblast growth factor 23 |
| FGFR | fibroblast growth factor receptor |
| FGFR1 | fibroblast growth factor receptor 1 |
| FGFR1c | fibroblast growth factor receptor 1c |
| G-3-P | glycerol-3-phosphate |
| GFR | glomerular filtration rate |
| HD | hemodialysis |
| ISRNM | international society of renal nutrition and metabolism |
| KDIGO | kidney disease improving global outcome |
| KF | kidney failure |
| MBD | metabolic bone disease |
| MNT | medical nutrition therapy |
| NaPi | sodium-dependent phosphate cotransporter |
| NaPi-IIb, Npt2b | sodium-dependent phosphate cotransporter IIb |
| NaPi-IIc, Npt2c | sodium-phosphate cotransporter IIc |
| NHE3 | sodium hydrogen exchanger 3 |
| PD | peritoneal dialysis |
| Pit-2, Ram-1 | sodium-potassium cotransporter type III |
| PT | proximal tubule |
| PTH | parathyroid hormone |
| QOL | quality of life |
| RANK-RANKL | receptor activator of nuclear factor kB-Receptor activator of nuclear factor kB ligand |
| RCT | randomized controlled trial |
| RD | registered dietician |
| RT | renal tubule |
| ROMK (1) | renal outer medullary potassium channel 1 |
| TRPV5 | transient receptor potential cation channel subfamily V member 5 |
| VSMC | vascular smooth muscle cells |
References
- Heaney, R.P. Phosphorus. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 447–458. [Google Scholar]
- Hu, M.C.; Moe, O.W. Phosphate and Cellular Senescence. Adv. Exp. Med. Biol. 2022, 1362, 55–72. [Google Scholar] [PubMed]
- Phosphorus. Facts Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/#en1 (accessed on 24 March 2025).
- The United States Renal Data System Annual Data Report 2024. Available online: https://usrds-adr.niddk.nih.gov/2024 (accessed on 24 March 2025).
- Shroff, R.; Long, D.A.; Shanahan, C. Mechanistic insights into vascular calcification in CKD. J. Am. Soc. Nephrol. 2013, 24, 179–189. [Google Scholar] [CrossRef]
- Shroff, R.; Long, D.A.; Shanahan, C.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Ren. Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef]
- Zhou, W.; Simic, P.; Zhou, I.Y.; Caravan, P.; Vela Parada, X.; Wen, D.; Washington, O.L.; Shvedova, M.; Pierce, K.A.; Clish, C.B.; et al. Kidney glycolysis serves as a mammalian phosphate sensor that maintains phosphate homeostasis. J. Clin. Investig. 2023, 133, e164610. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Giral, H.; Caldas, Y.; Levi, M.; Schiavi, S.C. Intestinal phosphate transport. Adv. Chronic Kidney Dis. 2011, 18, 85–90. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Berndt, T.J.; Kumar, R. Clinical Disturbances of Phosphate Homeostasis. In Seldin and Giebisch’s the Kidney, 5th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Walton, J.; Gray, T.K. Absorption of inorganic phosphate in the human small intestine. Clin. Sci. 1979, 56, 407–412. [Google Scholar] [CrossRef]
- Danisi, G.; Straub, R.W. Unidirectional influx of phosphate across the mucosal membrane of rabbit small intestine. Pflugers Arch. 1980, 385, 117–122. [Google Scholar] [CrossRef]
- Davis, G.R.; Zerwekh, J.E.; Parker, T.F.; Krejs, G.J.; Pak, C.Y.; Fordtran, J.S. Absorption of phosphate in the jejunum of patients with chronic renal failure before and after correction of vitamin D deficiency. Gastroenterology 1983, 85, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.; Debnam, E.S.; Unwin, R.J. The role of the gastrointestinal tract in phosphate homeostasis in health and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 481–487. [Google Scholar] [CrossRef]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009, 20, 2348–2358. [Google Scholar] [CrossRef]
- Marks, J.; Debnam, E.S.; Unwin, R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. Renal Physiol. 2010, 299, F285–F296. [Google Scholar] [CrossRef] [PubMed]
- Larsson, T.E.; Kameoka, C.; Nakajo, I.; Taniuchi, Y.; Yoshida, S.; Akizawa, T.; Smulders, R.A. NPT-IIb inhibition does not improve hyperphosphatemia in CKD. Kidney Int. Rep. 2018, 3, 73–80. [Google Scholar] [CrossRef]
- Saurette, M.; Alexander, R.T. Intestinal phosphate absorption: The paracellular pathway predominates? Exp. Biol. Med. 2019, 244, 646–654. [Google Scholar] [CrossRef]
- Knöpfel, T.; Himmerkus, N.; Günzel, D.; Bleich, M.; Hernando, N.; Wagner, C.A. Paracellular transport of phosphate along the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G233–G241. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.B.; Walling, M.W.; Corry, D.B. Phosphate transport across rat jejunum: Influence of sodium, pH, and 1,25-dihydroxyvitamin D3. Am. J. Physiol. 1986, 251 Pt 1, G90–G95. [Google Scholar] [CrossRef]
- King, A.J.; Siegel, M.; He, Y.; Nie, B.; Wang, J.; Koo-McCoy, S.; Minassian, N.A.; Jafri, Q.; Pan, D.; Kohler, J.; et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci. Transl. Med. 2018, 10, eaam6474. [Google Scholar] [CrossRef]
- Dudeja, P.K.; Rao, D.D.; Syed, I.; Joshi, V.; Dahdal, R.Y.; Gardner, C.; Risk, M.C.; Schmidt, L.; Bavishi, D.; Kim, K.E.; et al. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am. J. Physiol. 1996, 271 Pt 1, G483–G493. [Google Scholar] [CrossRef]
- Rosenbaum, D.P.; Yan, A.; Jacobs, J.W. Pharmacodynamics, safety, and tolerability of the NHE3 inhibitor tenapanor: Two trials in healthy volunteers. Clin. Drug Investig. 2018, 38, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Rosenbaum, D.P.; Yan, A.; Chertow, G.M. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: A randomized phase 3 trial. J. Am. Soc. Nephrol. 2019, 30, 641–652. [Google Scholar] [CrossRef]
- Tatsumi, S.; Miyagawa, A.; Kaneko, I.; Shiozaki, Y.; Segawa, H.; Miyamoto, K. Regulation of renal phosphate handling: Inter-organ communication in health and disease. J. Bone Min. Metab. 2016, 34, 1–10. [Google Scholar] [CrossRef]
- Lederer, E. Renal phosphate transporters. Curr. Opin. Nephrol. Hypertens. 2014, 23, 502–506. [Google Scholar] [CrossRef]
- Erben, R.G. Physiological Actions of Fibroblast Growth Factor-23. Front. Endocrinol. 2018, 9, 267. [Google Scholar] [CrossRef]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef]
- Lee, M.; Partridge, N.C. Parathyroid hormone signaling in bone and kidney. Curr. Opin. Nephrol. Hypertens. 2009, 18, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, M.; Arena, M.; Naticchia, A.; Gambaro, G.; Mazzaferro, S.; Fuster, D.; Ferraro, P.M. The Role of Diet in Bone and Mineral Metabolism and Secondary Hyperparathyroidism. Nutrients 2021, 13, 2328. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Z. Molecular Basis of Klotho: From Gene to Function in Aging. Endocrine Reviews 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Kuro-o, M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Dalton, G.D.; Xie, J.; An, S.-w.; Huang, C.-L. New Insights into the Mechanism of Action of Soluble Klotho. Front. Endocrinol. 2017, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging, and the Failing Kidney. Front. Endocrinol. 2020, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sun, Z. Estrogen inhibits renal Na-Pi Co-transporters and improves klotho deficiency-induced acute heart failure. Redox Biol. 2021, 47, 102173. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.K.; Hu, M.-C.; Kurosu, H.; Kuro-o, M.; Moe, O.; Huang, C.-L. Regulation of ROMK1 channel and renal K+ excretion by Klotho. Mol. Pharmacol. 2009, 76, 38–46. [Google Scholar] [CrossRef]
- Chang, Q.; Hoefs, S.; van der Kemp, A.W.; Topala, C.N.; Bindels, R.J.; Hoenderop, J.G. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 2005, 310, 490–493. [Google Scholar] [CrossRef]
- Wolf, M.T.; An, S.W.; Nie, M.; Bal, M.S.; Huang, C.L. Klotho up-regulates renal calcium channel transient receptor potential vanilloid 5 (TRPV5) by intra- and extracellular n-glycosylation-dependent mechanisms. J. Biol. Chem. 2014, 289, 35849–35857. [Google Scholar] [CrossRef]
- Ritz, E. Gross M-L Hyperphosphatemia in renal failure. Blood Purif. 2005, 23, 6–9. [Google Scholar] [CrossRef]
- Chen, W.; Bushinsky, D. Chronic kidney disease–mineral and bone disorder. In Handbook of Dialysis Therapy, 5th ed.; Nissenson, A.R., Fine, R.N.B.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 685–697.e1. [Google Scholar]
- Block, G.A.; Hulbert-Shearon, T.E.; Levin, N.W.; Port, F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef]
- Dhingra, R.; Sullivan, L.M.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B.; Gaziano Sr, J.M.; Vasan, R.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern Med. 2007, 167, 879–885. [Google Scholar] [CrossRef]
- Palmer, S.C.; Hayen, A.; Macaskill, P.; Pellegrini, F.; Craig, J.C.; Elder, G.J.; Strippoli, G.F. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 2011, 305, 1119–1127. [Google Scholar] [CrossRef]
- Tong, J.; Liu, M.; Li, H.; Luo, Z.; Zhong, X.; Huang, J.; Liu, R.; He, F.; Fu, J. Mortality and associated risk factors in dialysis patients with cardiovascular disease. Kidney Blood Press Res. 2016, 41, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii28–iii34. [Google Scholar] [CrossRef]
- Schwartz, S.; Trivedi, B.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 825–831. [Google Scholar] [CrossRef]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.; Port, F.K. Association of elevated serum PO4, Ca × PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2001, 12, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Dusso, A.S.; Slatopolsky, E. Role of calcium-phosphate product and bone-associated proteins on vascular calcification in renal failure. J. Am. Soc. Nephrol. 2001, 12, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bi, X.; Liu, Y.; Huang, Y.; Xiong, J.; Xu, X.; Xiao, T.; Yu, Y.; Jiang, W.; Huang, Y.; et al. High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-κb signaling pathway. Kidney Blood Press. Res. 2017, 42, 1205–1215. [Google Scholar] [CrossRef]
- Amann, K. Media Calcification and Intima Calcification Are Distinct Entities in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1599–1605. [Google Scholar] [CrossRef]
- El-Abbadi, M.M.; Pai, A.S.; Leaf, E.M.; Yang, H.-Y.; Bartley, B.A.; Quan, K.K.; Ingalls, C.M.; Liao, H.W.; Giachelli, C.M. Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009, 75, 1297–1307. [Google Scholar] [CrossRef]
- Pai, A.S.; Giachelli, C.M. Matrix remodeling in vascular calcification associated with chronic kidney disease. J. Am. Soc. Nephrol. 2010, 21, 1637–1640. [Google Scholar] [CrossRef]
- Luong, T.T.D.; Schelski, N.; Boehme, B.; Makridakis, M.; Vlahou, A.; Lang, F.; Pieske, B.; Alesutan, I.; Voelkl, J. Fibulin-3 attenuates phosphate-induced vascular smooth muscle cell calcification by inhibition of oxidative stress. Cell Physiol. Biochem. 2018, 46, 1305–1316. [Google Scholar] [CrossRef]
- Voelkl, J.; Lang, F.; Eckardt, K.; Amann, K.; Kuro-O, M.; Pasch, A.; Pieske, B.; Alesutan, I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol. Life Sci. 2019, 76, 2077–2091. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Welsh, P.; Papacosta, O.; Lennon, L.; Whincup, P.H.; Sattar, N. Elevated parathyroid hormone, but not vitamin D deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circulation Heart Fail. 2014, 7, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Vogt, I.; Haffner, D.; Leifheit-Nestler, M. FGF23 and Phosphate–Cardiovascular Toxins in CKD. Toxins 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Katz, R.; Kestenbaum, B.R.; de Boer, I.H.; Chonchol, M.; Mukamal, K.J.; Rifkin, D.; Siscovick, D.S.; Sarnak, M.J.; Shlipak, M.G. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J. Am. Coll. Cardiol. 2012, 60, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Edmonston, D.; Grabner, A.; Wolf, M. FGF23 and klotho at the intersection of kidney andcardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 11–24. [Google Scholar] [CrossRef]
- Zhong, Z.; Feng, S.; Fu, D.; Li, B.; Li, Z.; Mao, H. Serum fibroblast growth factor 23 concentration and the risk of mortality in patients undergoing peritoneal dialysis. Perit. Dial. Int. 2024, 44, 194. [Google Scholar] [CrossRef]
- Cheng, S.P.; Liu, C.L.; Liu, T.P.; Hsu, Y.C.; Lee, J.J. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. 2014, 2014, 709024. [Google Scholar] [CrossRef]
- Goldsmith, D.J.; Covic, A.A.; Venning, M.C.; Ackrill, P. Blood pressure reduction after parathyroidectomy for secondary hyperparathyroidism: Further evidence implicating calcium homeostasis in blood pressure regulation. Am. J. Kidney Dis. 1996, 27, 819–825. [Google Scholar] [CrossRef]
- Smogorzewski, M.; Perna, A.F.; Borum, P.R.; Massry, S.G. Fatty acid oxidation in the myocardium: Effects of parathyroid hormone and CRF. Kidney Int. 1988, 34, 797–803. [Google Scholar] [CrossRef]
- Rodríguez-Ayala, E.; Avila-Díaz, M.; Foyo-Niembro, E.; Amato, D.; Ramirez-San-Juan, E.; Paniagua, R. Effect of parathyroidectomy on cardiac fibrosis and apoptosis: Possible role of aldosterone. Nephron Physiol. 2006, 103, 112–118. [Google Scholar] [CrossRef]
- Saleh, F.N.; Schirmer, H.; Sundsfjord, J.; Jorde, R. Parathyroid hormone and left ventricular hypertrophy. Eur. Heart J. 2003, 24, 2054–2060. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Liu, H.L.; Qian, G. Vitamin D, parathyroid hormone, and heart failure in a Chinese elderly population. Endocr. Pract. 2015, 21, 30–40. [Google Scholar] [CrossRef]
- Hum, J.M.; O’bryan, L.M.; Tatiparthi, A.K.; Cass, T.A.; Clinkenbeard, E.L.; Cramer, M.S.; Bhaskaran, M.; Johnson, R.L.; Wilson, J.M.; Smith, R.C.; et al. Chronic Hyperphosphatemia and Vascular Calcification Are Reduced by Stable Delivery of Soluble Klotho. J. Am. Soc. Nephrol. 2016, 28, 1162–1174. [Google Scholar] [CrossRef]
- Saito, Y.; Yamagishi, T.; Nakamuraa, T.; Ohyamaa, Y.; Aizawaa, H.; Sugaa, T.; Matsumuraab, Y.; Masudaab, H.; Kurabayashia, M.; Kuro-Ob, M.; et al. Klotho protein protects against endothelial dysfunction. Biochem. Biophys. Res. Commun. 1998, 248, 324–329. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Brinza, C.; Ozbek, L.; Guldan, M.; Sisman, U.; Copur, S.; Covic, A.; Scripcariu, D.-V.; Burlacu, A.; Covic, A. The association between klotho and kidney and cardiovascular outcomes: A comprehensive systematic review and meta-analysis. Clin. Kidney J. 2024, 17, sfae255. [Google Scholar] [CrossRef] [PubMed]
- Edmonston, D.; Fuchs, M.A.; Burke, E.J.; Isakova, T.; Wolf, M.; on behalf of the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Klotho and clinical outcomes in CKD: Findings from the chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis. 2024, 84, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 520–528. [Google Scholar] [CrossRef]
- Voormolen, N.; Noordzij, M.; Grootendorst, D.C.; Beetz, I.; Sijpkens, Y.W.; van Manen, J.G.; Boeschoten, E.W.; Huisman, R.M.; Krediet, R.T.; Dekker, F.W.; et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol. Dial. Transpl. 2007, 222, 909–916. [Google Scholar] [CrossRef]
- Tonelli, M.; Curhan, G.; Pfeffer, M.; Sacks, F.; Thadhani, R.; Melamed, M.L.; Wiebe, N.; Muntner, P. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 2009, 120, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Xu, C.; Fan, Y.; Wang, Y.; Xiao, Y.B. Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS ONE 2014, 9, e102276. [Google Scholar] [CrossRef] [PubMed]
- Maique, J.; Flores, B.; Shi, M.; Shepard, S.; Zhou, Z.; Yan, S.; Moe, O.W.; Hu, M.C. High phosphate induces and Klotho attenuates kidney epithelial senescence and fibrosis. Front. Pharmacol. 2020, 11, 1273. [Google Scholar] [CrossRef]
- Shen, Z.-J.; Hu, J.; Shiizaki, K.; Kuro-O, M.; Malter, J.S. Phosphate Induced renal fibrosis requires the prolyl isomerase Pin1. PLoS ONE 2016, 11, e01500. [Google Scholar] [CrossRef]
- Sim, J.J.; Bhandari, S.K.; Smith, N.; Chung, J.; Liu, I.L.; Jacobsen, S.J.; Kalantar-Zadeh, K. Phosphorus and risk of renal failure in subjects with normal renal function. Am. J. Med. 2013, 126, 311–318. [Google Scholar] [CrossRef]
- Zoccali, C.; Ruggenenti, P.; Perna, A.; Leonardis, D.; Tripepi, R.; Tripepi, G.; Mallamaci, F.; Remuzzi, G.; REIN Study Group. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J. Am. Soc. Nephrol. 2011, 22, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Xie, X.; Wolf, M.; Disthabanchong, S.; Wang, J.; Zha, Y.; Lv, J.; Zhang, L.; Wang, H. Serum phosphorus and progression of CKD and mortality: A meta-analysis of cohort studies. Am. J. Kidney Dis. 2015, 66, 258–265. [Google Scholar] [CrossRef]
- Barrera-Baena, P.; Rodríguez-García, M.; Rodríguez-Rubio, E.; González-Llorente, L.; Ortiz, A.; Zoccali, C.; Locatelli, F.; Floege, J.; Cohen-Solal, M.; Ferreira, M.A.; et al. Serum phosphate is associated with increased risk of bone fragility fractures in haemodialysis patients. Nephrol. Dial. Transplant. 2023, 39, 618–626. [Google Scholar] [CrossRef]
- Tentori, F.; McCullough, K.; Kilpatrick, R.D.; Bradbury, B.D.; Robinson, B.M.; Kerr, P.G.; Pisoni, R.L. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014, 85, 166–173. [Google Scholar] [CrossRef]
- Fusaro, M.; Holden, R.; Lok, C.; Iervasi, G.; Plebani, M.; Aghi, A.; Gallieni, M.; Cozzolino, M. Phosphate and bone fracture risk in chronic kidney disease patients. Nephrol. Dial. Transpl. 2021, 36, 405–412. [Google Scholar] [CrossRef]
- Meleti, Z.; Shapiro, I.M.; Adams, C.S. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone 2000, 27, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Mozar, A.; Haren, N.; Chasseraud, M.; Louvet, L.; Mazière, C.; Wattel, A.; Mentaverri, R.; Morlière, P.; Kamel, S.; Brazier, M.; et al. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J. Cell. Physiol. 2008, 215, 47–54. [Google Scholar] [CrossRef]
- Guedes, M.; Bieber, B.; Dasgupta, I.; Vega, A.; Nitta, K.; Brunelli, S.; Hartman, J.; Raimann, J.G.; Robinson, B.M.; Pisoni, R.L. Serum Phosphorus Level Rises in US Hemodialysis Patients Over the Past Decade: A DOPPS Special Report. Kidney Med. 2022, 5, 100584. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, M.K. Phosphate elimination in modalities of hemodialysis and peritoneal dialysis. Blood Purif. 2010, 29, 137–144. [Google Scholar] [CrossRef] [PubMed]
- DeSoi, C.A.; Umans, J.G. Phosphate kinetics during high-flux hemodialysis. J. Am. Soc. Nephrol. 1993, 4, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Sugisaki, H.; Onohara, M.; Kunitomo, T. Dynamic behavior of plasma phosphate in chronic dialysis patients. Trans. Am. Soc. Artif. Intern. Organs. 1982, 28, 302–307. [Google Scholar] [PubMed]
- Zucchelli, P.; Santoro, A. Inorganic phosphate removal during different dialytic procedures. Int. J. Artif. Organs. 1987, 10, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.; Hillion, D.; Dongradi, G. Phosphate kinetics in dialysis patients. Nephrol. Dial. Transplant. 1991, 6 (Suppl. 2), 108–113. [Google Scholar] [PubMed]
- Man, N.K.; Chauveau, P.; Kuno, T.; Poignet, J.L.; Yanai, M. Phosphate removal during hemodialysis, hemodiafiltration, and hemofiltration. A reappraisal. ASAIO Trans. 1991, 37, M463–M465. [Google Scholar] [PubMed]
- Daugirdas, J.T. Removal of Phosphorus by Hemodialysis. Semin. Dial. 2015, 28, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Kooienga, L. Phosphorus balance with daily dialysis. Semin. Dial. 2007, 20, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Bellizzi, V.; Cioffi, M.; Iodice, C.; Giannattasio, P.; Andreucci, M.; Terracciano, V.; Di Iorio, B.R.; Conte, G.; De Nicola, L. Postdialytic rebound of serum phosphorus: Pathogenetic and clinical insights. J. Am. Soc. Nephrol. 2002, 13, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Stremke, E.R.; Trevino, L.; Doshi, S.; Moorthi, R.N.; Gallant, K.M.H.; Moe, S.M. Post-Dialysis Serum Phosphate Equilibrium in Hemodialysis Patients on a controlled diet and no binders. Hemodial. Int. 2022, 26, 255–263. [Google Scholar] [CrossRef]
- Perl, J.; Bargman, J.M. Peritoneal dialysis: From bench to bedside and bedside to bench. Am. J. Physiol. Renal Physiol. 2016, 311, F999–F1004. [Google Scholar] [CrossRef]
- Courivaud, C.; Davenport, A. Phosphate Removal by Peritoneal Dialysis: The Effect of Transporter Status and Peritoneal Dialysis Prescription. Perit. Dial. Int. 2016, 36, 85–93. [Google Scholar] [CrossRef]
- Bammens, B.; Evenepoel, P.; Verbeke, K.; Vanrenterghem, Y. Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int. 2003, 64, 2238–2243. [Google Scholar] [CrossRef]
- Debowska, M.; Gomez, R.; Pinto, J.; Waniewski, J.; Lindholm, B. Phosphate clearance in peritoneal dialysis. Sci. Rep. 2020, 10, 17504. [Google Scholar] [CrossRef]
- Peruzzo, D.; Guedes, M.; Larkin, J.W.; Yokoyama, G.; Dos Santos, T.L.; Pecoits-Filho, R.; Ribeiro, S.C.; Ramos, A.; Barretti, P.; de Moraes, T.P.; et al. Peritoneal dialysis modality transition and impact on phosphate and potassium serum levels. PLoS ONE 2021, 16, e0257140. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef]
- Calvo, M.S.; Uribarri, J. Dietary phosphorus intake and the risk for cardiovascular disease in the general population. Adv. Chronic Kidney Dis. 2011, 18, 266–272. [Google Scholar]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate Additives in Food—a Health Risk. Dtsch. Arztebl. Int. 2012, 109, 49–55. [Google Scholar] [CrossRef]
- Dang, Z.; He, Y.; Xie, R.; Chen, P.; Dong, F. Plant-based Diet and Chronic Kidney Disease: A Systematic Review and Meta-analysis. J. Ren. Nutr. 2025, 11. [Google Scholar] [CrossRef]
- Calvo, M.S.; Park, Y.K. Changing phosphorus content of the U.S. diet: Potential for adverse effects on bone. J. Nutr. 1996, 126, 1168S–1180S. [Google Scholar] [CrossRef]
- Huml, A.M.; Sullivan, C.M.; Leon, J.B.; Sehgal, A.R. The adequacy of phosphorus binder prescriptions among American hemodialysis patients. Ren Fail. 2012, 34, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Brown-Tortorici, A.R.; Narasaki, Y.; You, A.S.; Norris, K.C.; Streja, E.; Peralta, R.A.; Guerrero, Y.; Daza, A.; Arora, R.; Lo, R.; et al. The interplay between dietary phosphorus, protein intake, and mortality in a prospective hemodialysis cohort. Nutrients 2022, 14, 3070. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Re-evaluation of phosphoric acid–phosphates—di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA 2019, 17, e05674. [Google Scholar] [CrossRef]
- Capra, B.T.; Hudson, S.; Helder, M.; Laskaridou, E.; Johnson, A.L.; Gilmore, C.; Marinik, E.; Hedrick, V.E.; Savla, J.; David, L.A.; et al. Ultra-processed food intake, gut microbiome, and glucose homeostasis in mid-life adults: Background, design, and methods of a controlled feeding trial. Contemp. Clin. Trials. 2024, 137, 107427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- FoodNavigator-USA. Until Phosphorus Gets on the USDA’s Radar, Labeling Policy Won’t Change—NKF. Available online: https://www.foodnavigator-usa.com/Article/2014/07/17/Until-phosphorus-gets-on-the-USDA-s-radar-labeling-policy-won-t-change-NKF/ (accessed on 23 March 2025).
- Nelson, S.M.; Sarabia, S.R.; Christilaw, E.; Ward, E.C.; Lynch, S.K.; Adams, M.A.; Holden, R.M. Phosphate-containing prescription medications contribute to the daily phosphate intake in a third of hemodialysis patients. J. Ren. Nutr. 2017, 27, 91–96. [Google Scholar] [CrossRef]
- Sherman, R.A.; Ravella, S.; Kapoian, T. A dearth of data: The problem of phosphorus in prescription medications. Kidney Int. 2015, 87, 1097–1099. [Google Scholar] [CrossRef]
- Moe, S.M.; Chen, N.X.; Seifert, M.F.; Sinders, R.M.; Duan, D.; Chen, X.; Liang, Y.; Radcliff, J.S.; White, K.E.; Gattone, V.H., 2nd. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009, 75, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Esmaillzadeh, A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: A crossover, randomized clinical trial. J. Ren. Nutr. 2009, 19, 479–486. [Google Scholar] [CrossRef]
- Wu, T.; Chang, C.; Hsu, W.; Wang, I.; Hsu, C.; Cheng, S.; Liang, C.; Chang, C.; Huang, C. Nutritional status of vegetarians on maintenance haemodialysis. Nephrology 2011, 16, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Torres, R.; Young, L.; Murray, D.P.; Kheda, M.; Nahman, N.S., Jr. Dietary protein source and phosphate levels in patients on hemodialysis. J. Ren. Nutr. 2020, 30, 423–429. [Google Scholar] [CrossRef]
- Kistler, B.M.; Moore, L.W.; Benner, D.; Biruete, A.; Boaz, M.; Brunori, G.; Chen, J.; Drechsler, C.; Guebre-Egziabher, F.; Hensley, M.K.; et al. The International Society of Renal Nutrition and Metabolism commentary on the National Kidney Foundation and Academy of Nutrition and Dietetics KDOQI clinical practice guideline for nutrition in chronic kidney disease. J. Ren. Nutr. 2021, 31, 116–120.e1. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D. Preventing and correcting metabolic acidosis in chronic kidney disease: Implications for bone and mineral disease. Am. J. Kidney Dis. 2013, 61, 508–516. [Google Scholar] [CrossRef]
- Goraya, N.; Wesson, D.E. Whole-Food Low-Protein Plant-Based Nutrition to Prevent or Slow Progression of Chronic Kidney Disease. J. Ren. Nutr. 2020, 30, 480–488. [Google Scholar]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, F.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Zarantonello, D.; Brunori, G. Plant-Based Diets in Preventing and Mitigating Chronic Kidney Disease. J. Clin. Med. 2023, 12, 6137. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Shah, S.; Kalantar-Zadeh, K. Adequacy of plant-based proteins in chronic kidney disease. J. Ren. Nutr. 2019, 29, 112–117. [Google Scholar] [CrossRef]
- Consultant360. Dietary Modification in Renal Disease. Available online: https://www.consultant360.com/blog/consultant360/nephrology/dietary-modification-renal-disease (accessed on 23 March 2025).
- Escoffier. The True Costs of Processed Foods on Health and the Planet. Available online: https://www.escoffier.edu/blog/world-food-drink/true-costs-processed-foods-health-planet/#:~:text=So%20although%20processed%20food%20is,And%20agricultural%20byproducts%20are%20destroyed (accessed on 23 March 2025).
- Springmann, M.; Clark, M.A.; Rayner, M.; Scarborough, P.; Webb, P. The global and regional costs of healthy and sustainable dietary patterns: A modelling study. Lancet Planet. Health 2021, 5, e797–e807. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Itokawa, Y. Cooking losses of minerals in foods and its nutritional significance. J. Nutr. Sci. Vitaminol. 1990, 36 (Suppl. 1), S25–S32, discussion S33. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Rodrigues, A.M.; Lima, M.L. Do plant-based consumers spend more on food? Front. Nutr. 2021, 8, 732129. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegan diet and food costs among adults with overweight: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 2023, 6, e2332106. [Google Scholar] [CrossRef]
- GFI. Reducing the Price of Alternative Proteins. 2022. Available online: https://gfi.org/reducing-the-price-of-alternative-proteins/ (accessed on 23 March 2025).
- Food & Wine. Why Going Vegetarian Is a Recipe for a Cheaper Grocery Bill. 2024. Available online: https://www.foodandwine.com/vegetarian-cheapest-groceries-report-11685113 (accessed on 23 March 2025).
- The Guardian. “Don’t Be Scared of Beans“: How Readers are Handling US Grocery Inflation. 2024. Available online: https://www.theguardian.com/environment/article/2024/aug/28/inflation-groceries-tips (accessed on 23 March 2025).
- Jones, W.L. Demineralization of a wide variety of foods for the renal patient. J. Ren. Nutr. 2001, 11, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Naber, T.; Purohit, S. Chronic Kidney Disease: Role of Diet for a Reduction in the Severity of the Disease. Nutrients 2021, 13, 3277. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 4299. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Bellizzi, V.; Piccoli, G.B.; Shi, Y.; Lim, S.K.; Riaz, S.; Urbina Arronte, R.; Lau, W.P.; Fouque, D. Caring for Patients with Advanced Chronic Kidney Disease: Dietary Considerations. Nutr. Clin. Pract. 2023, 38, 22–34. [Google Scholar]
- US-DOPPS Practice Monitor. 2021. Available online: http://www.dopps.org/DPM (accessed on 24 March 2025).
- Fresenius Medical Care North America. PhosLo® Gelcaps (Calcium Acetate): 667 mg [Prescribing Information]; Fresenius Medical Care North America: Waltham, MA, USA, 2011. [Google Scholar]
- Fresenius Medical Care North America. VELPHORO ® (Sucroferric Oxyhydroxide) [Prescribing Information]; Fresenius Medical Care North America: Waltham, MA, USA, 2013. [Google Scholar]
- Shire US Inc. FOSRENAL® (Lanthanum Carbonate) [Prescribing Information]; Shire US Inc.: Lexington, MA, USA, 2016. [Google Scholar]
- Keryx Biopharmaceuticals Inc. AURYXIA® (Ferric Citrate) Tablets [Prescribing Information]; Keryx Biopharmaceuticals Inc.: Cambridge, MA, USA, 2017. [Google Scholar]
- Genzyme Corp. RENVELA® (Sevelamer Carbonate) [80Prescribing Information]; Genzyme Corp.: Cambridge, MA, USA, 2020. [Google Scholar]
- Salusky, I.B.; Foley, J.; Nelson, P.; Goodman, W.G. Aluminum accumulation during treatment with aluminum hydroxide and dialysis in children and young adults with chronic renal disease. N. Engl. J. Med. 1991, 324, 527–531. [Google Scholar] [CrossRef]
- Elliott, H.L.; Dryburgh, F.; Fell, G.S.; Sabet, S.; Macdougall, A.I. Aluminium toxicity during regular haemodialysis. BMJ 1978, 1, 1101–1103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parkinson, I.S.; Ward, M.K.; Feest, T.G.; Fawcett, R.W.; Kerr, D.N. Fracturing dialysis osteodystrophy and dialysis encephalopathy: An epidemiological survey. Lancet 1979, 1, 406–409. [Google Scholar] [CrossRef]
- Young, E.W.; Albert, J.M.; Satayathum, S.; Goodkin, D.A.; Pisoni, R.L.; Akiba, T.; Akizawa, T.; Kurokawa, K.; Bommer, J.; Piera, L.; et al. Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005, 67, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42 (Suppl. 3), S1–S201. [Google Scholar] [CrossRef]
- Janssen, M.J.; van der Kuy, A.; ter Wee, P.M.; van Boven, W.P. Aluminum hydroxide, calcium carbonate and calcium acetate in chronic intermittent hemodialysis patients. Clin. Nephrol. 1996, 45, 111–119. [Google Scholar]
- Navaneethan, S.D.; Palmer, S.C.; Craig, J.C.; Elder, G.J.; Strippoli, G.F. Benefits and harms of phosphate binders in CKD: A systematic review of randomized controlled trials. Am. J. Kidney Dis. 2009, 54, 619–637. [Google Scholar] [CrossRef]
- Suki, W.N.; Zabaneh, R.; Cangiano, J.L.; Reed, J.; Fischer, D.; Garrett, L.; Ling, B.; Chasan-Taber, S.; Dillon, M.; Blair, A.; et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007, 72, 1130–1137. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Culleton, B.; Lee, H.; Klarenbach, S.; Shrive, F.; Manns, B.; Alberta Kidney Disease Network. Systematic review of the clinical efficacy and safety of sevelamer in dialysis patients. Nephrol. Dial. Transplant. 2007, 22, 2856–2866. [Google Scholar] [CrossRef]
- Block, G.A.; Wheeler, D.C.; Persky, M.S.; Kestenbaum, B.; Ketteler, M.; Spiegel, D.M.; Allison, M.A.; Asplin, J.; Smits, G.; Hoofnagle, A.N.; et al. Effects of phosphate binders in moderate CKD. J. Am. Soc. Nephrol. 2012, 23, 1407–1415. [Google Scholar] [CrossRef]
- Hill, K.M.; Martin, B.R.; Wastney, M.E.; McCabe, G.P.; Moe, S.M.; Weaver, C.M.; Peacock, M. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2013, 83, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.M.; Brady, K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012, 81, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Bellasi, A.; Russo, D. Mortality in kidney disease patients treated with phosphate binders: A randomized study. Clin. J. Am. Soc. Nephrol. 2012, 7, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Molony, D.; Bell, C.; Cucciniello, E.; Bellizzi, V.; Russo, D.; Bellasi, A. Sevelamer versus calcium carbonate in incident hemodialysis patients: Results of an open-label 24-monthandomized clinical trial. Am. J. Kidney Dis. 2013, 62, 771–778. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, J.; Wang, T.; Hu, H.; Chen, Y. A network meta-analysis of therapies for hyperphosphatemia in CKD based on randomized trials. Sci. Rep. 2025, 15, 2012. [Google Scholar] [CrossRef]
- Perry, C.M.; Plosker, G.L. Sevelamer carbonate: A review in hyperphosphataemia in adults with chronic kidney disease. Drugs 2014, 74, 771–792. [Google Scholar] [CrossRef]
- Raggi, P.; Vukicevic, S.; Moysés, R.M.; Wesseling, K.; Spiegel, D.M. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. 1), S31–S40. [Google Scholar] [CrossRef]
- Vervloet, M.G.; Sezer, S.; Massy, Z.A.; Johansson, L.; Cozzolino, M.; Fouque, D.; on behalf of the ERA–EDTA Working Group on Chronic Kidney Disease–Mineral and Bone Disorders and the European Renal Nutrition Working Group. The role of phosphate in kidney disease. Nat. Rev. Nephrol. 2017, 13, 27–38. [Google Scholar] [CrossRef]
- Lenglet, A.; Fabresse, N.; Taupin, M.; Gomila, C.; Liabeuf, S.; Kamel, S.; Alvarez, J.C.; Drueke, T.B.; Massy, Z.A. Does the administration of sevelamer or nicotinamide modify uremic toxins or endotoxemia in chronic hemodialysis patients? Drugs 2019, 79, 855–862. [Google Scholar] [CrossRef]
- Wrong, O.; Harland, C. Sevelamer. Nephrol. Dial. Transpl. 2008, 23, 2101–2102. [Google Scholar] [CrossRef]
- Pai, A.B.; Shepler, B.M. Comparison of sevelamer hydrochloride and sevelamer carbonate: Risk of metabolic acidosis and clinical implications. Pharmacotherapy 2009, 29, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Delmez, J.; Block, G.; Robertson, J.; Chasan-Taber, S.; Blair, A.; Dillon, M.; Bleyer, A. A randomized, double-blind, crossover design study of sevelamer hydrochloride and sevelamer carbonate in patients on hemodialysis. Clin. Nephrol. 2007, 68, 386–391. [Google Scholar] [CrossRef]
- Fissell, R.B.; Karaboyas, A.; Bieber, B.A.; Sen, A.; Li, Y.; Lopes, A.A.; Akiba, T.; Bommer, J.; Ethier, J.; Jadoul, M.; et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: Findings from the DOPPS. Hemodial. Int. 2016, 20, 38–49. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Scott, L.J.; Cvetković, R.S.; Plosker, G.L. Sevelamer hydrochloride: A review of its use for hyperphosphataemia in patients with end-stage renal disease on haemodialysis. Drugs 2008, 68, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.J.; Limketkai, B.N.; Liu, T.C.; Nazari, K.; Park, J.Y.; Santiago, W.C.; Torbenson, M.S.; Voltaggio, L.Y.; Martha, M.; Arnold, C.A. Sevelamer Crystals in the Gastrointestinal Tract (GIT) A New Entity Associated with Mucosal Injury. Am. J. Surg. Pathol. 2013, 37, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Elkalashy, A.; Rainwater, R.R.; Ali, U.; Elbahnasawy, E.; Singh, M.; Karakala, N. Gastrointestinal mucosal cell injury caused by sevelamer crystals—Case series and literature review. J. Renal. Nutr. 2025. [Google Scholar] [CrossRef]
- Braunlin, W.; Zhorov, E.; Guo, A.; Apruzzese, W.; Xu, Q.; Hook, P.; Smisek, D.L.; Mandeville, W.H.; Holmes-Farley, S.R. Bile acid binding to sevelamer HCl. Kidney Int. 2002, 62, 611–619. [Google Scholar] [CrossRef]
- Burke, S.K.; Dillon, M.A.; Hemken, D.E.; Rezabek, M.S.; Balwit, J.M. Meta-analysis of the effect of sevelamer on phosphorus, calcium, PTH, and serum lipids in dialysis patients. Adv. Ren. Replace. Ther. 2003, 10, 133–145. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Jaber, B.L. Potential interaction between sevelamer and fat-soluble vitamins: A hypothesis. Am. J. Kidney Dis. 2012, 59, 165–167. [Google Scholar] [CrossRef]
- Vemuri, N.; Michelis, M.F.; Matalon, A. Conversion to lanthanum carbonate monotherapy effectively controls serum phosphorus with a reduced tablet burden: A multicenter open-label study. BMC Nephrol. 2011, 12, 49. [Google Scholar] [CrossRef]
- Dellanna, F.; Reichel, H.; Seibt, F. Efficacy and safety of lanthanum carbonate in German patients on dialysis. Clin. Nephrol. 2012, 78, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Keith, M.S.; Preston, P.; Copley, J.B. The real-world dose-relativity of sevelamer hydrochloride and lanthanum carbonate monotherapy in patients with end-stage renal disease. Adv. Ther. 2013, 30, 1100–1110. [Google Scholar] [CrossRef]
- Takahara, Y.; Matsuda, Y.; Takahashi, S.; Shigematsu, T.; on behalf of the Lanthanum Carbonate Study Group. Effcacy and safety of lanthanum carbonate in pre-dialysis CKD patients with hyperphosphatemia: A randomized trial. Clin. Nephrol. 2014, 82, 181–190. [Google Scholar] [CrossRef]
- Toida, T.; Fukudome, K.; Fujimoto, S.; Yamada, K.; Sato, Y.; Chiyotanda, S.; Kitamura, K. Effect of lanthanum carbonate vs. calcium carbonate on serum calcium in hemodialysis patients: A crossover study. Clin. Nephrol. 2012, 78, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, J.; Li, Z.; Fan, J. Efficacy and safety of lanthanum carbonate on chronic kidney disease mineral and bone disorder in dialysis patients: A systematic review. BMC Nephrol. 2013, 14, 226. [Google Scholar] [CrossRef]
- Lacour, B.; Lucas, A.; Auchere, D.; Ruellan, N.; De Serre Patey, N.M.; Drüeke, T.B. Chronic renal failure is associated with increased tissue deposition of lanthanum after 28-day oral administration. Kidney Int. 2005, 67, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.J.; Barnett, M.E.; Krause, R.; Siami, G.A. Lanthanum carbon- ate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease stage 5 receiving hemodialysis. Clin. Nephrol. 2009, 71, 286–295. [Google Scholar]
- Spasovski, G.B.; Sikole, A.; Gelev, S.; Masin-Spasovska, J.; Freemont, T.; Webster, I.; Gill, M.; Jones, C.; De Broe, M.E.; D’Haese, P.C. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol. Dial. Transplant 2006, 21, 2217–2224. [Google Scholar] [CrossRef]
- Hutchison, A.J.; Wilson, R.J.; Garafola, S.; Copley, J.B. Lanthanum carbonate: Safety data after 10 years. Nephrology 2016, 21, 987–994. [Google Scholar] [CrossRef]
- Lewis, J.B.; Sika, M.; Koury, M.J.; Chuang, P.; Schulman, G.; Smith, M.T.; Whittier, F.C.; Linfert, D.R.; Galphin, C.M.; Athreya, B.P.; et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J. Am. Soc. Nephrol. 2015, 26, 493–503. [Google Scholar] [CrossRef]
- Umanath, K.; Jalal, D.I.; Greco, B.A.; Umeukeje, E.M.; Reisin, E.; Manley, J.; Zeig, S.; Negoi, D.G.; Hiremath, A.N.; Blumenthal, S.S.; et al. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J. Am. Soc. Nephrol. 2015, 26, 2578–2587. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Hirakata, H.; Akiba, T.; Fukagawa, M.; Nakayama, M.; Sawada, K.; Kumagai, Y.; Block, G.A. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 543–552. [Google Scholar] [CrossRef]
- Van Buren, P.N.; Lewis, J.B.; Dwyer, J.P.; Greene, T.; Middleton, J.; Sika, M.; Umanath, K.; Abraham, J.D.; Arfeen, S.S.; Bowline, I.G.; et al. Collaborative Study Group. The Phosphate Binder Ferric Citrate and Mineral Metabolism and Inflammatory Markers in Maintenance Dialysis Patients: Results from Prespecified Analyses of a Randomized Clinical Trial. Am. J. Kidney Dis. 2015, 66, 479–488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Floege, J. Phosphate binders in chronic kidney disease: A systematic review of recent data. J. Nephrol. 2016, 29, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Funk, F.; Rakov, V.; Phan, O.; Teitelbaum, I. Preclinical pharmacokinetics, pharmacodynamics and safety of sucroferric oxyhydroxide. Curr. Drug Metab. 2014, 15, 953–965. [Google Scholar] [CrossRef]
- Floege, J.; Covic, A.C.; Ketteler, M.; Mann, J.F.; Rastogi, A.; Spinowitz, B.; Chong, E.M.; Gaillard, S.; Lisk, L.J.; Sprague, S.M.; et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol. Dial. Transplant. 2015, 30, 1037–1046. [Google Scholar] [CrossRef]
- Floege, J.; Covic, A.C.; Ketteler, M.; Rastogi, A.; Chong, E.M.; Gaillard, S.; Lisk, L.J.; Sprague, S.M. A phase III study of the efficacy and safety of a novel iron based phosphate binder in dialysis patients. Kidney Int. 2014, 86, 638–647. [Google Scholar] [CrossRef]
- Ketteler, M.; Sprague, S.M.; Covic, A.C.; Rastogi, A.; Spinowitz, B.; Rakov, V.; Walpen, S.; Floege, J. Effects of sucroferric oxyhydroxide and sevelamer carbonate on chronic kidney disease-mineral bone disorder parameters in dialysis patients. Nephrol. Dial. Transpl. 2019, 34, 1163–1170. [Google Scholar] [CrossRef]
- Covic, A.C.; Floege, J.; Ketteler, M.; Sprague, S.M.; Lisk, L.; Rakov, V.; Rastogi, A. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol. Dial. Transpl. 2017, 32, 1330–1338. [Google Scholar] [CrossRef][Green Version]
- Lioulios, G.; Stangou, M.; Sarafidis, P.A.; Tsouchnikas, I.; Minasidis, I.; Vainas, A.; Faitatzidou, D.; Sampani, E.; Papagianni, A. Chronic therapy with sucroferric oxyhydroxide does not affect iron and anemia markers in dialysis patients. Blood Purif. 2020, 49, 440–447. [Google Scholar] [CrossRef]
- Schiavi, S.C.; Tang, W.; Bracken, C.; O’brien, S.P.; Song, W.; Boulanger, J.; Ryan, S.; Phillips, L.; Liu, S.; Arbeeny, C.; et al. Npt2b deletion attenuates hyperphosphatemia associated with CKD. J. Am. Soc. Nephrol. 2012, 23, 1691–1700. [Google Scholar] [CrossRef]
- Müller, D.; Mehling, H.; Otto, B.; Bergmann-Lips, R.; Luft, F.; Jordan, J.; Kettritz, R.; Müller, D. Niacin lowers serum phosphate and increases HDL cholesterol in dialysis patients. Clin. J. Am. Soc. Nephrol. 2007, 2, 1249. [Google Scholar] [PubMed]
- Cheng, S.C.; Young, D.O.; Huang, Y.; Delmez, J.A.; Coyne, D.W. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 1131. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Isakova, T.; Larive, B.; Raphael, K.L.; Raj, D.S.; Cheung, A.K.; Sprague, S.M.; Fried, L.F.; Gassman, J.J.; Middleton, J.P.; et al. Effects of Nicotinamide and Lanthanum Carbonate on Serum Phosphate and Fibroblast Growth Factor-23 in CKD: The COMBINE Trial. J. Am. Soc. Nephrol. 2019, 30, 1096. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Wiecek, A.; Rosenkranz, A.R.; Ose, C.; Rekowski, J.; Lorenz, H.; Hellmann, B.; Karus, M.; Ruhmann, M.; Ammer, R. Modified-release nicotinamide for the treatment of hyperphosphataemia in haemodialysis patients: 52-week efficacy and safety results of the phase 3 randomized controlled NOPHOS trial. Nephrol. Dial. Transplant. 2023, 38, 982. [Google Scholar] [CrossRef]
- Gallant, K.M.H.; Sprague, S.M.; Rosenbaum, D.P.; Spiegel, D.M.; Kozuka, K.; Edelstein, S.; Chertow, G.M. Tenapanor: A phosphate absorption inhibitor for the management of hyperphosphatemia in patients with kidney failure. J. Ren. Nutr. 2025, 35, 25–34. [Google Scholar] [CrossRef]
- Pergola, P.E.; Rosenbaum, D.P.; Yang, Y.; Chertow, G.M. A randomized trial of tenapanor and phosphate binders as a dual-mechanism treatment for hyperphosphatemia in patients on maintenance dialysis (AMPLIFY). J. Am. Soc. Nephrol. 2021, 32, 1465–1473. [Google Scholar] [CrossRef]
- Clinical Data Supporting XPHOZAH. Available online: https://ir.ardelyx.com/news-releases/news-release-details/fda-approves-xphozahr-tenapanor-first-class-phosphate-absorption#:~:text=FDA%20approval%20of%20XPHOZAH%20is,met%20their%20primary%20and%20key (accessed on 24 March 2025).
- Chiu, Y.W.; Teitelbaum, I.; Misra, M.; de Leon, E.M.; Adzize, T.; Mehrotra, R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1089–1096. [Google Scholar] [CrossRef]
- Nagano, N.; Ito, K.; Ono, T.; Ariyoshi, Y.; Masima, S.; Kobayashi, H.; Ando, T.; Tsutsui, T.; Ogawa, T. Prescription characteristics of phosphate binders in a high pill burden for hemodialysis patients. Ren. Replace. Ther. 2021, 7, 5. [Google Scholar] [CrossRef]
- Forfang, D.; Edwards, D.P.; Kalantar-Zadeh, K. The Impact of Phosphorus Management Today on Quality of Life: Patient Perspectives. Kidney Med. 2022, 4, 100437. [Google Scholar] [CrossRef]
- Edmonston, D.L.; Isakova, T.; Dember, L.M.; Brunelli, S.; Young, A.; Brosch, R.; Beddhu Chakraborty, H.; Wolf, M. Design and Rationale of HiLo: A Pragmatic, Randomized Trial of Phosphate Management for Patients on Maintenance Hemodialysis. Am. J. Kidney Dis. 2020, 77, 920–930.e1. [Google Scholar] [CrossRef] [PubMed]
- Wald, R.; Walsh, M.W. In Search of the Optimal Target for Phosphate Control: Episode 1. J. Am. Soc. Nephrol. 2021, 32, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Greg, L.P.; Hedayati, S.S. Management of Traditional Cardiovascular Risk Factors in CKD: What Are the Data? Am. J. Kidney Dis. 2018, 72, 728–744. [Google Scholar] [CrossRef] [PubMed]
| Dietary Interventions | Comments |
| Limit processed meats, processed cheese, and dairy products | Contain highly bioavailable (80–100%) inorganic phosphates |
| Choose fresh meat or fish without added phosphates | Contain organic phosphates of intermediate bioavailability (40–60%) |
| Choose plant based food (Like legumes, soy products, nuts, whole grains) | Contains organic phosphate complexed with phytates. Has low bioavailability (20–40%) |
| Be aware of hidden phosphates in prescription/ over-the counter medicines and supplements | May contain considerable amount of highly bioavailable (80–100%) inorganic phosphates |
| Reduction of Intestinal Phosphate Absorption | |
| Phosphate Binders | Comments |
| Calcium acetate: Daily dose 1334–2001 mg) | High pill burden. Risk of calcium load and vascular calcification. Cheap |
| Calcium carbonate: Daily dose 1250–3750 mg) | High pill burden. Risk of calcium load and vascular calcification. Cheap |
| Sevelamer hydrochloride and sevelamer carbonate: Daily dose 2400–9600 mg | High pill burden. Gastrointestinal side effects. More expensive than Ca-based binders |
| Lanthanum carbonate: Daily dose 1500 mg | Low pill burden. Chewable tablets or powder preparations. Gastrointestinal side effects. No evidence of hepatotoxicity |
| Sucroferric oxyhydroxide: Daily dose 7.5–15 g | Low pill burden. Chewable. Low iron absorption. Primary gastrointestinal side effects. |
| Ferric citrate: Daily dose 630–1260 mg | Low pill burden. High iron absorption. May help with anemia management. Primary gastrointestinal side effects. Risk of aluminum toxicity as citrate increases absorption of aluminum |
| Intestinal Phosphate Transport Inhibitors | Comments |
| Nicotinamide | Blocks small intestinal active transport of phosphate via NaPi-IIb. Limited efficacy and poor tolerance due to side effects (Diarrhea, pruritus, and thrombocytopenia). Not recommended for hyperphosphatemia management |
| Tenapanor: Dose: 10–30 mg twice a day | Inhibits small intestinal paracellular transport of phosphate by blocking sodium/hydrogen exchanger 3. Used as an add-on therapy with phosphate binders. The major adverse effect is an increase in stool frequency and diarrhea |
| Removal of Phosphate by Dialysis | |
| Dialysis Modality | Comments |
| In-center hemodialysis 3-times a week | Inadequate in removing daily phosphate load. Patients need additional measures (diet and phosphate lowering agents) to manage hyperphosphatemia |
| Short-daily or nocturnal hemodialysis | Much better in controlling hyperphosphatemia |
| Peritoneal dialysis | Inadequate in removing daily phosphate load. Patients need additional measures (diet and phosphate-lowering agents) to manage hyperphosphatemia |
| Sources of Phosphate | Nature of Phosphate | Bioavailability | Examples |
|---|---|---|---|
| Plant-based foods | Organic Phosphates Complexed With Phytates | 20–40% | Nuts and seeds Legumes Whole grain Leafy greens Soy products |
| Animal-based foods | Organic Phosphates | 40–60% | Chicken/Poultry Red meat Fish/ sea food Milk/dairy products |
| Preservatives/additives | Inorganic Phosphates | 80–100% | Soft drinks Processed foods Canned foods |
| Medicines/supplements | Inorganic Phosphates | 80–100% | OTC * multivitamins/supplements Prescription medicines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, S.; Saxena, R. Hyperphosphatemia in Kidney Failure: Pathophysiology, Challenges, and Critical Role of Phosphorus Management. Nutrients 2025, 17, 1587. https://doi.org/10.3390/nu17091587
Raju S, Saxena R. Hyperphosphatemia in Kidney Failure: Pathophysiology, Challenges, and Critical Role of Phosphorus Management. Nutrients. 2025; 17(9):1587. https://doi.org/10.3390/nu17091587
Chicago/Turabian StyleRaju, Swetha, and Ramesh Saxena. 2025. "Hyperphosphatemia in Kidney Failure: Pathophysiology, Challenges, and Critical Role of Phosphorus Management" Nutrients 17, no. 9: 1587. https://doi.org/10.3390/nu17091587
APA StyleRaju, S., & Saxena, R. (2025). Hyperphosphatemia in Kidney Failure: Pathophysiology, Challenges, and Critical Role of Phosphorus Management. Nutrients, 17(9), 1587. https://doi.org/10.3390/nu17091587






