Relationship Between Maternal Iron Indices in the Second Trimester with Cord Blood Iron Indices and Pregnancy Outcomes: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size
2.4. Study Procedure
2.5. Laboratory Investigation
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. The Main Findings
4.2. Clinical Implications
4.3. Strengths, Limitations, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Sharma, E.; Marley, A.; Samaan, M.A.; Brookes, M.J. Iron Deficiency Anaemia: Pathophysiology, Assessment, Practical Management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.B.; Patel, N.; Hedges, M.A.; Benson, A.E.; Tomer, A.; Lo, J.O. Hematologic complications of pregnancy. Eur. J. Haematol. 2025, 114, 596–614. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Maršál, K. Intrauterine growth restriction. Curr. Opin. Obstet. Gynecol. 2002, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Clark, P.M. Fetal Undernutrition and Disease in Later Life. Rev. Reprod. 1997, 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Abe, S.K.; Rahman, M.S.; Kanda, M.; Narita, S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal Anemia and Risk of Adverse Birth and Health Outcomes in Low- and Middle-Income Countries: Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2016, 103, 495–504. [Google Scholar] [CrossRef]
- Rios, E.; Lipschitz, D.A.; Cook, J.D.; Smith, N.J. Relationship of maternal and infant iron stores as assessed by determination of plasma ferritin. Pediatrics 1975, 55, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, H.G.; Kroos, M.J.; Hoogendoorn, G.A.; Wallenburg, H.C.S. Serum Ferritin and Iron Stores during Pregnancy. Clin. Chim. Acta 1978, 83, 81–91. [Google Scholar] [CrossRef]
- Sachdev, H.; Gera, T.; Nestel, P. Effect of Iron Supplementation on Mental and Motor Development in Children: Systematic Review of Randomised Controlled Trials. Public Health Nutr. 2005, 8, 117–132. [Google Scholar] [CrossRef]
- Tamura, T.; Goldenberg, R.L.; Hou, J.; Johnston, K.E.; Cliver, S.P.; Ramey, S.L.; Nelson, K.G. Cord Serum Ferritin Concentrations and Mental and Psychomotor Development of Children at Five Years of Age. J. Pediatr. 2002, 140, 165–170. [Google Scholar] [CrossRef]
- Raffaeli, G.; Manzoni, F.; Cortesi, V.; Cavallaro, G.; Mosca, F.; Ghirardello, S. Iron Homeostasis Disruption and Oxidative Stress in Preterm Newborns. Nutrients 2020, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Lelic, M.; Bogdanovic, G.; Ramic, S.; Brkicevic, E. Influence of Maternal Anemia During Pregnancy on Placenta and Newborns. Med. Arch. 2014, 68, 184. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; Macdonald, D.J.; McDougall, A.N. Observations on Maternal and Fetal Ferritin Concentrations at Term. Br. J. Obstet. Gynaecol. 1978, 85, 338–343. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, A.P.; Charlton, R.W.; Bothwell, T.H.; Torrance, J.D. The relationship between maternal and infant iron status. Scand. J. Haematol. 1981, 25, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.; Hoffman, M.; Goudar, S.; Patel, A.; Saleem, S.; Ali, S.; Goldenberg, R.; Hibberd, P.; Moore, J.; Wallace, D.; et al. Maternal Anaemia and Maternal, Fetal, and Neonatal Outcomes in a Prospective Cohort Study in India and Pakistan. Br. J. Obstet. Gynaecol. 2019, 126, 737–743. [Google Scholar] [CrossRef]
- Yi, S.-W.; Han, Y.-J.; Ohrr, H. Anemia before Pregnancy and Risk of Preterm Birth, Low Birth Weight and Small-for-Gestational-Age Birth in Korean Women. Eur. J. Clin. Nutr. 2013, 67, 337–342. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily Oral Iron Supplementation during Pregnancy. Cochrane Database Syst. Rev. 2015, 7, 1–373. [Google Scholar] [CrossRef]
- Dewey, K.G.; Oaks, B.M. U-Shaped Curve for Risk Associated with Maternal Hemoglobin, Iron Status, or Iron Supplementation. Am. J. Clin. Nutr. 2017, 106, 1694S–1702S. [Google Scholar] [CrossRef]
- Derman, R.J.; Goudar, S.S.; Thind, S.; Bhandari, S.; Aghai, Z.; Auerbach, M.; Boelig, R.; Charantimath, U.S. RAPIDIRON: Reducing Anaemia in Pregnancy in India—A 3-Arm, Randomized-Controlled Trial Comparing the Effectiveness of Oral Iron with Single-Dose Intravenous Iron in the Treatment of Iron Deficiency Anaemia in Pregnant Women and Reducing Low Birth Weight Deliveries. Trials 2021, 22, 649. [Google Scholar] [CrossRef]
- Mangla, M.; Singla, D. Prevalence of Anaemia among Pregnant Women in Rural India: A Longitudinal Observational Study. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 3500–3505. [Google Scholar] [CrossRef]
- Bernhardt, G.V.; Jhancy, M.; Shivappa, P.; Bernhardt, K.; Pinto, J.R. Relationship between maternal and cord blood iron status in women and their new born pairs. Biomed. Pharmacol. J. 2021, 14, 317–322. [Google Scholar] [CrossRef]
- Swetha, K.; Tarakeswararao, P.; Saisunilkishore, M. Relationship between Maternal Iron and Cord Blood Iron Status: A Prospective Study. Indian J. Child Health. 2017, 4, 595–598. [Google Scholar] [CrossRef]
- Lee, S.; Guillet, R.; Cooper, E.M.; Westerman, M.; Orlando, M.; Kent, T.; Pressman, E.; O’Brien, K.O. Prevalence of Anemia and Associations between Neonatal Iron Status, Hepcidin, and Maternal Iron Status among Neonates Born to Pregnant Adolescents. Pediatr. Res. 2016, 79, 42–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Li, H.; Jin, L.; Zhang, Y.; Zhang, L.; Liu, J.; Ye, R.; Liu, J.; Ren, A. Maternal Haemoglobin Concentration and Risk of Preterm Birth in a Chinese Population. J. Obstet. Gynaecol. 2018, 38, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ananth, C.V.; Li, Z.; Smulian, J.C. Maternal Anaemia and Preterm Birth: A Prospective Cohort Study. Int. J. Epidemiol. 2009, 38, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, K. High and Low Hemoglobin Levels during Pregnancy: Differential Risks for Preterm Birth and Small for Gestational Age. Obstet. Gynecol. 2000, 96, 741–748. [Google Scholar] [CrossRef]
- Smith, C.; Teng, F.; Branch, E.; Chu, S.; Joseph, K.S. Maternal and Perinatal Morbidity and Mortality Associated with Anemia in Pregnancy. Obstet. Gynecol. 2019, 134, 1234–1244. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Zhang, B.; Kang, Y.; Cheng, Y.; Zeng, L.; Chen, F.; Mi, B.; Qu, P.; Zhao, D.; et al. Maternal Hemoglobin Concentrations and Birth Weight, Low Birth Weight (LBW), and Small for Gestational Age (SGA): Findings from a Prospective Study in Northwest China. Nutrients 2022, 14, 858. [Google Scholar] [CrossRef]
- Steer, P.J. Maternal Hemoglobin Concentration and Birth Weight. Am. J. Clin. Nutr. 2000, 71, 1285S–1287S. [Google Scholar] [CrossRef]
- Zondervan, H.A.; Voorhorst, F.J.; Robertson, E.A.; Kurver, P.H.J.; Massen, C. Is Maternal Whole Blood Viscosity a Factor in Fetal Growth? Eur. J. Obstet. Gynecol. Reprod. Biol. 1985, 20, 145–151. [Google Scholar] [CrossRef]
- Zondervan, H.A.; Oosting, J.; Hardeman, M.R.; Smorenberg-schoorl, M.E.; Treffers, P.E. The Influence of Maternal Whole Blood Viscosity on Fetal Growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 1987, 25, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Tikmani, S.S.; Saleem, S.; Patel, A.B.; Hibberd, P.L.; Goudar, S.S.; Dhaded, S.; Derman, R.J.; Moore, J.L.; McClure, E.M.; et al. Hemoglobin Concentrations and Adverse Birth Outcomes in South Asian Pregnant Women: Findings from a Prospective Maternal and Neonatal Health Registry. Reprod. Health 2020, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.; Siraj, M.d.S.; Islam, M.R.; Rahman, A.; Ekström, E.-C. Association between Maternal Plasma Ferritin Level and Infants’ Size at Birth: A Prospective Cohort Study in Rural Bangladesh. Glob. Health Action 2021, 14, 1870421. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.G.; Li, L.; Lee, S.J.; Hu, Y.H.; Kim, C.; Hwang, J.Y. Serum Ferritin Concentration in the Early Third Trimester of Pregnancy and Risk of Preterm Birth and Low Birth Weight Based on Gestational Age. J. Korean Soc. Matern. Child Health 2021, 25, 55–62. [Google Scholar] [CrossRef]
- Xiao, R.; Sorensen, T.K.; Frederick, I.O.; El-Bastawissi, A.; King, I.B.; Leisenring, W.M.; Williams, M.A. Maternal Second-trimester Serum Ferritin Concentrations and Subsequent Risk of Preterm Delivery. Paediatr. Perinat. Epidemiol. 2002, 16, 297–304. [Google Scholar] [CrossRef]
- Tao, Y.; Kang, J.; Liu, J.; Duan, J.; Wang, F.; Shi, Y.; Li, Y.; Wang, C.; Xu, D.; Qu, X.; et al. Association of Low Birthweight and Small for Gestational Age with Maternal Ferritin Levels: A Retrospective Cohort Study in China. Front. Nutr. 2022, 9, 1002702. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Tamura, T.; DuBard, M.; Johnston, K.E.; Copper, R.L.; Neggers, Y. Plasma Ferritin and Pregnancy Outcome. Am. J. Obstet. Gynecol. 1996, 175, 1356–1359. [Google Scholar] [CrossRef]
- Lao, T.T. Third Trimester Iron Status and Pregnancy Outcome in Non-Anaemic Women; Pregnancy Unfavourably Affected by Maternal Iron Excess. Hum. Reprod. 2000, 15, 1843–1848. [Google Scholar] [CrossRef]
- Iglesias Vázquez, L.; Arija, V.; Aranda, N.; Aparicio, E.; Serrat, N.; Fargas, F.; Ruiz, F.; Pallejà, M.; Coronel, P.; Gimeno, M.; et al. The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study. Nutrients 2019, 11, 2418. [Google Scholar] [CrossRef]
- Oaks, B.M.; Jorgensen, J.M.; Baldiviez, L.M.; Adu-Afarwuah, S.; Maleta, K.; Okronipa, H.; Sadalaki, J.; Lartey, A.; Ashorn, P.; Ashorn, U.; et al. Prenatal Iron Deficiency and Replete Iron Status Are Associated with Adverse Birth Outcomes, but Associations Differ in Ghana and Malawi. J. Nutr. 2019, 149, 513–521. [Google Scholar] [CrossRef]
- Okwara, J.E.; Nnabuo, L.C.; Nwosu, D.C.; Ahaneku, J.E.; Anolue, F.; Okwara, N.A.; Amah, U.K.; Meludu, S.C.; Dioka, C.E.; Okwara, E.C.; et al. Iron Status of Some Pregnant Women in Orlu Town-Eastern Nigeria. Niger. J. Med. 2013, 22, 15–18. [Google Scholar] [PubMed]
- Günther, F.; Straub, R.H.; Hartung, W.; Fleck, M.; Ehrenstein, B.; Schminke, L. Usefulness of Soluble Transferrin Receptor in the Diagnosis of Iron Deficiency Anemia in Rheumatoid Arthritis Patients in Clinical Practice. Int. J. Rheumatol. 2022, 2022, 7067262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Haas, J. Response of Serum Transferrin Receptor to Iron Supplementation in Iron-Depleted, Nonanemic Women. Am. J. Clin. Nutr. 1998, 67, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Næss-Andresen, M.-L.; Jenum, A.K.; Berg, J.P.; Falk, R.S.; Sletner, L. The Impact of Recommending Iron Supplements to Women with Depleted Iron Stores in Early Pregnancy on Use of Supplements, and Factors Associated with Changes in Iron Status from Early Pregnancy to Postpartum in a Multi-Ethnic Population-Based Cohort. BMC Pregnancy Childbirth 2023, 23, 350. [Google Scholar] [CrossRef] [PubMed]
- Kohli, U.A.; Rajput, M.; Venkatesan, S. Association of Maternal Hemoglobin and Iron Stores with Neonatal Hemoglobin and Iron Stores. Med. J. Armed Forces India 2021, 77, 158–164. [Google Scholar] [CrossRef]
- Shukla, A.; Srivastava, S.; Verma, G. Effect of Maternal Anemia on the Status of Iron Stores in Infants: A Cohort Study. J. Fam. Community Med. 2019, 26, 118. [Google Scholar] [CrossRef]
- Sharma, J.B.; Soni, D.; Murthy, N.S.; Malhotra, M. Effect of dietary habits on prevalence of anemia in pregnant women of Delhi. J. Obstet. Gynaecol. Res. 2003, 29, 73–78. [Google Scholar] [CrossRef]
- Avnon, T.; Anbar, R.; Lavie, I.; Ben-Mayor Bashi, T.; Paz Dubinsky, E.; Shaham, S.; Yogev, Y. Does vegan diet influence umbilical cord vitamin B12, folate, and ferritin levels? Arch. Gynecol. Obstet. 2020, 301, 1417–1422. [Google Scholar] [CrossRef]
| Maternal and Newborn Characteristics | Mean ± SD/Median (Q1, Q3)/n (%) | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age (years) | 22 (20, 26) | |
| Maternal Hb (at delivery) (g/dL) | 12.21 (11.40, 12.72) | |
| Gestational age (at delivery) | Term birth | 268 (91.78) |
| Preterm birth | 24 (8.22) | |
| Mode of delivery | Vaginal delivery | 172 (58.90) |
| C-section | 120 (41.10) | |
| Newborn characteristics | ||
| Gender | Male | 149 (51.03) |
| Female | 143 (48.97) | |
| Birth weight | Normal birth weight | 202 (69.18) |
| Low birth weight | 90 (30.82) | |
| Cord blood RBC indices | ||
| MCV (fL) | 107.63 ± 6.42 | |
| MCH (pg/cell) | 34.50 (33.40, 35.60) | |
| MCHC (g/dL) | 31.90 (31.40, 32.50) | |
| IRF (%) | 29.10 (23.90, 33.30) | |
| Ret-Hb (pg) | 32.30 (31.10, 33.30) | |
| Pregnancy complications | ||
| Prolonged labor | 48 (16.44) | |
| Antepartum hemorrhage | 6 (2.05) | |
| Severe postpartum hemorrhage | 1 (0.34) | |
| Eclampsia | 16 (5.48) | |
| Maternal anemia | 47 (16.2) | |
| Stillbirths | 4 (1.37) | |
| Newborns admitted to NICU | 15 (5.14) | |
| Pregnancy Outcome | Maternal Hb (g/dL) | Interaction F-Value (p-Value) | Within-Group F-Statistic | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| n | 12–16 GA | 20–24 GA | 26–30 GA | |||||
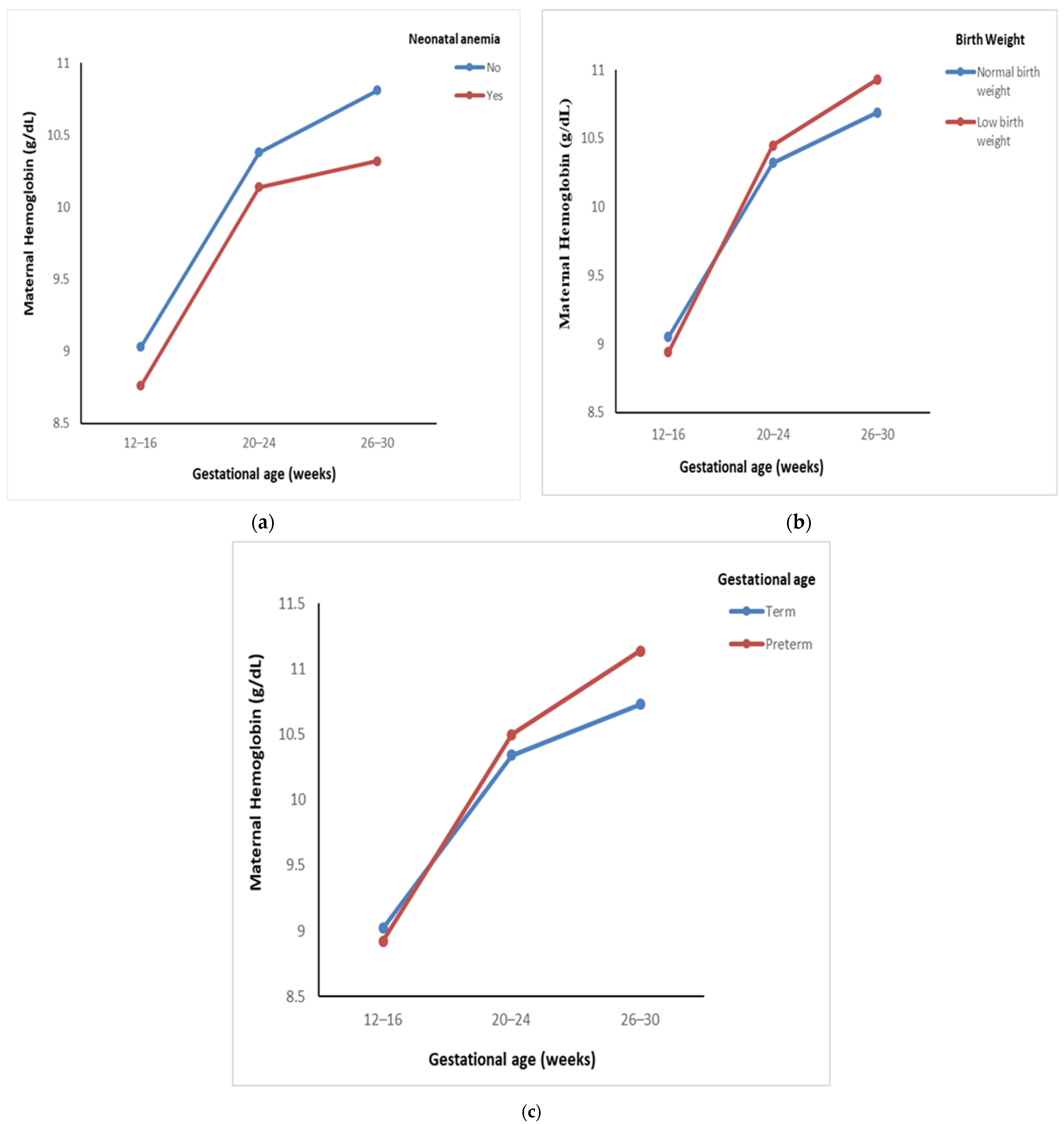
| Neonatal anemia | Yes | 27 | 8.76 ± 0.79 | 10.14 ± 1.03 | 10.32 ± 1.13 | 0.62 $ | 19.59 | <0.001 * |
| No | 253 | 9.03 ± 0.78 | 10.38 ± 1.02 | 10.81 ± 1.26 | (0.49) | 203.46 | <0.001 * | |
| Low birth weight | Yes | 97 | 8.94 ± 0.75 | 10.45 ± 1.11 | 10.93 ± 1.39 | 3.28 $ | 100.77 | <0.001 * |
| No | 218 | 9.05 ± 0.78 | 10.32 ± 0.99 | 10.69 ± 1.18 | (0.05) | 155.71 | <0.001 * | |
| Preterm birth | Yes | 28 | 8.92 ± 0.81 | 10.50 ± 1.07 | 11.14 ± 1.31 | 2.52 $ | 35.00 | <0.001 * |
| No | 287 | 9.02 ± 0.77 | 10.34 ± 1.03 | 10.73 ± 1.24 | (0.10) | 220.26 | <0.001 * | |
| Pregnancy Outcome | Maternal TSAT (%) | Interaction F-Value (p-Value) | Within-Group F-Statistic | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| n | 12–16 GA | 20–24 GA | 26–30 GA | |||||
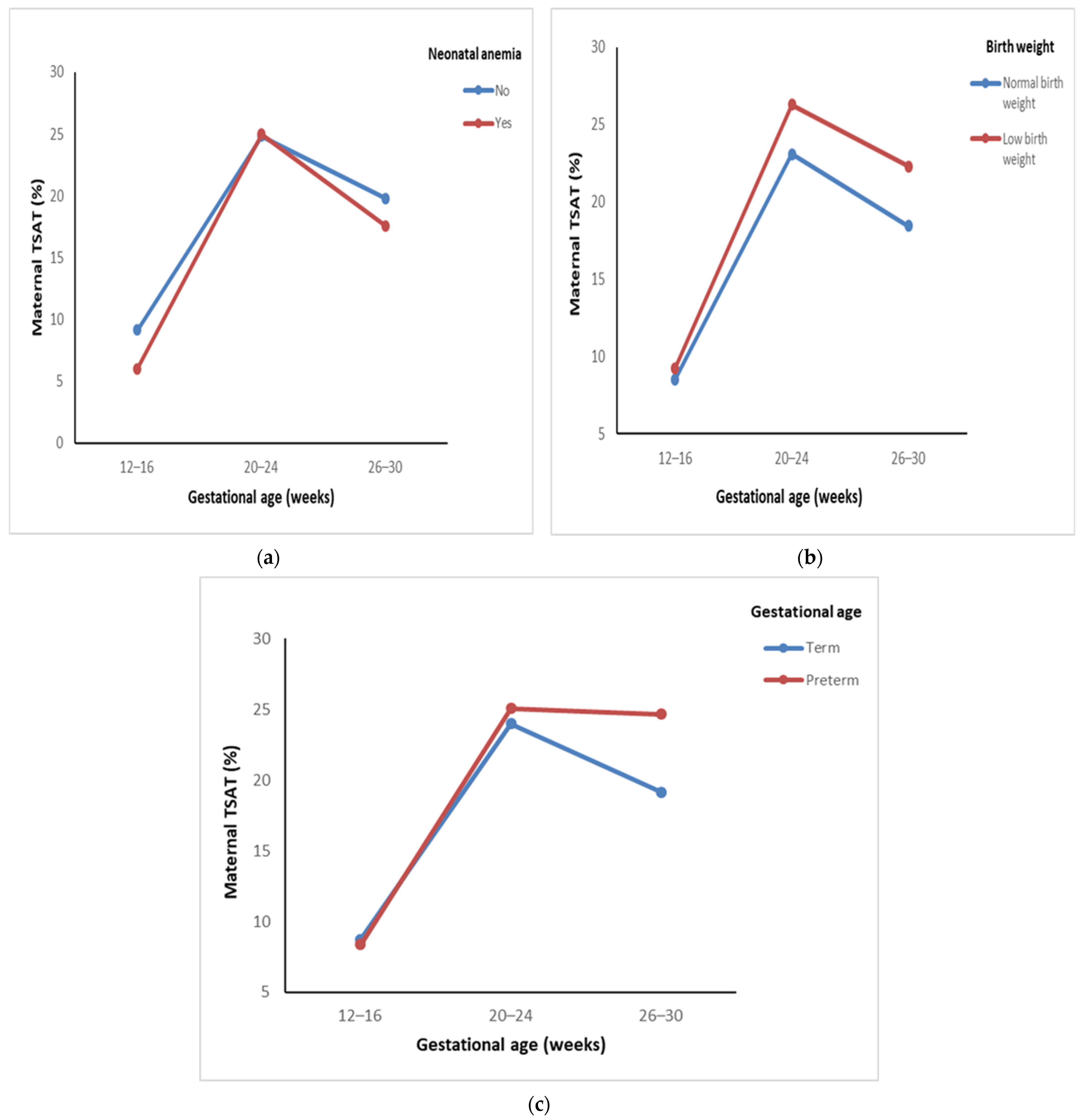
| Neonatal anemia | Yes | 27 | 6.02 ± 2.66 | 24.99 ± 16.96 | 17.57 ± 11.92 | 0.37 $ | 12.77 | <0.001 * |
| No | 253 | 9.14 ± 10.14 | 24.83 ± 19.48 | 19.79 ± 15.14 | (0.68) | 87.71 | <0.001 * | |
| Low birth weight | Yes | 97 | 9.24 ± 12.26 | 26.27 ± 25.13 | 22.30 ± 17.94 | 1.03 $ | 47.48 | <0.001 * |
| No | 218 | 8.52 ± 7.69 | 23.09 ± 14.75 | 18.44 ± 13.02 | (0.36) | 70.90 | <0.001 * | |
| Preterm birth | Yes | 28 | 8.36 ± 7.80 | 25.07 ± 15.75 | 24.67 ± 19.96 | 1.37 $ | 17.21 | <0.001 * |
| No | 287 | 8.78 ± 9.47 | 23.97 ± 18.86 | 19.14 ± 14.14 | (0.26) | 101.32 | <0.001 * | |
| Pregnancy Outcome | Maternal Ferritin (ng/mL) | Interaction F-Value (p-Value) | Within-Group F-Statistic | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| n | 12–16 GA | 20–24 GA | 26–30 GA | |||||
| Neonatal anemia | Yes | 27 | 8.86 ± 8.49 | 31.52 ± 16.47 | 30.51 ± 28.54 | 0.10 | 6.68 | 0.001 * |
| No | 253 | 11.15 ± 15.06 | 36.87 ± 38.72 | 35.44 ± 34.28 | (0.90) | 79.63 | <0.001 * | |
| Low birth weight | Yes | 97 | 12.93 ± 18.77 | 39.86 ± 49.53 | 36.52 ± 29.82 | 0.32 | 31.00 | <0.001 * |
| No | 218 | 11.62 ± 21.09 | 35.03 ± 30.23 | 33.51 ± 33.70 | (0.72) | 55.70 | <0.001 * | |
| Preterm birth | Yes | 28 | 15.64 ± 21.50 | 51.67 ± 83.26 | 40.71 ± 37.44 | 1.71 | 14.08 | <0.001 * |
| No | 287 | 11.67 ± 20.28 | 35.04 ± 29.03 | 33.83 ± 32.03 | (0.18) | 74.30 | <0.001 * | |
| Pregnancy Outcome | Maternal sTfR (µg/mL) | Interaction F-Value (p-Value) | Within-Group F-Statistic | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | 12–16 GA | 26–30 GA | |||||
| Neonatal anemia | Yes | 13 | 7.72 ± 1.33 | 5.87 ± 0.81 | 0.04 $ | 14.68 | 0.001 * |
| No | 91 | 7.51 ± 1.61 | 5.76 ± 1.11 | (0.85) | 91.89 | <0.001 * | |
| Low birth weight | Yes | 34 | 7.41 ± 1.50 | 5.79 ± 1.18 | 0.33 $ | 29.81 | <0.001 * |
| No | 71 | 7.62 ± 1.62 | 5.79 ± 1.04 | (0.57) | 79.20 | <0.001 * | |
| Preterm birth | Yes | 5 | 7.74 ± 1.13 | 6.04 ± 1.06 | 0.01 $ | 4.82 | 0.03 * |
| No | 100 | 7.54 ± 1.60 | 5.78 ± 1.09 | (0.94) | 103.52 | <0.001 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akshaykirthan, J.P.; Somannavar, M.S.; Deepthy, M.S.; Charantimath, U.; Yogeshkumar, S.; Patil, A.; Bellad, M.B.; Derman, R.; Goudar, S.S. Relationship Between Maternal Iron Indices in the Second Trimester with Cord Blood Iron Indices and Pregnancy Outcomes: A Prospective Cohort Study. Nutrients 2025, 17, 1584. https://doi.org/10.3390/nu17091584
Akshaykirthan JP, Somannavar MS, Deepthy MS, Charantimath U, Yogeshkumar S, Patil A, Bellad MB, Derman R, Goudar SS. Relationship Between Maternal Iron Indices in the Second Trimester with Cord Blood Iron Indices and Pregnancy Outcomes: A Prospective Cohort Study. Nutrients. 2025; 17(9):1584. https://doi.org/10.3390/nu17091584
Chicago/Turabian StyleAkshaykirthan, J. P., Manjunath S. Somannavar, M. S. Deepthy, Umesh Charantimath, S. Yogeshkumar, Amaresh Patil, Mrutyunjaya B. Bellad, Richard Derman, and Shivaprasad S. Goudar. 2025. "Relationship Between Maternal Iron Indices in the Second Trimester with Cord Blood Iron Indices and Pregnancy Outcomes: A Prospective Cohort Study" Nutrients 17, no. 9: 1584. https://doi.org/10.3390/nu17091584
APA StyleAkshaykirthan, J. P., Somannavar, M. S., Deepthy, M. S., Charantimath, U., Yogeshkumar, S., Patil, A., Bellad, M. B., Derman, R., & Goudar, S. S. (2025). Relationship Between Maternal Iron Indices in the Second Trimester with Cord Blood Iron Indices and Pregnancy Outcomes: A Prospective Cohort Study. Nutrients, 17(9), 1584. https://doi.org/10.3390/nu17091584






