Associations of Ultra-Processed Food Intake and Its Circulating Metabolomic Signature with Mental Disorders in Middle-Aged and Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Assessment
2.3. Metabolic Profiling
2.4. Outcome
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fact Sheet—Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 7 March 2025).
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E.; Gilbody, S.; Torous, J.; Teasdale, S.B.; Jackson, S.E.; et al. A meta-review of “lifestyle psychiatry”: The role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020, 19, 360–380. [Google Scholar] [CrossRef]
- Matison, A.P.; Mather, K.A.; Flood, V.M.; Reppermund, S. Associations between nutrition and the incidence of depression in middle-aged and older adults: A systematic review and meta-analysis of prospective observational population-based studies. Ageing Res. Rev. 2021, 70, 101403. [Google Scholar] [CrossRef] [PubMed]
- Guu, T.W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R.K.; Hamazaki, K.; Freeman, M.P.; Maes, M.; Matsuoka, Y.J.; Belmaker, R.H.; et al. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother. Psychosom. 2019, 88, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, Z.; Hou, Y.; Yang, H.; Wang, X.; Xu, C. The associations of dietary patterns with depressive and anxiety symptoms: A prospective study. BMC Med. 2023, 21, 307. [Google Scholar] [CrossRef]
- Alfaro-González, S.; Garrido-Miguel, M.; Pascual-Morena, C.; Pozuelo-Carrascosa, D.P.; Fernández-Rodríguez, R.; Martínez-Hortelano, J.A.; Mesas, A.E.; Martínez-Vizcaíno, V. The Association Between Adherence to the Mediterranean Diet and Depression and Anxiety Symptoms in University Students: The Mediating Role of Lean Mass and the Muscle Strength Index. Nutrients 2025, 17, 346. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Marino, M.; Puppo, F.; Del Bo, C.; Vinelli, V.; Riso, P.; Porrini, M.; Martini, D. A Systematic Review of Worldwide Consumption of Ultra-Processed Foods: Findings and Criticisms. Nutrients 2021, 13, 2778. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef] [PubMed]
- Canhada, S.L.; Vigo, Á.; Giatti, L.; Fonseca, M.J.; Lopes, L.J.; Cardoso, L.O.; Monteiro, C.A.; Schmidt, M.I.; Duncan, B.B. Associations of Ultra-Processed Food Intake with the Incidence of Cardiometabolic and Mental Health Outcomes Go Beyond Specific Subgroups-The Brazilian Longitudinal Study of Adult Health. Nutrients 2024, 16, 4291. [Google Scholar] [CrossRef] [PubMed]
- Samuthpongtorn, C.; Nguyen, L.H.; Okereke, O.I.; Wang, D.D.; Song, M.; Chan, A.T.; Mehta, R.S. Consumption of Ultraprocessed Food and Risk of Depression. JAMA Netw. Open 2023, 6, e2334770. [Google Scholar] [CrossRef] [PubMed]
- Witek, K.; Wydra, K.; Filip, M. A High-Sugar Diet Consumption, Metabolism and Health Impacts with a Focus on the Development of Substance Use Disorder: A Narrative Review. Nutrients 2022, 14, 2940. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, D.; Zhang, Y.; Wang, J.; Dong, L.; Hu, Y.; Wang, S. Long-term exposure to advanced lipid peroxidation end products impairs cognitive function through microbiota-gut-brain axis. Food Chem. 2024, 461, 140864. [Google Scholar] [CrossRef]
- Wiss, D.A.; LaFata, E.M. Ultra-Processed Foods and Mental Health: Where Do Eating Disorders Fit into the Puzzle? Nutrients 2024, 16, 1955. [Google Scholar] [CrossRef]
- Oliveras-Cañellas, N.; Castells-Nobau, A.; de la Vega-Correa, L.; Latorre-Luque, J.; Motger-Albertí, A.; Arnoriaga-Rodriguez, M.; Garre-Olmo, J.; Zapata-Tona, C.; Coll-Martínez, C.; Ramió-Torrentà, L.; et al. Adipose tissue coregulates cognitive function. Sci. Adv. 2023, 9, eadg4017. [Google Scholar] [CrossRef]
- Gao, X.; Geng, T.; Jiang, M.; Huang, N.; Zheng, Y.; Belsky, D.W.; Huang, T. Accelerated biological aging and risk of depression and anxiety: Evidence from 424,299 UK Biobank participants. Nat. Commun. 2023, 14, 2277. [Google Scholar] [CrossRef]
- Galante, J.; Adamska, L.; Young, A.; Young, H.; Littlejohns, T.J.; Gallacher, J.; Allen, N. The acceptability of repeat Internet-based hybrid diet assessment of previous 24-h dietary intake: Administration of the Oxford WebQ in UK Biobank. Br. J. Nutr. 2016, 115, 681–686. [Google Scholar] [CrossRef]
- Pirruccello, J.P.; Lin, H.; Khurshid, S.; Nekoui, M.; Weng, L.C.; Vasan, R.S.; Isselbacher, E.M.; Benjamin, E.J.; Lubitz, S.A.; Lindsay, M.E.; et al. Development of a Prediction Model for Ascending Aortic Diameter Among Asymptomatic Individuals. JAMA 2022, 328, 1935–1944. [Google Scholar] [CrossRef]
- Buergel, T.; Steinfeldt, J.; Ruyoga, G.; Pietzner, M.; Bizzarri, D.; Vojinovic, D.; Upmeier Zu Belzen, J.; Loock, L.; Kittner, P.; Christmann, L.; et al. Metabolomic profiles predict individual multidisease outcomes. Nat. Med. 2022, 28, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Negeri, Z.F.; Levis, B.; Sun, Y.; He, C.; Krishnan, A.; Wu, Y.; Bhandari, P.M.; Neupane, D.; Brehaut, E.; Benedetti, A.; et al. Accuracy of the Patient Health Questionnaire-9 for screening to detect major depression: Updated systematic review and individual participant data meta-analysis. BMJ 2021, 375, n2183. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, A.; Hüsing, P.; Gumz, A.; Wingenfeld, K.; Härter, M.; Schramm, E.; Löwe, B. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J. Affect. Disord. 2020, 265, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Cao, Z.; Cui, L.; Li, F.; Lu, Z.; Hou, Y.; Yang, H.; Wang, X.; Xu, C. The association between coffee consumption and risk of incident depression and anxiety: Exploring the benefits of moderate intake. Psychiatry Res. 2023, 326, 115307. [Google Scholar] [CrossRef]
- Pan, C.; Ye, J.; Wen, Y.; Chu, X.; Jia, Y.; Cheng, B.; Cheng, S.; Liu, L.; Yang, X.; Liang, C.; et al. The associations between sleep behaviors, lifestyle factors, genetic risk and mental disorders: A cohort study of 402 290 UK Biobank participants. Psychiatry Res. 2022, 311, 114488. [Google Scholar] [CrossRef]
- Han, H.; Cao, Y.; Feng, C.; Zheng, Y.; Dhana, K.; Zhu, S.; Shang, C.; Yuan, C.; Zong, G. Association of a Healthy Lifestyle With All-Cause and Cause-Specific Mortality Among Individuals With Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes Care 2022, 45, 319–329. [Google Scholar] [CrossRef]
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385 292 UK biobank participants. Eur. Heart J. 2020, 41, 1182–1189. [Google Scholar] [CrossRef]
- Li, X.; Xue, Q.; Wang, M.; Zhou, T.; Ma, H.; Heianza, Y.; Qi, L. Adherence to a Healthy Sleep Pattern and Incident Heart Failure: A Prospective Study of 408 802 UK Biobank Participants. Circulation 2021, 143, 97–99. [Google Scholar] [CrossRef]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hyppönen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef]
- Austin, P.C.; Latouche, A.; Fine, J.P. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat. Med. 2020, 39, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Z.; Zhu, J.H.; Liu, B.P.; Jia, C.X. The joint associations of physical activity and ultra-processed food consumption with depression: A cohort study in the UK Biobank. J. Affect. Disord. 2024, 367, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Nedic Erjavec, G.; Konjevod, M.; Nikolac Perkovic, M.; Svob Strac, D.; Tudor, L.; Barbas, C.; Grune, T.; Zarkovic, N.; Pivac, N. Short overview on metabolomic approach and redox changes in psychiatric disorders. Redox Biol. 2018, 14, 178–186. [Google Scholar] [CrossRef]
- Palmer, E.R.; Morales-Muñoz, I.; Perry, B.I.; Marwaha, S.; Warwick, E.; Rogers, J.C.; Upthegrove, R. Trajectories of Inflammation in Youth and Risk of Mental and Cardiometabolic Disorders in Adulthood. JAMA Psychiatry 2024, 81, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bao, Y.; Zhao, J.; Wang, Z.; Gao, Q.; Ma, M.; Xie, Z.; He, M.; Deng, X.; Ran, J. Associations of Triglycerides and Atherogenic Index of Plasma with Brain Structure in the Middle-Aged and Elderly Adults. Nutrients 2024, 16, 672. [Google Scholar] [CrossRef]
- Li, Y.; Hua, L.; Ran, Q.; Gu, J.; Bao, Y.; Sun, J.; Wu, L.; He, M.; Zhang, Y.; Gu, J.; et al. Plasma Polyunsaturated Fatty Acid Levels and Mental Health in Middle-Aged and Elderly Adults. Nutrients 2024, 16, 4065. [Google Scholar] [CrossRef]
- Jansen, R.; Milaneschi, Y.; Schranner, D.; Kastenmuller, G.; Arnold, M.; Han, X.; Dunlop, B.W.; Rush, A.J.; Kaddurah-Daouk, R.; Penninx, B. The metabolome-wide signature of major depressive disorder. Mol. Psychiatry 2024, 29, 3722–3733. [Google Scholar] [CrossRef]
- Yin, B.; Cai, Y.; Teng, T.; Wang, X.; Liu, X.; Li, X.; Wang, J.; Wu, H.; He, Y.; Ren, F.; et al. Identifying plasma metabolic characteristics of major depressive disorder, bipolar disorder, and schizophrenia in adolescents. Transl. Psychiatry 2024, 14, 163. [Google Scholar] [CrossRef]
- Srour, B.; Kordahi, M.C.; Bonazzi, E.; Deschasaux-Tanguy, M.; Touvier, M.; Chassaing, B. Ultra-processed foods and human health: From epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022, 7, 1128–1140. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Hidese, S.; Nogawa, S.; Saito, K.; Kunugi, H. Food allergy is associated with depression and psychological distress: A web-based study in 11,876 Japanese. J. Affect. Disord. 2019, 245, 213–218. [Google Scholar] [CrossRef]
- Song, Z.; Song, R.; Liu, Y.; Wu, Z.; Zhang, X. Effects of ultra-processed foods on the microbiota-gut-brain axis: The bread-and-butter issue. Food Res. Int. 2023, 167, 112730. [Google Scholar] [CrossRef]
- Chen, H.; Gao, L.; Liu, J.; Ji, C.; Dang, X.; Zhou, Z.; Luo, W. Comparison of Gut Microbiota in Overwintering Bees: Apis cerana vs. Apis mellifera. Microbiol. Res. 2024, 15, 2425–2434. [Google Scholar]
- Naughton, M.; Dinan, T.G.; Scott, L.V. Corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal axis in psychiatric disease. Handb. Clin. Neurol. 2014, 124, 69–91. [Google Scholar] [CrossRef] [PubMed]
- McHugh, R.K.; Votaw, V.R.; Sugarman, D.E.; Greenfield, S.F. Sex and gender differences in substance use disorders. Clin. Psychol. Rev. 2018, 66, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Martínez Steele, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in Consumption of Ultraprocessed Foods Among US Youths Aged 2-19 Years, 1999-2018. Jama 2021, 326, 519–530. [Google Scholar] [CrossRef]
- Marrón-Ponce, J.A.; Tolentino-Mayo, L.; Hernández, F.M.; Batis, C. Trends in Ultra-Processed Food Purchases from 1984 to 2016 in Mexican Households. Nutrients 2018, 11, 45. [Google Scholar] [CrossRef]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef]
- Baker, P.; Friel, S. Food systems transformations, ultra-processed food markets and the nutrition transition in Asia. Glob. Health 2016, 12, 80. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Bueno, N.B.; DiFeliceantonio, A.G.; Roberto, C.A.; Jiménez-Murcia, S.; Fernandez-Aranda, F. Social, clinical, and policy implications of ultra-processed food addiction. BMJ 2023, 383, e075354. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Rossato, S.L.; Hang, D.; Khandpur, N.; Wang, K.; Lo, C.H.; Willett, W.C.; Giovannucci, E.L.; Song, M. Association of ultra-processed food consumption with all cause and cause specific mortality: Population based cohort study. BMJ 2024, 385, e078476. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Barquera, S.; Corvalan, C.; Hofman, K.J.; Monteiro, C.; Ng, S.W.; Swart, E.C.; Taillie, L.S. Towards unified and impactful policies to reduce ultra-processed food consumption and promote healthier eating. Lancet Diabetes Endocrinol. 2021, 9, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, P.; Ng, S.W. Promoting Healthier Purchases: Ultraprocessed Food Taxes and Minimally Processed Foods Subsidies for the Low Income. Am. J. Prev. Med. 2024, 67, 3–14. [Google Scholar] [CrossRef]
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and Challenges in Integrating Nutritional Care into Oncology Practice: Results from a National Survey on Behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, M.; Huang, N.; Guo, X.; Huang, T. Long-Term Air Pollution, Genetic Susceptibility, and the Risk of Depression and Anxiety: A Prospective Study in the UK Biobank Cohort. Environ. Health Perspect. 2023, 131, 17002. [Google Scholar] [CrossRef]
- Yang, T.; Wang, J.; Huang, J.; Kelly, F.J.; Li, G. Long-term Exposure to Multiple Ambient Air Pollutants and Association With Incident Depression and Anxiety. JAMA Psychiatry 2023, 80, 305–313. [Google Scholar] [CrossRef]
- Hao, G.; Zuo, L.; Xiong, P.; Chen, L.; Liang, X.; Jing, C. Associations of PM2.5 and road traffic noise with mental health: Evidence from UK Biobank. Environ. Res. 2022, 207, 112221. [Google Scholar] [CrossRef]
- Perez-Cornago, A.; Pollard, Z.; Young, H.; van Uden, M.; Andrews, C.; Piernas, C.; Key, T.J.; Mulligan, A.; Lentjes, M. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. Eur. J. Nutr. 2021, 60, 4019–4030. [Google Scholar] [CrossRef]
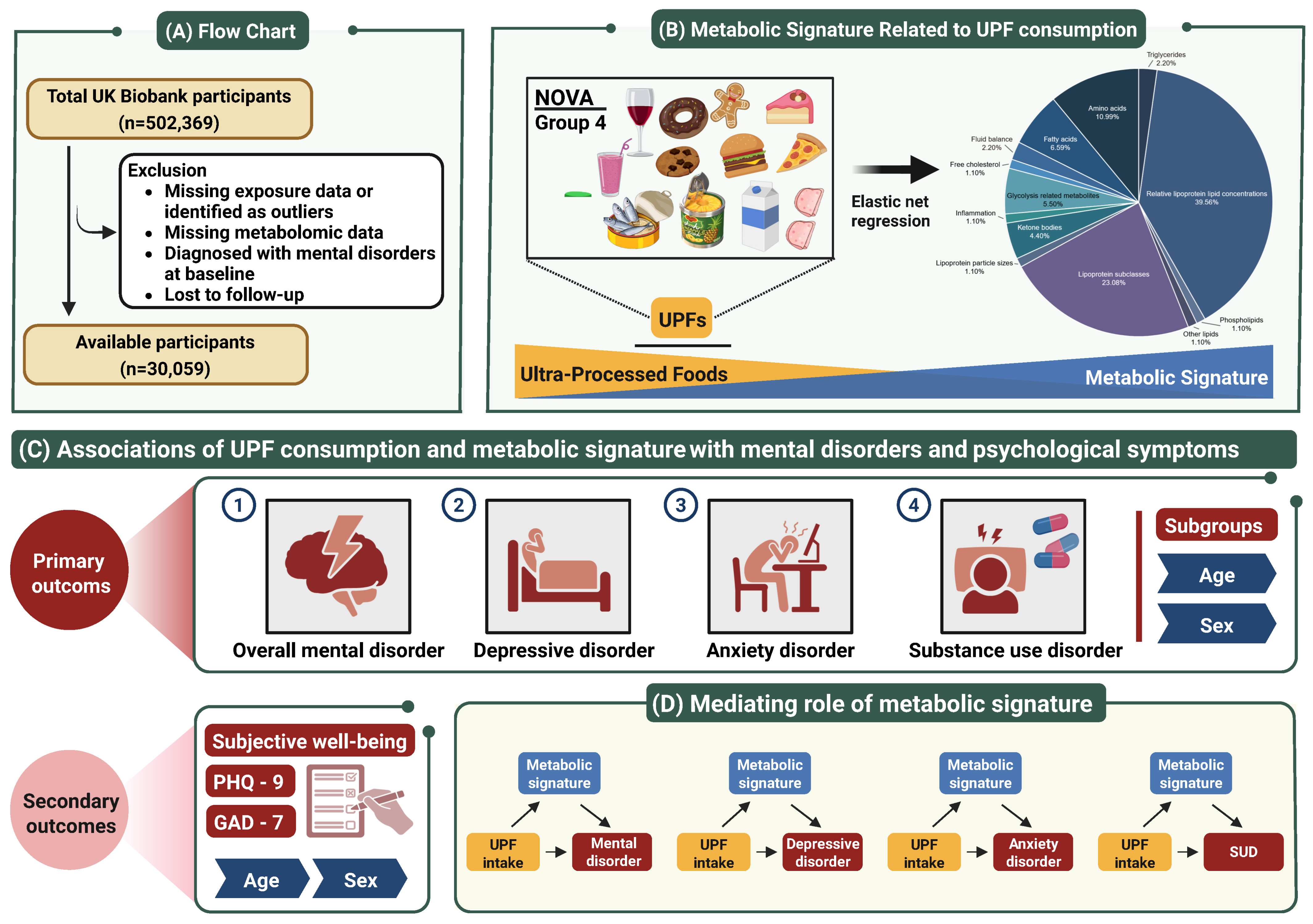
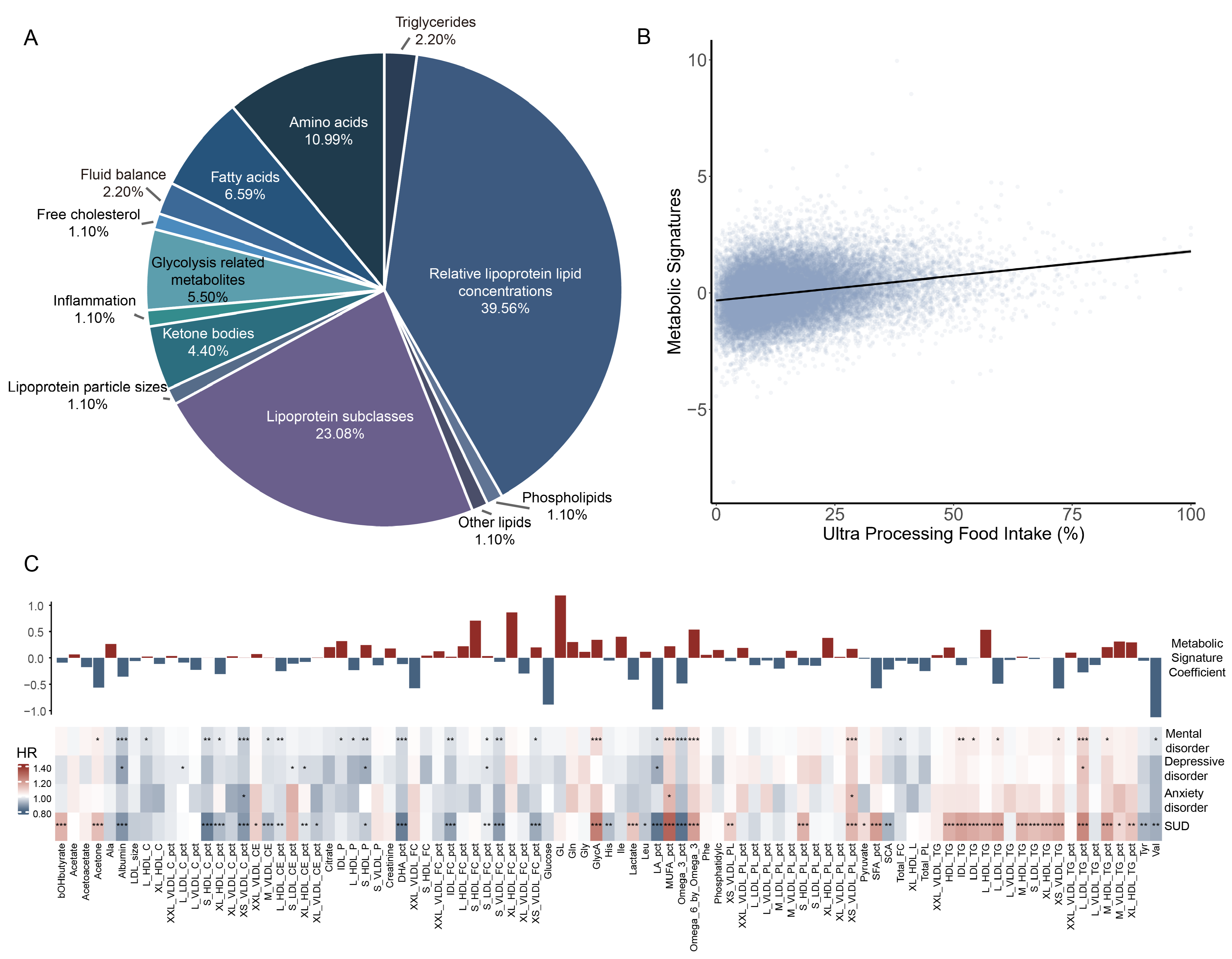

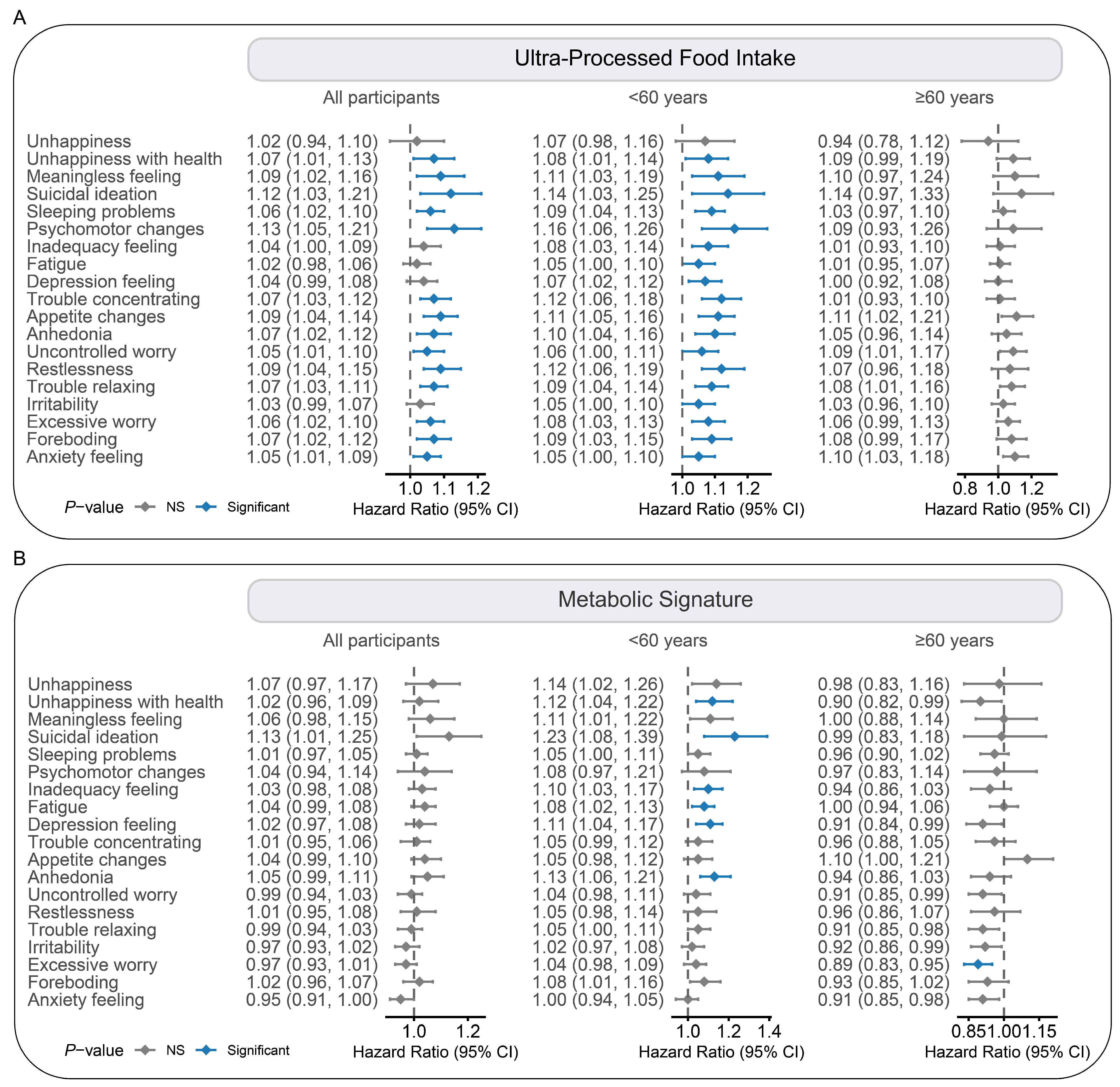
| Characteristics | All Participants | Ultra-Processed Food Intake Level a | p Value b | ||
|---|---|---|---|---|---|
| Low (n = 19,455) | Moderate (n = 19,454) | High (n = 19,514) | |||
| Age | 56.5 (8.1) | 57.1 (7.8) | 57.1 (7.8) | 55.3 (8.4) | <0.001 |
| Sex | <0.001 | ||||
| Female | 15,956 (53.1%) | 5446 (54.4%) | 5359 (53.5%) | 5151 (51.3%) | |
| Male | 14,103 (46.9%) | 4564 (45.6%) | 4650 (46.5%) | 4889 (48.7%) | |
| IMD c | <0.001 | ||||
| Low | 15,041 (50.0%) | 5221 (52.2%) | 5194 (51.9%) | 4626 (46.1%) | |
| High | 15,018 (50.0%) | 4789 (47.8%) | 4815 (48.1%) | 5414 (53.9%) | |
| BMI | 27.1 (4.7) | 26.6 (4.4) | 26.8 (4.4) | 27.9 (5.0) | <0.001 |
| WHR d | <0.001 | ||||
| Low | 15,578 (51.8%) | 5471 (54.7%) | 5312 (53.1%) | 4795 (47.8%) | |
| High | 14,481 (48.2%) | 4539 (45.3%) | 4697 (46.9%) | 5245 (52.2%) | |
| Healthy lifestyle | |||||
| Never smoking e | 17,485 (58.2%) | 5564 (55.6%) | 5959 (59.5%) | 5962 (59.4%) | <0.001 |
| Moderate drinking f | 20,277 (67.5%) | 6136 (61.3%) | 6782 (67.8%) | 7359 (73.3%) | <0.001 |
| Regular physical activity g | 16,717 (55.6%) | 5735 (57.3%) | 5592 (55.9%) | 5390 (53.7%) | <0.001 |
| Healthy sleep pattern h | 16,930 (56.3%) | 5792 (57.9%) | 5669 (56.6%) | 5469 (54.5%) | <0.001 |
| Healthy diet i | 11,708 (39.0%) | 4603 (46.0%) | 3913 (39.1%) | 3192 (31.8%) | <0.001 |
| Prevalent disease | |||||
| Hypertension | 7943 (26.4%) | 2613 (26.1%) | 2566 (25.6%) | 2764 (27.5%) | 0.007 |
| Diabetes | 1378 (4.6%) | 443 (4.4%) | 383 (3.8%) | 552 (5.5%) | <0.001 |
| Coronary artery disease | 606 (2.0%) | 197 (2.0%) | 191 (1.9%) | 218 (2.2%) | 0.381 |
| Disease | Categorical a | Continuous b | ||||
|---|---|---|---|---|---|---|
| Low | Moderate | High | p Trend | HR (95% CI) | p Value | |
| Ultra-processed food intake | ||||||
| Mental disorder | ||||||
| Basic c | reference | 1.02 (0.96, 1.08) | 1.08 (1.02, 1.14) | 0.006 | 1.04 (1.02, 1.07) | <0.001 |
| MV d | reference | 1.03 (0.97, 1.09) | 1.08 (1.02, 1.14) | 0.011 | 1.04 (1.02, 1.06) | <0.001 |
| MV + MA e | reference | 1.02 (0.96, 1.07) | 1.05 (1.00, 1.12) | 0.068 | 1.03 (1.01, 1.05) | 0.006 |
| Depressive disorder | ||||||
| Basic | reference | 1.12 (0.94, 1.33) | 1.39 (1.18, 1.65) | <0.001 | 1.16 (1.10, 1.22) | <0.001 |
| MV | reference | 1.12 (0.94, 1.33) | 1.35 (1.14, 1.60) | <0.001 | 1.14 (1.08, 1.20) | <0.001 |
| MV + MA | reference | 1.10 (0.92, 1.31) | 1.31 (1.10, 1.55) | 0.002 | 1.12 (1.06, 1.18) | <0.001 |
| Anxiety disorder | ||||||
| Basic | reference | 1.17 (0.99, 1.38) | 1.35 (1.15, 1.59) | <0.001 | 1.13 (1.07, 1.19) | <0.001 |
| MV | reference | 1.16 (0.98, 1.38) | 1.32 (1.12, 1.56) | 0.001 | 1.12 (1.06, 1.18) | <0.001 |
| MV + MA | reference | 1.14 (0.96, 1.35) | 1.28 (1.08, 1.51) | 0.005 | 1.10 (1.04, 1.16) | 0.001 |
| SUD | ||||||
| Basic | reference | 0.95 (0.83, 1.09) | 0.96 (0.84, 1.10) | 0.53 | 1.04 (0.99, 1.09) | 0.100 |
| MV | reference | 1.03 (0.90, 1.18) | 1.03 (0.90, 1.18) | 0.684 | 1.06 (1.01, 1.11) | 0.020 |
| MV + MA | reference | 1.00 (0.87, 1.15) | 0.96 (0.84, 1.11) | 0.597 | 1.02 (0.97, 1.07) | 0.377 |
| Metabolic signature score | ||||||
| Mental disorder | ||||||
| Basic | reference | 1.08 (1.02, 1.14) | 1.23 (1.16, 1.30) | <0.001 | 1.10 (1.07, 1.12) | <0.001 |
| MV | reference | 1.07 (1.01, 1.13) | 1.18 (1.11, 1.25) | <0.001 | 1.08 (1.05, 1.10) | <0.001 |
| MV + MA | reference | 1.07 (1.01, 1.13) | 1.17 (1.10, 1.24) | <0.001 | 1.07 (1.04, 1.10) | <0.001 |
| Depressive disorder | ||||||
| Basic | reference | 1.13 (0.94, 1.34) | 1.44 (1.21, 1.71) | <0.001 | 1.21 (1.13, 1.30) | <0.001 |
| MV | reference | 1.10 (0.92, 1.31) | 1.32 (1.10, 1.58) | 0.002 | 1.16 (1.08, 1.24) | <0.001 |
| MV + MA | reference | 1.07 (0.90, 1.28) | 1.27 (1.06, 1.52) | 0.008 | 1.13 (1.05, 1.21) | 0.001 |
| Anxiety disorder | ||||||
| Basic | reference | 1.19 (1.00, 1.41) | 1.44 (1.21, 1.71) | <0.001 | 1.18 (1.10, 1.26) | <0.001 |
| MV | reference | 1.17 (0.98, 1.38) | 1.37 (1.15, 1.63) | <0.001 | 1.15 (1.07, 1.23) | <0.001 |
| MV + MA | reference | 1.14 (0.96, 1.36) | 1.32 (1.10, 1.57) | 0.002 | 1.12 (1.05, 1.21) | 0.001 |
| SUD | ||||||
| Basic | reference | 1.02 (0.88, 1.19) | 1.61 (1.40, 1.86) | <0.001 | 1.28 (1.21, 1.35) | <0.001 |
| MV | reference | 1.05 (0.90, 1.23) | 1.60 (1.39, 1.85) | <0.001 | 1.25 (1.19, 1.32) | <0.001 |
| MV + MA | reference | 1.06 (0.91, 1.23) | 1.61 (1.39, 1.86) | <0.001 | 1.25 (1.18, 1.32) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Zhu, T.; Gu, J.; Hua, L.; Sun, J.; Deng, X.; Ran, J. Associations of Ultra-Processed Food Intake and Its Circulating Metabolomic Signature with Mental Disorders in Middle-Aged and Older Adults. Nutrients 2025, 17, 1582. https://doi.org/10.3390/nu17091582
Yuan S, Zhu T, Gu J, Hua L, Sun J, Deng X, Ran J. Associations of Ultra-Processed Food Intake and Its Circulating Metabolomic Signature with Mental Disorders in Middle-Aged and Older Adults. Nutrients. 2025; 17(9):1582. https://doi.org/10.3390/nu17091582
Chicago/Turabian StyleYuan, Shenghao, Tengfei Zhu, Jiawei Gu, Li Hua, Jinli Sun, Xiaobei Deng, and Jinjun Ran. 2025. "Associations of Ultra-Processed Food Intake and Its Circulating Metabolomic Signature with Mental Disorders in Middle-Aged and Older Adults" Nutrients 17, no. 9: 1582. https://doi.org/10.3390/nu17091582
APA StyleYuan, S., Zhu, T., Gu, J., Hua, L., Sun, J., Deng, X., & Ran, J. (2025). Associations of Ultra-Processed Food Intake and Its Circulating Metabolomic Signature with Mental Disorders in Middle-Aged and Older Adults. Nutrients, 17(9), 1582. https://doi.org/10.3390/nu17091582







