The Obesity–Epigenetics–Microbiome Axis: Strategies for Therapeutic Intervention
Abstract
1. Introduction
2. Epigenetics and Development of Obesity
2.1. Altered DNAmet in Obesity
2.2. Histone Modifications in Obesity
2.3. Altered miRNAs in Obesity
3. Gut Microbiota and Obesity
4. Mutual Connections Between Gut Microbial Changes and Epigenetic Alterations
5. Therapeutic Strategies for Prevention or Treatment of Obesity by Microbiome Mediated Epigenetic Modulations
5.1. Caloric Restriction (CR) and Physical Activity and Their Influence on Gut Microbiome
5.2. Dietary Methyl Donors and GM
5.3. GM-Derived Metabolites for Treatment of Obesity
5.3.1. Short Chain Fatty Acids (SCFAs) for Treatment of Obesity
5.3.2. Indole and Its Derivatives for Treatment of Obesity and Obesity-Related Disorders
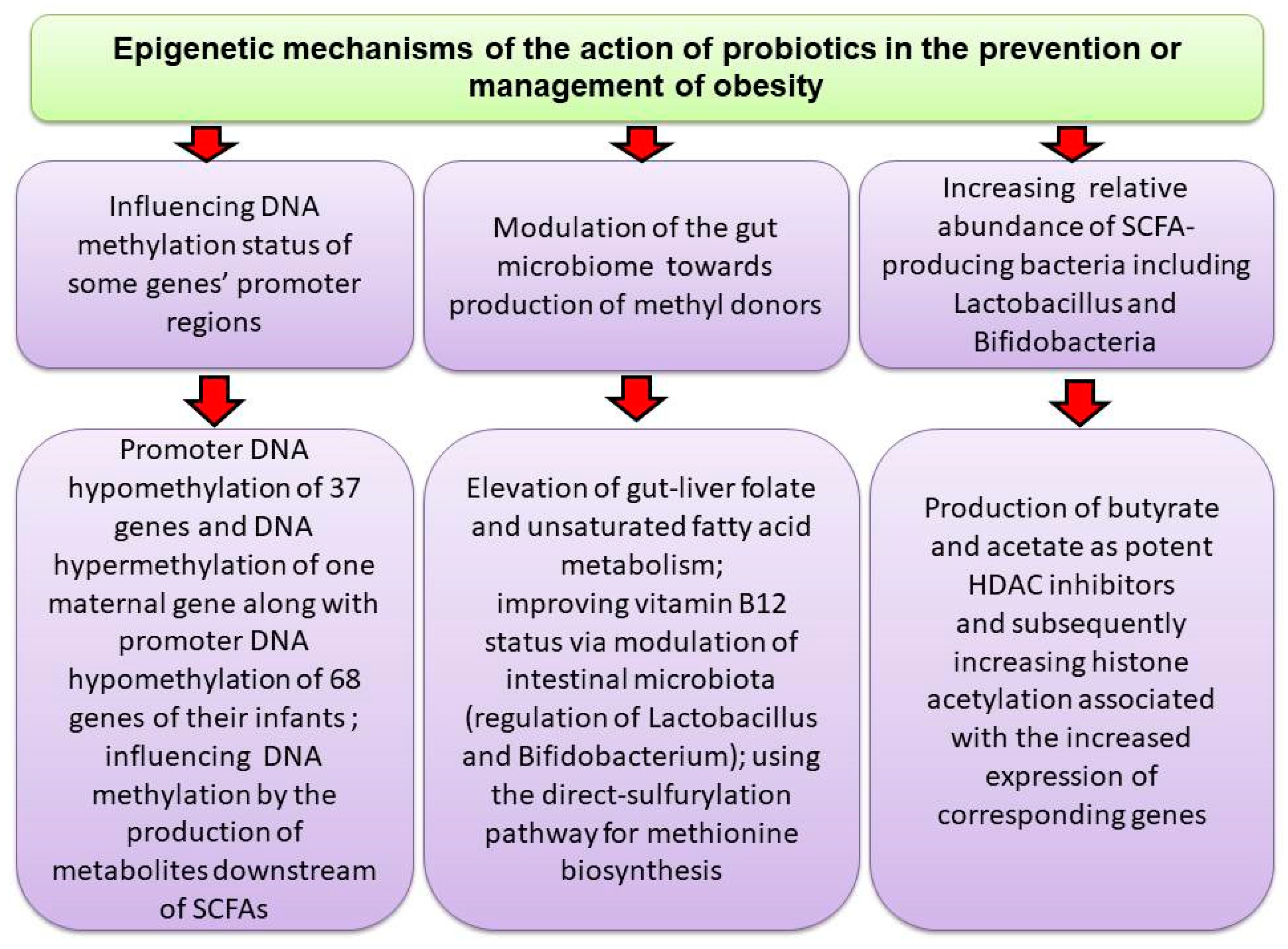
5.4. Probiotics
5.5. Engineered Probiotics by Synthetic Biology Approaches for Management of Obesity
5.6. Prebiotics/Postbiotics
5.7. Antibiotics and Gut Microbiota Changes
6. Existing Challenges and Research Directions
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| OB | obesity |
| GM | gut microbiome |
| T2DM | type 2 diabetes |
| BMI | body mass index |
| NAFLD | nonalcoholic fatty liver disease |
| TNF-α | tumor necrosis factor alpha |
| IL-6 | interleukin 6 |
| IL-1β | interleukin-1 beta |
| PPARγ | peroxisome proliferator-activated receptor γ |
| GI | gastrointestinal |
| ncRNAs | non-coding RNAs |
| miRNAs | microRNAs |
| DNAmet | DNA methylation |
| DMRs | differentially methylated regions |
| HFD | high-fat diet |
| HDACs | histone deacetylases |
| DNMTs | DNA methyltransferases |
| 5mC | 5-methylcytosine |
| VAT | visceral adipose tissue |
| SAT | subcutaneous adipose tissue |
| WAT | white adipose tissue |
| SCFAs | short-chain fatty acids |
| CR | caloric restriction |
| LDL | low-density lipoprotein |
| SB | sodium butyrate |
| GLP-1 | glucagon-like peptide-1 |
| STAT3 | signal transducer and activator of transcription 3 |
| GSH/GSSG ratio | glutathione (GSH)/glutathione disulfide (GSSG) ratio |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| FFAR | free fatty acid receptor |
| IBA | indole-3-butyric acid |
| IAA | indole-3-acetic acid |
| IPA | indole-3-propionic acid |
| IA | indoleacrylic acid |
| I3A | indole-3-aldehyde |
| ALT | alanine aminotransferase |
| Microbiota | A microbial community including commensal, symbiotic and pathogenic microorganisms; typically defined based on the habitat that it occupies (e.g., the oral or gut microbiota) |
| Microbiome | The aggregate genomes and genes present in the members of microbial community in the body |
| Dysbiosis | A perturbation in microbial balance in the gastrointestinal tract that causes host maladaptation and disease |
| Alpha diversity | Demonstration of the number of different species within a specific community or individual sample |
| Beta diversity | Demonstration of the similarity of one community or individual sample to another |
| Short chain fatty acids | Metabolites created by bacteria during the metabolization of cellulose or other polysaccharides |
| MicroRNA | A class of non-coding RNA in the form of tiny fragment with a crucial regulatory function |
| Histone modification | The occurrence of acetylation, methylation, and phosphorylation on the N-terminal tail of histones H3 and H4 for controlling gene transcription |
| Histone deacetylases (HDAC) | A class of enzymes in charge of the removal of an acetyl group from lysine residues on the histone tail and subsequently affecting the interplay between histones and DNA |
| Polyphenols | Naturally occurring plant ingredients including phenol groups |
| Probiotics | Live microorganisms capable of creating a health benefit on the host when administered in adequate concentrations |
| Prebiotics | Substrates, fermented nondigestible food ingredients, or substances that are selectively consumed by health-promoting bacteria for creating a health benefit via increasing their growth and/or activity |
| Postbiotics | Bacterial fragments with or without bioactive compounds as a product of microbial growth with a health benefit for the host |
| Caloric restriction (CR) | The decrease in caloric intake without the induction of malnutrition |
| Glucagon-like peptide-1 (GLP-1) | An incretin released by the intestines that promotes insulin secretion and sensitivity, improves satiety, and suppresses secretion of glucagon |
| Lipopolysaccharide (LPS) | An endotoxin and the main component of the outer membrane of Gram-negative bacteria involved in the release of cytokines and activation of the innate immune system |
References
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Cohen, R.V.; le Roux, C.W.; Sumithran, P. Obesity in adults. Lancet 2024, 404, 972–987. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Obesity and Lipotoxicity; Springer: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar]
- Magkos, F.; Sørensen, T.I.; Raubenheimer, D.; Dhurandhar, N.V.; Loos, R.J.; Bosy-Westphal, A.; Clemmensen, C.; Hjorth, M.F.; Allison, D.B.; Taubes, G. On the pathogenesis of obesity: Causal models and missing pieces of the puzzle. Nat. Metab. 2024, 6, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Devericks, E.N.; Carson, M.S.; McCullough, L.E.; Coleman, M.F.; Hursting, S.D. The obesity-breast cancer link: A multidisciplinary perspective. Cancer Metastasis Rev. 2022, 41, 607–625. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of obesity-induced inflammation on cardiovascular diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The role of obesity in type 2 diabetes mellitus—An overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The interplay between obesity and inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Varra, F.-N.; Varras, M.; Varra, V.-K.; Theodosis-Nobelos, P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation-mediating treatment options. Mol. Med. Rep. 2024, 29, 95. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Q.; Yu, L.; Shi, H.; Xue, B.; Shi, H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight 2016, 1, e87748. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, X.; Teng, Y.; Zhao, T.; Lin, L.; Li, Y.; Shang, H.; Jin, Y.; Jin, Q. Adipocytes-derived Exosomal microRNA-1224 inhibits M2 macrophage polarization in obesity-induced adipose tissue inflammation via MSI2-mediated Wnt/β-catenin Axis. Mol. Nutr. Food Res. 2022, 66, 2100889. [Google Scholar] [CrossRef]
- Fischer, I.; Irmler, M.; Meyer, C.; Sachs, S.; Neff, F.; Hrabě de Angelis, M.; Beckers, J.; Tschöp, M.; Hofmann, S.; Ussar, S. A history of obesity leaves an inflammatory fingerprint in liver and adipose tissue. Int. J. Obes. 2018, 42, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Venkatesan, M.; Sabarathinam, S. Efficacy of Microbiome-Targeted Interventions in Obesity Management—A Comprehensive Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2025, 19, 103208. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, X.; Mei, H.; Chang, X.; He, P.; Sun, L.; Xiao, H.; Wang, S.; Li, R. Association between gut microbiota and short-chain fatty acids in children with obesity. Sci. Rep. 2025, 15, 483. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, A.; Lee, S.; Feart, C.; Garcia-Esquinas, E.; Gomez-Cabrero, D.; Lopez-Garcia, E.; Morzel, M.; Neyraud, E.; Rodriguez-Artalejo, F.; Streich, R. Alterations in the oral microbiome associated with diabetes, overweight, and dietary components. Front. Nutr. 2022, 9, 914715. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Yuan, X.; Chang, C.; Chen, X.; Li, K. Emerging trends and focus of human gastrointestinal microbiome research from 2010–2021: A visualized study. J. Transl. Med. 2021, 19, 327. [Google Scholar] [CrossRef]
- Parker, A.; Lawson, M.A.; Vaux, L.; Pin, C. Host-microbe interaction in the gastrointestinal tract. Environ. Microbiol. 2018, 20, 2337–2353. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut microbiome composition in obese and non-obese persons: A systematic review and meta-analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Tang, R.; Liu, R.; Zha, H.; Cheng, Y.; Ling, Z.; Li, L. Gut microbiota induced epigenetic modifications in the non-alcoholic fatty liver disease pathogenesis. Eng. Life Sci. 2024, 24, 2300016. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Tzeng, H.-T.; Lee, W.-C. Impact of transgenerational nutrition on nonalcoholic fatty liver disease development: Interplay between gut microbiota, epigenetics and immunity. Nutrients 2024, 16, 1388. [Google Scholar] [CrossRef] [PubMed]
- Saez, V.M.; Perdicaro, D.; Cremonini, E.; Costantino, V.; Fontana, A.; Oteiza, P.; Prieto, M.V. Grape pomace extract attenuates high fat diet-induced endotoxemia and liver steatosis in mice. Food Funct. 2025, 16, 2515–2529. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, N.M.; Tupper, C.; Jialal, I. Genetics, epigenetic mechanism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Selleri, L.; Rijli, F.M. Shaping faces: Genetic and epigenetic control of craniofacial morphogenesis. Nat. Rev. Genet. 2023, 24, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Gökbuget, D.; Blelloch, R. Epigenetic control of transcriptional regulation in pluripotency and early differentiation. Development 2019, 146, dev164772. [Google Scholar] [CrossRef]
- Weiner, A.K.; Katz, L.A. Epigenetics as driver of adaptation and diversification in microbial eukaryotes. Front. Genet. 2021, 12, 642220. [Google Scholar] [CrossRef]
- Vaissière, T.; Sawan, C.; Herceg, Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat. Res. Rev. Mutat. Res. 2008, 659, 40–48. [Google Scholar] [CrossRef]
- Radford, E.J. An introduction to epigenetic mechanisms. Prog. Mol. Biol. Transl. Sci. 2018, 158, 29–48. [Google Scholar]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Shvedunova, M.; Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef]
- Calestagne-Morelli, A.; Ausio, J. Long-range histone acetylation: Biological significance, structural implications, and mechanisms. Biochem. Cell Biol. 2006, 84, 518–527. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, T.; Doss C, G.P. Non-coding RNAs in human health and disease: Potential function as biomarkers and therapeutic targets. Funct. Integr. Genom. 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Nikpay, M.; Ravati, S.; Dent, R.; McPherson, R. Epigenome-wide study identified methylation sites associated with the risk of obesity. Nutrients 2021, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- Haberman, M.; Menashe, T.; Cohen, N.; Kisliouk, T.; Yadid, T.; Marco, A.; Meiri, N.; Weller, A. Paternal high-fat diet affects weight and DNA methylation of their offspring. Sci. Rep. 2024, 14, 19874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, W.; Zhang, S.; Wu, H.; Gao, Y.; Zhang, J.; Zheng, J. Maternal high-fat diet orchestrates offspring hepatic cholesterol metabolism via MEF2A hypermethylation-mediated CYP7A1 suppression. Cell. Mol. Biol. Lett. 2024, 29, 154. [Google Scholar] [CrossRef]
- Vander Velden, J.W.; Osborne, D.M. Prolonged diet-induced obesity modifies DNA methylation and gene expression in the hippocampus. Neurosci. Lett. 2022, 780, 136656. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Cheng, Y.; Jin, L.-Y.; Zhou, Y.; Pang, H.-Y.; Zhu, H.; Yan, C.-C.; Yan, Y.-S.; Yu, J.-E.; Sheng, J.-Z. Paternal obesity impairs hepatic gluconeogenesis of offspring by altering Igf2/H19 DNA methylation. Mol. Cell. Endocrinol. 2021, 529, 111264. [Google Scholar] [CrossRef]
- Sukur, G.; Uysal, F.; Cinar, O. High-fat diet induced obesity alters Dnmt1 and Dnmt3a levels and global DNA methylation in mouse ovary and testis. Histochem. Cell Biol. 2023, 159, 339–352. [Google Scholar] [CrossRef]
- Bozdemir, N.; Kablan, T.; Altintas, M.O.; Sukur, G.; Cinar, O.; Uysal, F. Altered DNA methylation and Dnmt expression in obese uterus may cause implantation failure. J. Mol. Histol. 2024, 55, 427–436. [Google Scholar] [CrossRef]
- Keyhan, S.; Burke, E.; Schrott, R.; Huang, Z.; Grenier, C.; Price, T.; Raburn, D.; Corcoran, D.L.; Soubry, A.; Hoyo, C. Male obesity impacts DNA methylation reprogramming in sperm. Clin. Epigenetics 2021, 13, 17. [Google Scholar] [CrossRef]
- Chen, N.; Miao, L.; Lin, W.; Zou, D.; Huang, L.; Huang, J.; Shi, W.; Li, L.; Luo, Y.; Liang, H. Integrated DNA methylation and gene expression analysis identified S100A8 and S100A9 in the pathogenesis of obesity. Front. Cardiovasc. Med. 2021, 8, 631650. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Carreira, M.C.; Boughanem, H.; Moreno-Navarrete, J.M.; Nicoletti, C.F.; Oliver, P.; de Luis, D.; Nonino, C.B.; Portillo, M.P.; Martinez-Olmos, M.A. Adipose tissue and blood leukocytes ACE2 DNA methylation in obesity and after weight loss. Eur. J. Clin. Investig. 2022, 52, e13685. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-B.; Ni, J.; Yao, R.; Goetzinger, K.R.; Harman, C.; Reece, E.A.; Wang, B.; Yang, P. Maternal obesity increases DNA methylation and decreases RNA methylation in the human placenta. Reprod. Toxicol. 2022, 107, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Murphy, K.E.; Briollais, L.; McGowan, P.O.; Matthews, S.G. DNA methylation profiles in the blood of newborn term infants born to mothers with obesity. PLoS ONE 2022, 17, e0267946. [Google Scholar] [CrossRef]
- Baca, P.; Barajas-Olmos, F.; Mirzaeicheshmeh, E.; Zerrweck, C.; Guilbert, L.; Sánchez, E.C.; Flores-Huacuja, M.; Villafán, R.; Martínez-Hernández, A.; García-Ortiz, H. DNA methylation and gene expression analysis in adipose tissue to identify new loci associated with T2D development in obesity. Nutr. Diabetes 2022, 12, 50. [Google Scholar] [CrossRef]
- Sehgal, R.; Perfilyev, A.; Männistö, V.; Ågren, J.; Nilsson, E.; Käkelä, P.; Ling, C.; de Mello, V.D.; Pihlajamäki, J. Liver saturated fat content associates with hepatic DNA methylation in obese individuals. Clin. Epigenetics 2023, 15, 21. [Google Scholar] [CrossRef]
- Lecorguillé, M.; McAuliffe, F.M.; Twomey, P.J.; Viljoen, K.; Mehegan, J.; Kelleher, C.C.; Suderman, M.; Phillips, C.M. Maternal glycaemic and insulinemic status and newborn DNA methylation: Findings in women with overweight and obesity. J. Clin. Endocrinol. Metab. 2023, 108, 85–98. [Google Scholar] [CrossRef]
- Han, F.; Zhu, S.; Kong, X.; Wang, W.; Wu, Y. Integrated genetic and epigenetic analyses uncovered GLP1R association with metabolically healthy obesity. Int. J. Obes. 2024, 48, 324–329. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, P.; Huang, X.; Zhang, L.; Liu, L.; Xiang, W.; Liu, L.; He, X. Alterations of DNA methylation profile in peripheral blood of children with simple obesity. Health Inf. Sci. Syst. 2024, 12, 26. [Google Scholar] [CrossRef]
- Jesse, T.G.; Becer, E.; Kalkan, R. Identification of the Relationship Between DNA Methylation of Circadian Rhythm Genes and Obesity. Biochem. Genet. 2024, 62, 281–293. [Google Scholar] [CrossRef]
- Noronha, N.Y.; Diani, L.M.; Rodrigues, G.d.S.; Noma, I.H.Y.; Pereira, V.A.B.; Pinhel, M.A.d.S.; Watanabe, L.M.; Morais, D.A.; Barbosa Jr, F.; Nonino, C.B. Serum Cobalt Concentration and DNA Methylation Signatures in Women with Obesity. Obesities 2024, 4, 85–92. [Google Scholar] [CrossRef]
- Boonrong, C.; Roytrakul, S.; Shantavasinkul, P.C.; Sritara, P.; Sirivarasai, J. Role of Dietary Factors on DNA Methylation Levels of TNF-Alpha Gene and Proteome Profiles in Obese Men. Nutrients 2024, 16, 877. [Google Scholar] [CrossRef] [PubMed]
- Lariviere, D.; Craig, S.J.; Paul, I.M.; Hohman, E.E.; Savage, J.S.; Wright, R.O.; Chiaromonte, F.; Makova, K.D.; Reimherr, M.L. Methylation profiles at birth linked to early childhood obesity. J. Dev. Orig. Health Dis. 2024, 15, e7. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L.M.; Pereira, V.A.B.; Noronha, N.Y.; de Souza Pinhel, M.A.; Wolf, L.S.; de Oliveira, C.C.; Plaça, J.R.; Noma, I.H.Y.; da Silva Rodrigues, G.; de Souza, V.C.O. The influence of serum selenium in differential epigenetic and transcriptional regulation of CPT1B gene in women with obesity. J. Trace Elem. Med. Biol. 2024, 83, 127376. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, Z.; Yang, J.; Liu, J.; Lin, H.; Yin, N. Depot-specific acetylation profiles of adipose tissues—Therapeutic targets for metabolically unhealthy obesity. Diabetol. Metab. Syndr. 2025, 17, 36. [Google Scholar] [CrossRef]
- Wu, J.; Singh, K.; Shing, V.; Gupta, A.; Arenberg, B.C.; Huffstutler, R.D.; Lee, D.-Y.; Sack, M.N. Mitochondrial fatty acid oxidation regulates monocytic type I interferon signaling via histone acetylation. Sci. Adv. 2025, 11, eadq9301. [Google Scholar] [CrossRef]
- Alawathugoda, T.T.; Sheikh, M.A.; Challagandla, A.K.; Dheen, S.T.; Emerald, B.S.; Ansari, S.A. Maternal obesity alters histone modifications mediated by the interaction between EZH2 and AMPK, impairing neural differentiation in the developing embryonic brain cortex. J. Biol. Chem. 2025, 301, 108173. [Google Scholar] [CrossRef]
- Glendining, K.A.; Jasoni, C.L. Maternal high fat diet-induced obesity modifies histone binding and expression of oxtr in offspring hippocampus in a sex-specific manner. Int. J. Mol. Sci. 2019, 20, 329. [Google Scholar] [CrossRef]
- Jannat Ali Pour, N.; Meshkani, R.; Toolabi, K.; Mohassel Azadi, S.; Zand, S.; Emamgholipour, S. Adipose tissue mRNA expression of HDAC1, HDAC3 and HDAC9 in obese women in relation to obesity indices and insulin resistance. Mol. Biol. Rep. 2020, 47, 3459–3468. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Chen, Y.; Cheng, Y.; Li, J.; Zheng, L.; Zeng, X.; Luo, T. Diet-induced obesity is associated with altered expression of sperm motility-related genes and testicular post-translational modifications in a mouse model. Theriogenology 2020, 158, 233–238. [Google Scholar] [CrossRef]
- Çakır, I.; Hadley, C.K.; Pan, P.L.; Bagchi, R.A.; Ghamari-Langroudi, M.; Porter, D.T.; Wang, Q.; Litt, M.J.; Jana, S.; Hagen, S. Histone deacetylase 6 inhibition restores leptin sensitivity and reduces obesity. Nat. Metab. 2022, 4, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, Y.; Gao, L.; Yang, Z.; Lin, J.; Ren, S.; Li, F.; Chen, J.; Wang, Z.; Dong, Z. PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics 2022, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, N.; Mohammadi, S.; Yousefi, Z.; Golpour, P.; Taheri, A.; Maleki, M.H.; Nourbakhsh, M.; Nourbakhsh, M.; Azar, M.R. Assessment of global histone acetylation in pediatric and adolescent obesity: Correlations with SIRT1 expression and metabolic-inflammatory profiles. PLoS ONE 2023, 18, e0293217. [Google Scholar] [CrossRef] [PubMed]
- Fofana, M.; Li, Z.; Li, H.; Li, W.; Wu, L.; Lu, L.; Liu, Q. Decreased Ubiquitination and Acetylation of Histones 3 and 4 are Associated with Obesity-Induced Disorders of Spermatogenesis in Mice. Toxics 2024, 12, 296. [Google Scholar] [CrossRef]
- Elkhawaga, S.Y.; Ismail, A.; Elsakka, E.G.; Doghish, A.S.; Elkady, M.A.; El-Mahdy, H.A. miRNAs as cornerstones in adipogenesis and obesity. Life Sci. 2023, 315, 121382. [Google Scholar] [CrossRef]
- Liu, X.; Sun, H.; Zheng, L.; Zhang, J.; Su, H.; Li, B.; Wu, Q.; Liu, Y.; Xu, Y.; Song, X. Adipose-derived miRNAs as potential biomarkers for predicting adulthood obesity and its complications: A systematic review and bioinformatic analysis. Obes. Rev. 2024, 25, e13748. [Google Scholar] [CrossRef]
- Makarenkov, N.; Haim, Y.; Yoel, U.; Pincu, Y.; Tarnovscki, T.; Liberty, I.F.; Kukeev, I.; Baraf, L.; Dukhno, O.; Zilber, O. Circulating miRNAs Detect High vs. Low Visceral Adipose Tissue Inflammation in Patients Living with Obesity. J. Clin. Endocrinol. Metab. 2024, 109, 858–867. [Google Scholar] [CrossRef]
- Mir, F.A.; Mall, R.; Iskandarani, A.; Ullah, E.; Samra, T.A.; Cyprian, F.; Parray, A.; Alkasem, M.; Abdalhakam, I.; Farooq, F. Characteristic MicroRNAs linked to dysregulated metabolic pathways in Qatari adult subjects with obesity and metabolic syndrome. Front. Endocrinol. 2022, 13, 937089. [Google Scholar] [CrossRef]
- Simino, L.A.; Panzarin, C.; Fontana, M.F.; de Fante, T.; Geraldo, M.V.; Ignácio-Souza, L.M.; Milanski, M.; Torsoni, M.A.; Ross, M.G.; Desai, M. MicroRNA Let-7 targets AMPK and impairs hepatic lipid metabolism in offspring of maternal obese pregnancies. Sci. Rep. 2021, 11, 8980. [Google Scholar] [CrossRef]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba-Ssalamah, A.; de Gier, C.; Valent, I.; Item, C.B.; Greber-Platzer, S.; Zeyda, M. Circulating microRNAs 34a, 122, and 192 are linked to obesity-associated inflammation and metabolic disease in pediatric patients. Int. J. Obes. 2021, 45, 1763–1772. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Carpenter, A.A.; Loche, E.; Pantaleão, L.C.; Fernandez-Twinn, D.S.; Schoonejans, J.M.; Blackmore, H.L.; Ashmore, T.J.; Pisani, L.P.; Tadross, J.A. Effects of maternal diet-induced obesity on metabolic disorders and age-associated miRNA expression in the liver of male mouse offspring. Int. J. Obes. 2022, 46, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Eritja, À.; Caus, M.; Belmonte, T.; de Gonzalo-Calvo, D.; García-Carrasco, A.; Martinez, A.; Martínez, M.; Bozic, M. microRNA Expression Profile in Obesity-Induced Kidney Disease Driven by High-Fat Diet in Mice. Nutrients 2024, 16, 691. [Google Scholar] [CrossRef] [PubMed]
- Masoumi-Ardakani, Y.; Eghbalian, M.; Fallah, H.; Jafari, A.; Shahouzehi, B. Exploring serum miR-33b as a novel diagnostic marker for hypercholesterolemia and obesity: Insights from a pilot case-control study. BMC Endocr. Disord. 2025, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hou, Y.; Yu, W.; Wang, J.; Chu, X.; Zhang, X.; Pang, H.; Ma, D.; Tang, Y.; Li, M. Adipose tissue-derived microRNA-450a-5p induces type 2 diabetes mellitus by downregulating DUSP10. Mol. Biomed. 2025, 6, 7. [Google Scholar] [CrossRef]
- Nalvarte, I.; Rüegg, J.; Guerrero-Bosagna, C. Intrinsic and extrinsic factors that influence epigenetics. In Epigenetics and Assisted Reproduction; CRC Press: Boca Raton, FL, USA, 2018; pp. 99–114. [Google Scholar]
- Bombin, A.; Yan, S.; Bombin, S.; Mosley, J.D.; Ferguson, J.F. Obesity influences composition of salivary and fecal microbiota and impacts the interactions between bacterial taxa. Physiol. Rep. 2022, 10, e15254. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Q.; Zheng, W.; Steinwandel, M.; Blot, W.J.; Shu, X.-O.; Long, J. Oral microbiome and obesity in a large study of low-income and African-American populations. J. Oral Microbiol. 2019, 11, 1650597. [Google Scholar] [CrossRef]
- Nagpal, R.; Newman, T.M.; Wang, S.; Jain, S.; Lovato, J.F.; Yadav, H. Obesity-linked gut microbiome dysbiosis associated with derangements in gut permeability and intestinal cellular homeostasis independent of diet. J. Diabetes Res. 2018, 2018, 3462092. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Wu, Y.; Chi, X.; Zhang, Q.; Chen, F.; Deng, X. Characterization of the salivary microbiome in people with obesity. PeerJ 2018, 6, e4458. [Google Scholar] [CrossRef]
- Sohail, M.U.; Elrayess, M.A.; Al Thani, A.A.; Al-Asmakh, M.; Yassine, H.M. Profiling the oral microbiome and plasma biochemistry of obese hyperglycemic subjects in Qatar. Microorganisms 2019, 7, 645. [Google Scholar] [CrossRef]
- Ke, X.; Walker, A.; Haange, S.-B.; Lagkouvardos, I.; Liu, Y.; Schmitt-Kopplin, P.; Von Bergen, M.; Jehmlich, N.; He, X.; Clavel, T. Synbiotic-driven improvement of metabolic disturbances is associated with changes in the gut microbiome in diet-induced obese mice. Mol. Metab. 2019, 22, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Maya-Lucas, O.; Murugesan, S.; Nirmalkar, K.; Alcaraz, L.D.; Hoyo-Vadillo, C.; Pizano-Zárate, M.L.; García-Mena, J. The gut microbiome of Mexican children affected by obesity. Anaerobe 2019, 55, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cuesta, M.C.; Del Campo, R.; Garriga-García, M.; Peláez, C.; Requena, T. Taxonomic characterization and short-chain fatty acids production of the obese microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 598093. [Google Scholar] [CrossRef] [PubMed]
- Therdtatha, P.; Song, Y.; Tanaka, M.; Mariyatun, M.; Almunifah, M.; Manurung, N.E.P.; Indriarsih, S.; Lu, Y.; Nagata, K.; Fukami, K. Gut microbiome of Indonesian adults associated with obesity and type 2 diabetes: A cross-sectional study in an Asian city, Yogyakarta. Microorganisms 2021, 9, 897. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Thomas, C.; Minty, M.; Canceill, T.; Loubières, P.; Azalbert, V.; Tercé, F.; Champion, C.; Burcelin, R.; Barthet, P.; Laurencin-Dalicieux, S. Obesity drives an oral microbiota signature of female patients with periodontitis: A pilot study. Diagnostics 2021, 11, 745. [Google Scholar] [CrossRef]
- Dong, T.S.; Guan, M.; Mayer, E.A.; Stains, J.; Liu, C.; Vora, P.; Jacobs, J.P.; Lagishetty, V.; Chang, L.; Barry, R.L. Obesity is associated with a distinct brain-gut microbiome signature that connects Prevotella and Bacteroides to the brain’s reward center. Gut Microbes 2022, 14, 2051999. [Google Scholar] [CrossRef]
- Ma, X.; Brinker, E.; Graff, E.C.; Cao, W.; Gross, A.L.; Johnson, A.K.; Zhang, C.; Martin, D.R.; Wang, X. Whole-genome shotgun metagenomic sequencing reveals distinct gut microbiome signatures of obese cats. Microbiol. Spectr. 2022, 10, e0083722. [Google Scholar] [CrossRef]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; Zhong, Y.; Turner, D.; Lan, R.S.; Pack, L.M.; Piccolo, B.D.; Chintapalli, S.V.; Abraham, A. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr. Obes. 2022, 17, e12921. [Google Scholar] [CrossRef]
- Ma, T.; Wu, Z.; Lin, J.; Shan, C.; Abasijiang, A.; Zhao, J. Characterization of the oral and gut microbiome in children with obesity aged 3 to 5 years. Front. Cell. Infect. Microbiol. 2023, 13, 1102650. [Google Scholar] [CrossRef]
- Vallès, Y.; Arshad, M.; Abdalbaqi, M.; Inman, C.K.; Ahmad, A.; Drou, N.; Gunsalus, K.C.; Ali, R.; Tahlak, M.; Abdulle, A. The infants’ gut microbiome: Setting the stage for the early onset of obesity. Front. Microbiol. 2024, 15, 1371292. [Google Scholar] [CrossRef] [PubMed]
- Stols-Gonçalves, D.; Tristão, L.S.; Henneman, P.; Nieuwdorp, M. Epigenetic markers and microbiota/metabolite-induced epigenetic modifications in the pathogenesis of obesity, metabolic syndrome, type 2 diabetes, and non-alcoholic fatty liver disease. Curr. Diabetes Rep. 2019, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Molina, B.; Sánchez-Alcoholado, L.; Cabrera-Mulero, A.; Lopez-Dominguez, R.; Carmona-Saez, P.; Garcia-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Gut microbiota composition is associated with the global DNA methylation pattern in obesity. Front. Genet. 2019, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Salas-Perez, F.; Assmann, T.S.; Ramos-Lopez, O.; Martínez, J.A.; Riezu-Boj, J.I.; Milagro, F.I. Crosstalk between gut microbiota and epigenetic markers in obesity development: Relationship between Ruminococcus, BMI, and MACROD2/SEL1L2 methylation. Nutrients 2023, 15, 1550. [Google Scholar] [CrossRef]
- Yan, H.; Qin, Q.; Chen, J.; Yan, S.; Li, T.; Gao, X.; Yang, Y.; Li, A.; Ding, S. Gut microbiome alterations in patients with visceral obesity based on quantitative computed tomography. Front. Cell. Infect. Microbiol. 2022, 11, 823262. [Google Scholar] [CrossRef]
- Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K.; Wade, P.A. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Galuppo, B.; Cline, G.; Van Name, M.; Shabanova, V.; Wagner, D.; Kien, C.L.; Santoro, N. Colonic fermentation and acetate production in youth with and without obesity. J. Nutr. 2021, 151, 3292–3298. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Li, J.; Wang, X.; Zhang, M.; Du, M.; Jiang, W.; Li, C. Butyrate and propionate are negatively correlated with obesity and glucose levels in patients with type 2 diabetes and obesity. Diabetes Metab. Syndr. Obes. 2024, 17, 1533–1541. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Li, L.; Liang, X.; Wu, Y.; Wang, X.; Ma, H.; Cheng, J.; Zhang, A.; Tang, P. Gut microbiota induces DNA methylation via SCFAs predisposing obesity-prone individuals to diabetes. Pharmacol. Res. 2022, 182, 106355. [Google Scholar] [CrossRef]
- Kern, L.; Kviatcovsky, D.; He, Y.; Elinav, E. Impact of caloric restriction on the gut microbiota. Curr. Opin. Microbiol. 2023, 73, 102287. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Grenkowitz, S.; Schlickeiser, S.; Sandforth, A.; Friedrich, M.; Kunkel, D.; Glauben, R.; Brachs, S.; Mai, K.; Thürmer, A. Effects of caloric restriction on the gut microbiome are linked with immune senescence. Microbiome 2022, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.-L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N. The intestinal microbiome predicts weight loss on a calorie-restricted diet and is associated with improved hepatic steatosis. Front. Nutr. 2021, 8, 718661. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, M.; You, X.; Zhao, J.; Chen, L.; Wang, L.; Luo, Y.; Chen, Y. Gut microbiota mediates the anti-obesity effect of calorie restriction in mice. Sci. Rep. 2018, 8, 13037. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Skurk, T.; Hastreiter, L.; Lagkouvardos, I.; Fischer, S.; Büttner, J.; Kellerer, T.; Clavel, T.; Rychlik, M.; Haller, D. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017, 7, 11955. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Y.; Li, T.; Zhu, H.; Huang, F.; Yang, C.; Guo, F. Calorie restriction on normal body weight mice prevents body weight regain on a follow-up high-fat diet by shaping an obesity-resistant-like gut microbiota profile. Food Funct. 2022, 13, 7684–7696. [Google Scholar] [CrossRef]
- Dong, T.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.; Dreskin, B.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N. A high protein calorie restriction diet alters the gut microbiome in obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Li, Q.; Song, C.; Han, N.; Yan, T.; Zhang, L.; Ren, D.; Zhao, Y.; Yang, X. Caloric restriction, friend or foe: Effects on metabolic status in association with the intestinal microbiome and metabolome. J. Agric. Food Chem. 2022, 70, 14061–14072. [Google Scholar] [CrossRef]
- Aragón-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Álvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of exercise on gut microbiota in obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef]
- Nagano, T.; Yano, H. Effect of dietary cellulose nanofiber and exercise on obesity and gut microbiota in mice fed a high-fat-diet. Biosci. Biotechnol. Biochem. 2020, 84, 613–620. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, R.J.; Konstanti, P.; Smidt, H.; Hermus, A.R.; Thijssen, D.H.; Hopman, M.T. Eight-week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obesity 2021, 29, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zuo, Y.; Wen, S.; Wang, X.; Liu, Y.; Li, T. Impact of exercise training on gut microbiome imbalance in obese individuals: A study based on Mendelian randomization analysis. Front. Physiol. 2024, 14, 1264931. [Google Scholar] [CrossRef] [PubMed]
- Torquati, L.; Gajanand, T.; Cox, E.; Willis, C.; Zaugg, J.; Keating, S.; Coombes, J. Effects of exercise intensity on gut microbiome composition and function in people with type 2 diabetes. Eur. J. Sport Sci. 2023, 23, 530–541. [Google Scholar] [CrossRef]
- Poursalehi, D.; Lotfi, K.; Mirzaei, S.; Asadi, A.; Akhlaghi, M.; Saneei, P. Association between methyl donor nutrients and metabolic health status in overweight and obese adolescents. Sci. Rep. 2022, 12, 17045. [Google Scholar] [CrossRef]
- Du, J.; Zhang, P.; Luo, J.; Shen, L.; Zhang, S.; Gu, H.; He, J.; Wang, L.; Zhao, X.; Gan, M. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes 2021, 13, 1862612. [Google Scholar] [CrossRef]
- Jin, L.; Duan, B.; Liu, J.; Zhang, X.; Li, X.; Zhu, X.; Xin, Y.; Li, Y.; Alsareii, S.A.; Wang, Q. Dietary Betaine Attenuates Obesity: Involvement of Hepatic MicroRNAs. Sci. Adv. Mater. 2021, 13, 2319–2326. [Google Scholar] [CrossRef]
- Kushwaha, V.; Rai, P.; Varshney, S.; Gupta, S.; Khandelwal, N.; Kumar, D.; Gaikwad, A.N. Sodium butyrate reduces endoplasmic reticulum stress by modulating CHOP and empowers favorable anti-inflammatory adipose tissue immune-metabolism in HFD fed mice model of obesity. Food Chem. Mol. Sci. 2022, 4, 100079. [Google Scholar] [CrossRef]
- Cavaliere, G.; Catapano, A.; Trinchese, G.; Cimmino, F.; Penna, E.; Pizzella, A.; Cristiano, C.; Lama, A.; Crispino, M.; Mollica, M.P. Butyrate improves neuroinflammation and mitochondrial impairment in cerebral cortex and synaptic fraction in an animal model of diet-induced obesity. Antioxidants 2022, 12, 4. [Google Scholar] [CrossRef]
- Zhu, W.; Peng, K.; Zhao, Y.; Xu, C.; Tao, X.; Liu, Y.; Huang, Y.; Yang, X. Sodium butyrate attenuated diet-induced obesity, insulin resistance and inflammation partly by promoting fat thermogenesis via intro-adipose sympathetic innervation. Front. Pharmacol. 2022, 13, 938760. [Google Scholar] [CrossRef]
- Wang, X.; Duan, C.; Li, Y.; Lu, H.; Guo, K.; Ge, X.; Chen, T.; Shang, Y.; Liu, H.; Zhang, D. Sodium butyrate reduces overnutrition-induced microglial activation and hypothalamic inflammation. Int. Immunopharmacol. 2022, 111, 109083. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik Jr, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar] [CrossRef]
- Arnoldussen, I.A.; Wiesmann, M.; Pelgrim, C.E.; Wielemaker, E.M.; van Duyvenvoorde, W.; Amaral-Santos, P.; Verschuren, L.; Keijser, B.J.; Heerschap, A.; Kleemann, R. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int. J. Obes. 2017, 41, 935–944. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef]
- Fang, W.; Xue, H.; Chen, X.; Chen, K.; Ling, W. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. J. Nutr. 2019, 149, 747–754. [Google Scholar] [CrossRef]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef]
- Tang, X.; Sun, Y.; Li, Y.; Ma, S.; Zhang, K.; Chen, A.; Lyu, Y.; Yu, R. Sodium butyrate protects against oxidative stress in high-fat-diet-induced obese rats by promoting GSK-3β/Nrf2 signaling pathway and mitochondrial function. J. Food Biochem. 2022, 46, e14334. [Google Scholar] [CrossRef]
- Fu, Q.; Li, T.; Zhang, C.; Ma, X.; Meng, L.; Liu, L.; Shao, K.; Wu, G.; Zhu, X.; Zhao, X. Butyrate mitigates metabolic dysfunctions via the ERα-AMPK pathway in muscle in OVX mice with diet-induced obesity. Cell Commun. Signal. 2023, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zheng, M.; Hu, M.; Fang, X.; Geng, D.; Liu, S.; Wang, L.; Zhang, J.; Guan, L.; Zheng, P. Butyrate ameliorates quinolinic acid–induced cognitive decline in obesity models. J. Clin. Investig. 2023, 133, e154612. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.; Han, Y.; Song, S.; Kwon, S.Y.; Na, K.; Lindroth, A.M.; Park, Y.J. Anti-obesity effect of butyrate links to modulation of gut microbiome and epigenetic regulation of muscular circadian clock. J. Nutr. Biochem. 2024, 127, 109590. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, M.; Cheng, X.; Kang, G.; Cao, X.; Huang, H. Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice. Microb. Cell Factories 2020, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, X.; Bai, L.; Gao, M.; Kang, G.; Cao, X.; Huang, H. Positive interventional effect of engineered butyrate-producing bacteria on metabolic disorders and intestinal flora disruption in obese mice. Microbiol. Spectr. 2022, 10, e0114721. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Yan, H.; Wang, Q.; Wang, H. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition 2021, 87–88, 111198. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef]
- Petersen, K.F.; Impellizeri, A.; Cline, G.W.; Shulman, G.I. The effects of increased acetate turnover on glucose-induced insulin secretion in lean and obese humans. J. Clin. Transl. Sci. 2019, 3, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fan, X.; Lu, Y.; Chen, D.; Zhao, Y.; Qi, K. Dietary acetic acid suppress high-fat diet-induced obesity in mice by altering taurine conjugated bile acids metabolism. Curr. Res. Food Sci. 2022, 5, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Mandaliya, D.K.; Patel, S.; Seshadri, S. The combinatorial effect of acetate and propionate on high-fat diet induced diabetic inflammation or metaflammation and T cell polarization. Inflammation 2021, 44, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Eslick, S.; Williams, E.J.; Berthon, B.S.; Wright, T.; Karihaloo, C.; Gately, M.; Wood, L.G. Weight loss and short-chain fatty acids reduce systemic inflammation in monocytes and adipose tissue macrophages from obese subjects. Nutrients 2022, 14, 765. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Liang, A.; Fan, X.; Wang, R.; Li, P.; Qi, K. Effects of SCFA on the DNA methylation pattern of adiponectin and resistin in high-fat-diet-induced obese male mice. Br. J. Nutr. 2018, 120, 385–392. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Kappo, K.A.; Petzke, K.J.; Kipp, A.P.; Blaut, M.; Klaus, S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol. Nutr. Food Res. 2016, 60, 2611–2621. [Google Scholar] [CrossRef]
- Han, K.; Meadows, A.M.; Rodman, M.J.; Russo, A.C.; Sharma, R.; Singh, K.; Hassanzadeh, S.; Dagur, P.K.; Huffstutler, R.D.; Krause, F.N. Propionate functions as a feeding state-dependent regulatory metabolite to counter proinflammatory signaling linked to nutrient load and obesity. J. Leukoc. Biol. 2024, 115, 738–749. [Google Scholar] [CrossRef]
- Miyamoto, J.; Ando, Y.; Yamano, M.; Nishida, A.; Murakami, K.; Kimura, I. Acidipropionibacterium acidipropionici, a propionate-producing bacterium, contributes to GPR41 signaling and metabolic regulation in high-fat diet-induced obesity in mice. Front. Nutr. 2025, 12, 1542196. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D′Angelo, C.; Massi-Benedetti, C.; Fallarino, F. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS ONE 2013, 8, e80604. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Xin, F.-Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chimerel, C.; Emery, E.; Summers, D.; Keyser, U.; Gribble, F.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, M.; Zhao, J.; Song, Y.; Du, W.; Shi, J. The mechanism underlying the influence of indole-3-propionic acid: A relevance to metabolic disorders. Front. Endocrinol. 2022, 13, 841703. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; Liu, L.; Mao, A.; Kan, H.; Yu, F.; Ma, X.; Feng, L.; Zhou, T. The gut microbiota-derived metabolite indole-3-propionic acid enhances leptin sensitivity by targeting STAT3 against diet-induced obesity. Clin. Transl. Med. 2024, 14, e70053. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Bai, J.; Zhao, N.; Wang, Q.; Zhou, R.; Li, G.; Hu, C.; Li, X.; Tao, K. Role of indole-3-acetic acid in NAFLD amelioration after sleeve gastrectomy. Obes. Surg. 2021, 31, 3040–3052. [Google Scholar] [CrossRef]
- DiMattia, Z.; Damani, J.J.; Van Syoc, E.; Rogers, C.J. Effect of probiotic supplementation on intestinal permeability in overweight and obesity: A systematic review of randomized controlled trials and animal studies. Adv. Nutr. 2024, 15, 100162. [Google Scholar] [CrossRef]
- Judkins, T.C.; Archer, D.L.; Kramer, D.C.; Solch, R.J. Probiotics, nutrition, and the small intestine. Curr. Gastroenterol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Ghalichi, F.; Ahrabi, S.S.; Jamilian, P.; Jamilian, P.; Ghoreishi, Z. Anti-obesity properties of probiotics; a considerable medical nutrition intervention: Findings from an umbrella meta-analysis. Eur. J. Pharmacol. 2022, 928, 175069. [Google Scholar] [CrossRef]
- Wang, X.; Ba, T.; Cheng, Y.; Zhang, P.; Chang, X. Probiotics alleviate adipose inflammation in high-fat diet–induced obesity by restoring adipose invariant natural killer T cells. Nutrition 2021, 89, 111285. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Janchot, K.; Kanacharoen, S.; Lertmongkolaksorn, T.; Wongsaroj, L.; Somboonna, N.; Ngamwongsatit, N.; Leelahavanichkul, A. Lactiplantibacillus plantarum dfa1 outperforms enterococcus faecium dfa1 on anti-obesity in high fat-induced obesity mice possibly through the differences in gut dysbiosis attenuation, despite the similar anti-inflammatory properties. Nutrients 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Vähämiko, S.; Laiho, A.; Lund, R.; Isolauri, E.; Salminen, S.; Laitinen, K. The impact of probiotic supplementation during pregnancy on DNA methylation of obesity-related genes in mothers and their children. Eur. J. Nutr. 2019, 58, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.-K.; Tang, M.; Lei, L.; Li, J.-R.; Sun, H.; Jiang, J.; Dong, B.; Li, H.-Y.; Jiang, J.-D. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 2024, 16, 2304159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ye, H.; Yang, X.; Shen, L.; Dang, X.; Liu, X.; Gong, Y.; Wu, Q.; Wang, L.; Ge, X. Probiotic Clostridium butyricum ameliorates cognitive impairment in obesity via the microbiota-gut-brain axis. Brain Behav. Immun. 2024, 115, 565–587. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, S.I.; Kim, N.; Nam, R.H.; Jang, J.Y.; Na, H.Y.; Shin, C.M.; Lee, D.H.; Min, H.; Kim, Y.-R. Effect of Clostridium butyricum on high-fat diet-induced intestinal inflammation and production of short-chain fatty acids. Dig. Dis. Sci. 2023, 68, 2427–2440. [Google Scholar] [CrossRef]
- Vu, V.; Muthuramalingam, K.; Singh, V.; Hyun, C.; Kim, Y.M.; Unno, T.; Cho, M. Effects of β-glucan, probiotics, and synbiotics on obesity-associated colitis and hepatic manifestations in C57BL/6J mice. Eur. J. Nutr. 2022, 61, 793–807. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Zhao, L.; Yu, D.; Meng, K.; Yang, P. Lactobacillus plantarum FRT4 alleviated obesity by modulating gut microbiota and liver metabolome in high-fat diet-induced obese mice. Food Nutr. Res. 2022, 66, 7974. [Google Scholar] [CrossRef]
- Won, S.-M.; Chen, S.; Lee, S.Y.; Lee, K.E.; Park, K.W.; Yoon, J.-H. Lactobacillus sakei ADM14 induces anti-obesity effects and changes in gut microbiome in high-fat diet-induced obese mice. Nutrients 2020, 12, 3703. [Google Scholar] [CrossRef]
- Joung, H.; Chu, J.; Kim, B.-K.; Choi, I.-S.; Kim, W.; Park, T.-S. Probiotics ameliorate chronic low-grade inflammation and fat accumulation with gut microbiota composition change in diet-induced obese mice models. Appl. Microbiol. Biotechnol. 2021, 105, 1203–1213. [Google Scholar] [CrossRef]
- Benvenuti, L.; D′Antongiovanni, V.; Pellegrini, C.; Fornai, M.; Bernardini, N.; Ippolito, C.; Segnani, C.; Di Salvo, C.; Colucci, R.; Martelli, A. Dietary supplementation with the probiotic SF68 reinforces intestinal epithelial barrier in obese mice by improving butyrate bioavailability. Mol. Nutr. Food Res. 2023, 67, 2200442. [Google Scholar] [CrossRef]
- Yavorov-Dayliev, D.; Milagro, F.I.; López-Yoldi, M.; Clemente, I.; Riezu-Boj, J.I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici (pA1c®) alleviates obesity-related dyslipidemia and inflammation in Wistar rats by activating beta-oxidation and modulating the gut microbiota. Food Funct. 2023, 14, 10855–10867. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; El-Bacha, T.; Rosado, E.L. Modulation of the gut microbiota by probiotics and symbiotics is associated with changes in serum metabolite profile related to a decrease in inflammation and overall benefits to metabolic health: A double-blind randomized controlled clinical trial in women with obesity. Food Funct. 2021, 12, 2161–2170. [Google Scholar] [PubMed]
- Chen, A.-C.; Fang, T.-J.; Ho, H.-H.; Chen, J.-F.; Kuo, Y.-W.; Huang, Y.-Y.; Tsai, S.-Y.; Wu, S.-F.; Lin, H.-C.; Yeh, Y.-T. A multi-strain probiotic blend reshaped obesity-related gut dysbiosis and improved lipid metabolism in obese children. Front. Nutr. 2022, 9, 922993. [Google Scholar] [CrossRef]
- Kang, M.; Choe, D.; Kim, K.; Cho, B.-K.; Cho, S. Synthetic biology approaches in the development of engineered therapeutic microbes. Int. J. Mol. Sci. 2020, 21, 8744. [Google Scholar] [CrossRef]
- Patra, D. Synthetic Biology-Enabled Engineering of Probiotics for Precision and Targeted Therapeutic Delivery Applications. Exon 2024, 1, 54–66. [Google Scholar] [CrossRef]
- Nazir, A.; Hussain, F.H.N.; Raza, A. Advancing microbiota therapeutics: The role of synthetic biology in engineering microbial communities for precision medicine. Front. Bioeng. Biotechnol. 2024, 12, 1511149. [Google Scholar] [CrossRef]
- Bober, J.R.; Beisel, C.L.; Nair, N.U. Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. Annu. Rev. Biomed. Eng. 2018, 20, 277–300. [Google Scholar] [CrossRef]
- Dou, J.; Bennett, M.R. Synthetic biology and the gut microbiome. Biotechnol. J. 2018, 13, 1700159. [Google Scholar] [CrossRef]
- Chua, K.J.; Kwok, W.C.; Aggarwal, N.; Sun, T.; Chang, M.W. Designer probiotics for the prevention and treatment of human diseases. Curr. Opin. Chem. Biol. 2017, 40, 8–16. [Google Scholar] [CrossRef]
- Goh, Y.J.; Barrangou, R. Harnessing CRISPR-Cas systems for precision engineering of designer probiotic lactobacilli. Curr. Opin. Biotechnol. 2019, 56, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The potential impact of probiotics on human health: An update on their health-promoting properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Yoshikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Tsuji, A.; Matsuda, S. Encouraging tactics with genetically modified probiotics to improve immunity for the prevention of immune-related diseases including cardio-metabolic disorders. Biomolecules 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Mays, Z.J.; Nair, N.U. Synthetic biology in probiotic lactic acid bacteria: At the frontier of living therapeutics. Curr. Opin. Biotechnol. 2018, 53, 224–231. [Google Scholar] [CrossRef]
- Zhu, L.-B.; Zhang, Y.-C.; Huang, H.-H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Duan, F.F.; Liu, J.H.; March, J.C. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes 2015, 64, 1794–1803. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Chen, Z.; Guo, Y.; McMillan, C.; Flynn, C.R.; Davies, S.S. Two-week administration of engineered Escherichia coli establishes persistent resistance to diet-induced obesity even without antibiotic pre-treatment. Appl. Microbiol. Biotechnol. 2019, 103, 6711–6723. [Google Scholar] [CrossRef]
- Chen, X.; Gao, M.; Wang, L.; Qiang, G.; Wu, Y.; Huang, H.; Kang, G. A synthetic microbial consortium protects against obesity by regulating vitamin B6 metabolism. Gut Microbes 2024, 16, 2304901. [Google Scholar] [CrossRef]
- Rasaei, N.; Heidari, M.; Esmaeili, F.; Khosravi, S.; Baeeri, M.; Tabatabaei-Malazy, O.; Emamgholipour, S. The effects of prebiotic, probiotic or synbiotic supplementation on overweight/obesity indicators: An umbrella review of the trials’ meta-analyses. Front. Endocrinol. 2024, 15, 1277921. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Zhang, L.; Chen, C.; Wei, Y.; Shen, Z.-a. Effects of oral supplementation of probiotics on body weight and visceral fat in obese patients: A meta-analysis and systematic review. Sci. Rep. 2025, 15, 6355. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. An update on prebiotics and on their health effects. Foods 2024, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a tool for the prevention and treatment of obesity and diabetes: Classification and ability to modulate the gut microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Y.; Zhou, C.; Zhao, Q.; Zhong, H.; Zhu, X.; Fu, T.; Pan, L.; Shang, Q.; Yu, G. Dietary polysaccharide from Enteromorpha clathrata attenuates obesity and increases the intestinal abundance of butyrate-producing bacterium, Eubacterium xylanophilum, in mice fed a high-fat diet. Polymers 2021, 13, 3286. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lyu, Y.; Xu, H.; Luo, H.; Yin, X.; Zheng, H. Raspberry polysaccharides attenuate hepatic inflammation and oxidative stress in diet-induced obese mice by enhancing butyrate-mediated intestinal barrier function. Int. J. Biol. Macromol. 2024, 262, 130007. [Google Scholar] [CrossRef]
- Naudhani, M.; Thakur, K.; Ni, Z.-J.; Zhang, J.-G.; Wei, Z.-J. Formononetin reshapes the gut microbiota, prevents progression of obesity and improves host metabolism. Food Funct. 2021, 12, 12303–12324. [Google Scholar] [CrossRef]
- Wen, J.-J.; Li, M.-Z.; Hu, J.-L.; Wang, J.; Wang, Z.-Q.; Chen, C.-H.; Yang, J.-R.; Huang, X.-J.; Xie, M.-Y.; Nie, S.-P. Different dietary fibers unequally remodel gut microbiota and charge up anti-obesity effects. Food Hydrocoll. 2023, 140, 108617. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, H.; Fan, K.; Xu, H.; Huang, Y.; Zheng, Y.; Xu, Q.; Li, C.; Zhao, L.; Li, Y. Targeting gut microbiota and host metabolism with Dendrobium officinale dietary fiber to prevent obesity and improve glucose homeostasis in diet-induced obese mice. Mol. Nutr. Food Res. 2022, 66, 2100772. [Google Scholar] [CrossRef]
- Wang, B.; Yu, H.; He, Y.; Wen, L.; Gu, J.; Wang, X.; Miao, X.; Qiu, G.; Wang, H. Effect of soybean insoluble dietary fiber on prevention of obesity in high-fat diet fed mice via regulation of the gut microbiota. Food Funct. 2021, 12, 7923–7937. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Gao, J.; Wu, X.; Cui, C.; Wei, H.; Peng, J.; Zheng, R. The effect of functional fiber on microbiota composition in different intestinal segments of obese mice. Int. J. Mol. Sci. 2021, 22, 6525. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, N.; Yang, L.; Hong, Z.; Cai, B.; Le, Q.; Yang, T.; Shi, L.; He, J.; Cui, C.-B. Insoluble dietary fiber derived from brown seaweed Laminaria japonica ameliorate obesity-related features via modulating gut microbiota dysbiosis in high-fat diet–fed mice. Food Funct. 2021, 12, 587–601. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, M.; Wang, H.; Li, N.; Lu, Z.; Li, L.; Hui, S.; Xu, H. Gut microbiota and short chain fatty acids partially mediate the beneficial effects of inulin on metabolic disorders in obese ob/ob mice. J. Food Biochem. 2022, 46, e14063. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Han, J.; Li, C.; Tang, J.; Wang, X.; Ma, Y.; Chen, Y.; Xiao, D.; Guo, X. Tibetan highland barley fiber improves obesity and regulates gut microbiota in high-fat diet-fed mice. Food Biosci. 2023, 53, 102620. [Google Scholar] [CrossRef]
- Thompson, S.V.; Bailey, M.A.; Taylor, A.M.; Kaczmarek, J.L.; Mysonhimer, A.R.; Edwards, C.G.; Reeser, G.E.; Burd, N.A.; Khan, N.A.; Holscher, H.D. Avocado consumption alters gastrointestinal bacteria abundance and microbial metabolite concentrations among adults with overweight or obesity: A randomized controlled trial. J. Nutr. 2021, 151, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, Y.; Li, J.; Wu, T. Swimming combined with corn peptide counteracts high-fat diet-induced obesity through rescuing imbalanced gut flora. Adv. Exerc. Health Sci. 2024, 1, 260–269. [Google Scholar] [CrossRef]
- Shih, M.-K.; Tain, Y.-L.; Chen, Y.-W.; Hsu, W.-H.; Yeh, Y.-T.; Chang, S.K.; Liao, J.-X.; Hou, C.-Y. Resveratrol butyrate esters inhibit obesity caused by perinatal exposure to bisphenol a in female offspring rats. Molecules 2021, 26, 4010. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin alleviates obesity-associated metabolic syndrome by regulating the composition of the gut microbiome. Front. Microbiol. 2022, 12, 805827. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Lu, F.; Wang, X.; Liao, X.; Hu, X.; Zhang, Y. Beneficial effects of a chlorophyll-rich spinach extract supplementation on prevention of obesity and modulation of gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2019, 60, 103436. [Google Scholar] [CrossRef]
- Liu, X.; Hu, G.; Wang, A.; Long, G.; Yang, Y.; Wang, D.; Zhong, N.; Jia, J. Black tea reduces diet-induced obesity in mice via modulation of gut microbiota and gene expression in host tissues. Nutrients 2022, 14, 1635. [Google Scholar] [CrossRef]
- Youn, H.-Y.; Seo, K.-H.; Kim, H.-J.; Kim, Y.-S.; Kim, H. Effect of postbiotics derived from kefir lactic acid bacteria-mediated bioconversion of citrus pomace extract and whey on high-fat diet-induced obesity and gut dysbiosis. Food Res. Int. 2022, 162, 111930. [Google Scholar] [CrossRef]
- Yao, H.; Fan, C.; Lu, Y.; Fan, X.; Xia, L.; Li, P.; Wang, R.; Tang, T.; Wang, Y.; Qi, K. Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice. Genes Nutr. 2020, 15, 12. [Google Scholar] [CrossRef]
- Nandy, D.; Craig, S.J.; Cai, J.; Tian, Y.; Paul, I.M.; Savage, J.S.; Marini, M.E.; Hohman, E.E.; Reimherr, M.L.; Patterson, A.D. Metabolomic profiling of stool of two-year old children from the INSIGHT study reveals links between butyrate and child weight outcomes. Pediatr. Obes. 2022, 17, e12833. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tun, H.M.; Ng, S.C.; Wai, H.K.-F.; Zhang, X.; Parks, J.; Field, C.J.; Mandhane, P.; Moraes, T.J.; Simons, E. Maternal smoking during pregnancy increases the risk of gut microbiome-associated childhood overweight and obesity. Gut Microbes 2024, 16, 2323234. [Google Scholar] [CrossRef] [PubMed]
- Durazzi, F.; Sala, C.; Castellani, G.; Manfreda, G.; Remondini, D.; De Cesare, A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 2021, 11, 3030. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.M.; Leong, L.E.; Rogers, G.B. Sample storage conditions significantly influence faecal microbiome profiles. Sci. Rep. 2015, 5, 16350. [Google Scholar] [CrossRef]


| Sample Type | Affected Genes | Main Outcome | Ref. |
|---|---|---|---|
| Sperm | TP53AIP1, SPATA21, PTPRN2, and ZNF33A | 3264 differentially methylated CpG sites between normal weight and obese men; hypomethylation of TP53AIP1 and SPATA21 in obese vs. normal weight men | [42] |
| Visceral adipose tissue (VAT) | S100A8 and S100A9 | Hypomethylation and overexpression of S100A8 and S100A9 in obese subjects | [43] |
| VAT | Angiotensin-converting enzyme 2 (ACE2) gene | Hypermethylation of ACE2 gene in obese vs. normal subjects | [44] |
| Placenta | METTL-3/-14, WTAP, RBM15B, and KIAA1429 | Elevated levels of 5-methylcytosine (5mC) and reduced activity of Ten-Eleven Translocation (TETs) enzymes; reduced N6-methyladenosine (m6A) levels and RBM15B, WTAP, and KIAA1429 expression in the placenta with maternal OB | [45] |
| Blood | PTPRN2 and MAD1L1 | 1725 differentially methylated regions (DMRs) in male neonates from women with OB vs. normal weight women (1173 regions hypermethylated and 552 hypomethylated) | [46] |
| VAT | ATP11A, LPL, and EHD2 | 11120 differentially methylated CpGs and 96 DMRs in women with OB and T2DM vs. without T2DM | [47] |
| Liver | CACNA1B, CNR1, GNAI3, PRKCA, GNGT2, GNG12, ADCY6, and DRD2 | Altered DNAmet of 3169 CpGs in OB | [48] |
| Blood | PCSK7, RNF214, SYN3, JARID2, OCA2, and POLR2C | Lower DNAmet at cg03158092 and cg05985988 sites are linked to insulin resistance and beta-cell function in early pregnancy; DNAmet of cg12082129 and cg11955198 sites correlate with higher insulin concentrations in late pregnancy | [49] |
| Peripheral blood leukocytes | GLP1R | Association between OB and DNAmet of the intronic region of GLP1R | [50] |
| Blood | TFAM and PIEZO1 | Lower levels of DNAmet in obese vs. normal weight children; altered DNAmet of cg05831083 and cg14926485 sites in obese vs. normal weight children | [51] |
| Blood | BMAL1 | DNAmet of BMAL1 is associated with obese phenotype | [52] |
| Leukocytes | AHDC1, ANXA7, MED12L, TBXAS1, and ENGASE | Lower levels of DNAmet in the top differentially methylated positions in OB | [53] |
| Blood | TNF-α | Lower DNAmet of TNF-α in OB vs. normal weight individuals | [54] |
| Cord blood and placenta | PLIN4, UBE2F, and PPP1R16B | Association between DNAmet profiles of certain genes, including PLIN4, UBE2F, and PPP1R16B in cord blood and infant weight | [55] |
| Leukocytes | CPT1B | Higher DNAmet of CPT1B gene (involved in lipid oxidation), is linked to lower serum selenium in obese vs. normal weight individuals | [56] |
| Type of Histone Modification/Study Subjects | Sample Type | Affected Genes | Key Findings | Ref. |
|---|---|---|---|---|
| Histone acetylation/mouse | Hippocampus | oxytocin receptor (Oxtr) | increased H3K9Ac (an active histone mark) binding at the Oxtr promoter in male offspring of maternal HFD | [60] |
| Histone deacetylase enzymes/Human | Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) | HDAC3 and HDAC9 | Reduced levels of HDAC3 in the SAT and HDAC9 in the VAT in obese vs. normal wight women | [61] |
| Various histone modifications (acetylation, propionylation, and crotonylation/mouse | Testis | AKAP4, ODF2, PRKACB, SPAG6, LDHC, PGK2 and GAPDHS | Reduced levels of testicular H4K8ac, H3K122ac, H3K23pr and H4K8cr in obese mice vs. controls | [62] |
| Histone deacetylation enzyme/mouse | Adipose tissue | Leptin | Increased activity of the cytosolic histone deacetylase 6 (HDAC6) in obese mice | [63] |
| Histone acetylation and methylation/mouse and human | White adipose tissue (WAT) | Bmal1, PPAR-γ, and Slc1a5 | Reduced H3K27ac and H3K4me3 at Bmal1 promoter due to decreased methionine and glutamine levels in obese WAT | [64] |
| Histone acetylation/Human | Peripheral blood mononuclear cells | SIRT1 | Higher histone acetylation and decreased expression of SIRT1 in OB vs. control subjects | [65] |
| Histone acetylation/mouse | Testis | GRTH/DDX25, CRM1, HMGB2, PGK2, and tACE | Decreased H3AcK18 and H4tetraAck (histone H4AcK5, K8, K12 and K16), and aberrant protamine 1 deposition | [66] |
| Affected miRNA/Study Subjects | Sample Type | Affected Genes | Key Findings | Ref. |
|---|---|---|---|---|
| miRNA Let-7/mouse | Liver | AMPK | Overexpression of Let-7 in the newborn mice from obese dams | [71] |
| miRNA 192/human | Serum | TNFα, IL-1Ra, and procalcitonin | miRNA 192 upregulation in metabolically unhealthy OB | [72] |
| miR-582-3p and miR-582-5p/mouse | Liver | AMPKα, SAPK/JNK, Tgfβ1, Map3k14, Bax/Bcl-2, and Col1a1 | Correlation between maternal OB and elevated hepatic miR-582-3p and miR-582-5p | [73] |
| miR-5099, miR-551b-3p, miR-223-3p, miR-146a-3p and miR-21a-3p/mouse | Kidney | Adiposity-related pro-inflammatory and pro-fibrotic genes (MCP1, RANTES, TNFα, and iNOS | Differences in the expression of nine miRNAs upon HFD feeding vs. standard diet | [74] |
| miR-33b/human | Serum | ABCA1, CROT, HADHB, and NPC1 | Hyperexpression of miR-33b in obese vs. control subjects | [75] |
| microRNA-450a-5p/mouse | Serum, liver, and white adipose tissue | DUSP10 | Increased expression of microRNA-450a-5p in obese mice | [76] |
| Subjects/Case # or Condition | Type of Microbiome/Sample | Key Finding | Ref. |
|---|---|---|---|
| Human/lean and obese, young, Chinese | Gut microbiome (GM)/fecal samples | Reduced level of Bacteroides thetaiotaomicron, a glutamate-fermenting commensal, in obese vs. normal subjects | [81] |
| Human/33 adults with OB and 29 normal weight controls | Oral microbiome/saliva | Reduced bacterial diversity and richness in OB; increased abundance of Solobacterium, Mogibacterium, Prevotella, Granulicatella, Peptostreptococcus, and Catonella, and reduced abundance of Capnocytophaga, Haemophilus, Corynebacterium, and Staphylococcus in OB | [82] |
| Human/obese hyperglycemic individuals in Qatar | Oral microbiome/saliva | Increased Firmicutes/Bacteroidetes ratio and reduced Fusobacteria phylum in OB vs. controls subjects | [83] |
| Mice/HFD-fed mice at 12 weeks | GM/fecal and cecal samples | Reduced abundance of Lactobacillaceae, Bifidobacteriaceae, Erysipelotrichaceae and Verrucomicrobiaceae following HFD consumption | [84] |
| Human/Mexican children, 9–11 years-old (10 normal and 10 obese) | GM/fecal samples | Higher Ruminococcus spp. in normal weight but Prevotella spp. in OB; 19-fold increase in Human herpesvirus 4 in feces of obese children; inverse relationship between Oscillospiraceae family and cholesterol level in OB | [85] |
| Human/26 subjects (13 normoweight vs. 13 obese) | GM/fecal samples | Increased Collinsella, Clostridium XIVa, and Catenibacterium; decreased Clostridium sensu stricto, Romboutsia, Oscillibacter and Alistipes in OB | [86] |
| Human/Indonesian adults (n = 21) | GM/fecal samples | Reduced bacterial diversity and higher primary bile acids concentration in OB | [87] |
| Human/21 adults with OB vs. 21 controls | GM/fecal samples | Decreased gut microbiota diversity and Firmicutes/Bacteroidetes ratio in OB; increased Megamonas, Prevotella, Fusobacterium, and Blautia but decreased incertae_sedis, Lachnospiracea_ Gemmiger, Clostridium XlVa, and Faecalibacterium in OB | [88] |
| Human/normo-weight vs. obese | Oral microbiome/saliva | Greater abundance of the Capnocytophaga genus in OB | [89] |
| Human/male and female adults | GM/fecal samples | Elevated Prevotella/Bacteroides ratio and reduced fecal tryptophan level in OB | [90] |
| Obese cats and normal weight cats | GM/fecal samples | Reduced diversity and abundance of Firmicutes, and reduced ratio of Firmicutes/Bacteroidetes in obese cats | [91] |
| Human/Infants of women with OB | GM/stool samples at 1, 6, and 12 months | Reduced levels of SCFA-producing bacteria (Ruminococcus and Turicibacter) and fecal butyric acid in obese vs. normal infants at 1 month age; decreased levels of Lachnospiraceae at 6 months age | [92] |
| Human/30 obese and 30 normal weight children aged 3–5 years | Oral and GM/saliva and fecal samples | Increased abundance of Filifactor and Butyrivibrio in the saliva and Faecalibacterium, Tyzzerella, and Klebsiella in the fecal samples in OB | [93] |
| Human/infants born to obese and normoweight mothers (23/group) | GM/stool samples | Higher Bacillota/Bacteroidota ratio at 6 months of age with maternal OB | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohesara, S.; Mostafavi Abdolmaleky, H.; Pirani, A.; Pettinato, G.; Thiagalingam, S. The Obesity–Epigenetics–Microbiome Axis: Strategies for Therapeutic Intervention. Nutrients 2025, 17, 1564. https://doi.org/10.3390/nu17091564
Nohesara S, Mostafavi Abdolmaleky H, Pirani A, Pettinato G, Thiagalingam S. The Obesity–Epigenetics–Microbiome Axis: Strategies for Therapeutic Intervention. Nutrients. 2025; 17(9):1564. https://doi.org/10.3390/nu17091564
Chicago/Turabian StyleNohesara, Shabnam, Hamid Mostafavi Abdolmaleky, Ahmad Pirani, Giuseppe Pettinato, and Sam Thiagalingam. 2025. "The Obesity–Epigenetics–Microbiome Axis: Strategies for Therapeutic Intervention" Nutrients 17, no. 9: 1564. https://doi.org/10.3390/nu17091564
APA StyleNohesara, S., Mostafavi Abdolmaleky, H., Pirani, A., Pettinato, G., & Thiagalingam, S. (2025). The Obesity–Epigenetics–Microbiome Axis: Strategies for Therapeutic Intervention. Nutrients, 17(9), 1564. https://doi.org/10.3390/nu17091564






