Overnight Fasting and Body Weight: Emulated Target Trial Using Cancer Prevention Study-3 Data
Highlights
- Stratified analyses revealed sex-specific differences in the effect of overnight fasting duration on body weight: fasting for ≥12 hours overnight was linked to higher body weight compared to fasting for <12 hours; however, in women, fasting for ≥12 hours showed no difference in body weight compared to fasting for <12 hours.
- Stratified analyses revealed sex-specific differences in the association between overnight fasting duration and body weight: among men, fasting for ≥12 hours overnight was linked to higher body weight compared to fasting for <12 hours, whereas no such difference was observed among women.
- Preliminary data suggest that, compared to shorter overnight fasting, longer overnight fasting may be associated with higher body weight in men but not in women; however, these findings need to be replicated before informing recommendations.
Abstract
1. Introduction
2. Materials and Methods
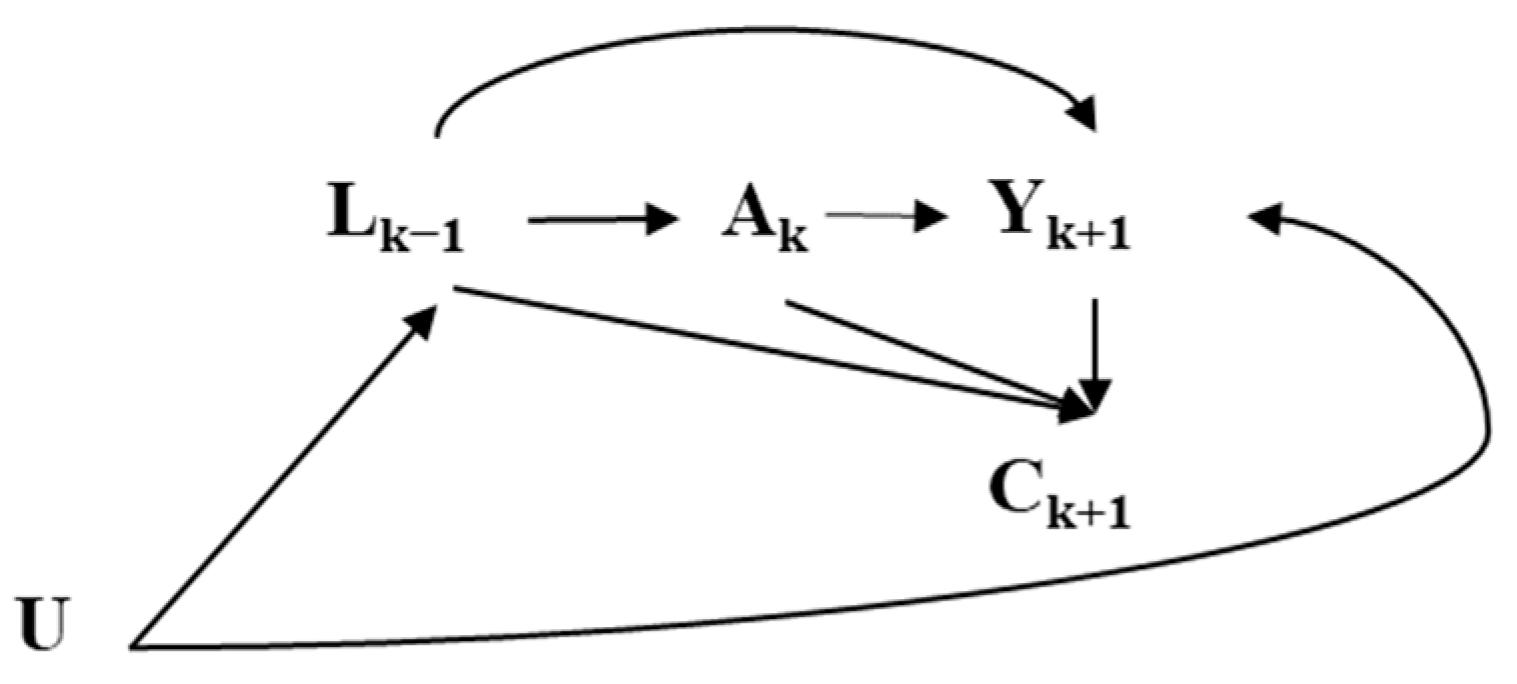
2.1. Target Trial
- Overnight fasting trial: <12 h vs. ≥12 h;
- Before-sleep fasting trial: <4 h vs. ≥4 h;
- After-sleep fasting trial: <1 h vs. ≥1 h.
2.2. Target Trial Emulation Using Observational Data
2.3. Modifications to the Target Trial Protocol
2.4. Statistical Analyses
3. Results
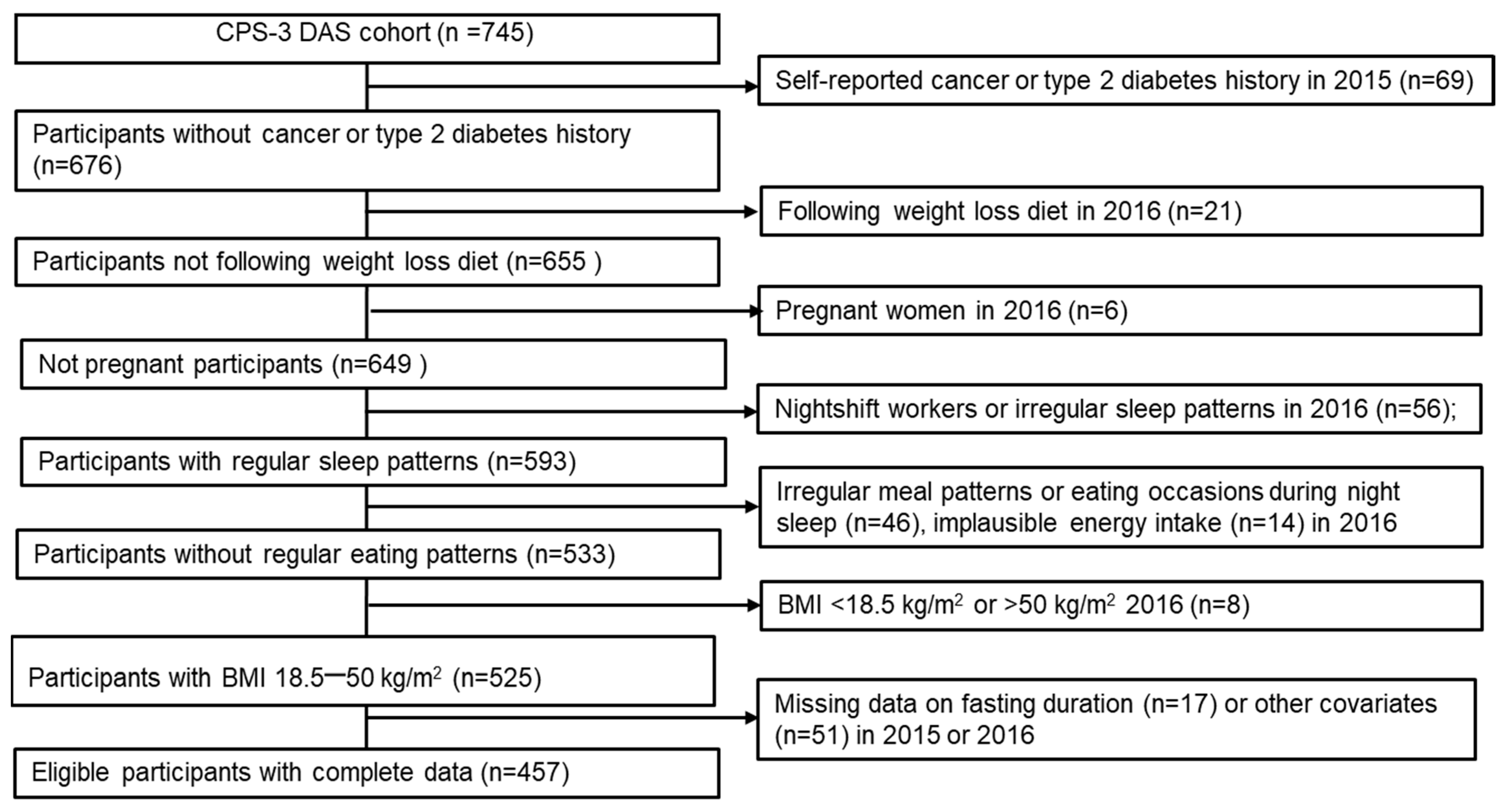
3.1. Study Population
3.2. Emulated Target Trial Estimates
3.3. Subgroup Analyses
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPS-3 DAS | Cancer Prevention Study-3 The Diet Assessment Substudy |
| MET | Metabolic Equivalent of Task |
| BMI | Body Mass Index |
Appendix A
| Intervention Strategy | Estimated Mean 1 and Mean Difference (95% CI) (kg), Stratified by Pre-Baseline Fasting Exposure | |||
|---|---|---|---|---|
| Stratified by overnight fasting 2 | <12 h (n = 246) | ≥12 h (n = 211) | ||
| <12 h overnight fasting | 79.4 (76.9, 82.2) | 0 (Referent) | 77.8 (73.5, 84.1) | 0 (Referent) |
| ≥12 h overnight fasting | 82.5 (74.2, 93.6) | 3.1 (−5.0, 14.3) | 78.3 (75.6, 81.0) | 0.6 (−6.7, 5.2) |
| Stratified by before-sleep fasting 3 | <4 h (n = 230) | ≥4 h (n = 227) | ||
| <4 h before-sleep fasting | 78.6 (76.4, 81.0) | 0 (Referent) | 76.3 (73.3, 79.5) | 0 (Referent) |
| ≥4 h before-sleep fasting | 79.9 (76.4, 83.4) | 1.4 (−2.3, 5.1) | 79.2 (76.0, 82.0) | 2.9 (−0.4, 5.8) |
| Stratified by after-sleep fasting 4 | <1 h (n = 239) | ≥1 h (n = 218) | ||
| <1 h after-sleep fasting | 78.4 (75.7, 82.6) | 0 (Referent) | 77.4 (73.4, 81.4) | 0 (Referent) |
| ≥1 h after-sleep fasting | 77.5 (73.9, 80.9) | −0.9 (−5.3, 3.0) | 80.8 (77.6, 83.8) | 3.4 (−1.6, 7.5) |
| Sensitivity Analysis | Estimated Mean Body Weight 1 (95% CI)/Mean Difference (95% CI) (kg) | |||||
|---|---|---|---|---|---|---|
| Overnight Fasting | Before-Sleep Fasting | After-Sleep Fasting | ||||
| <12 h | ≥12 h | <4 h | ≥4 h | <1 h | ≥1 h | |
| 0. Primary | 78.9 (75.5, 82.9)/Reference | 79.4 (76.2, 822)/0.4 (−4.1, 4.7) | 77.5 (75.7, 79.3)/Reference | 79.4 (76.9, 81.5)/1.9 (−0.4, 4.1) | 78.9 (75.6, 84.1)/Reference | 79.8 (77.6, 81.9)/0.9 (−4.3, 4.4) |
| 1. Imputation 2,3 | 80.5 (77.5, 83.4)/Reference | 79.7 (76.6, 83.2)/−0.8 (−4.0, 3.0) | 78.1 (76.4, 80.0)/Reference | 80.0 (78.0, 82.2)/1.9 (−0.1, 3.9) | 79.6 (76.6, 83.3)/Reference | 79.5 (77.4, 81.7)/−0.1 (−3.6, 3.1) |
| 2. Truncation 4 | 79.7 (75.4, 83.9)/Reference | 79.6 (76.4, 82.2)/−0.1 (−4.6, 4.6) | 77.6 (75.7, 79.2)/Reference | 79.4 (76.9, 81.5)/1.8 (−0.4, 4.0) | 78.6 (72.6, 89.3)/Reference | 79.8 (77.6, 81.9)/1.2 (−9.2, 7.1) |
| 3. Interaction 5 | 79.1 (75.6, 82.7)/Reference | 79.3 (75.9, 82.4)/0.5 (−3.9, 4.5) | 77.5 (75.6, 79.3)/Reference | 79.4 (76.9, 81.6)/1.9 (−0.4, 4.1) | 78.9 (75.7, 82.0)/Reference | 80.0 (77.9, 81.9)/0.8 (−4.6, 4.4) |
| 4. Further adjustment for physical activity 6 | 78.7 (73.5, 86.0)/Reference | 79.2 (74.3, 83.6)/0.4 (−8.7, 7.3) | 77.0 (74.7, 79.0)/Reference | 80.3 (76.9, 84.4)/3.3 (−0.1, 8.1) | 78.5 (74.3, 85.6)/Reference | 79.5 (75.9, 83.2)/1.0 (−7.1, 6.4) |
| 5. Further adjustment for before- or after-sleep durations 7 | - | - | 77.5 (75.5, 79.4)/Reference | 79.4 (76.9, 81.5)/1.9 (−0.4, 4.1) | 78.9 (75.7, 84.0)/Reference | 79.8 (77.7, 81.9)/0.9 (−4.0, 4.5) |
| 6. Further adjustment for the number of eating occasions 8 | 79.0 (75.4, 83.3)/ Reference | 79.3 (76.0, 82.3)/0.3 (−4.7, 4.7) | 77.4 (75.5, 79.2)/ Reference | 79.4 (77.0, 81.6)/2.0 (−0.4, 4.2) | 78.9 (75.7, 84.6)/ Reference | 79.9 (77.8, 82.0)/1.0 (−4.2, 4.5) |
References
- Wirth, M.D.; Zhao, L.; Turner-Mcgrievy, G.M.; Ortaglia, A. Associations between fasting duration, timing of first and last meal, and cardiometabolic endpoints in the national health and nutrition examination survey. Nutrients 2021, 13, 2686. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Adult Obesity Prevalence Maps; U.S. Dept of Health and Human Services: Washington, DC, USA, 2023.
- Jakicic, J.M.; Powell, K.E.; Campbell, W.W.; Dipietro, L.; Pate, R.R.; Pescatello, L.S.; Collins, K.A.; Bloodgood, B.; Piercy, K.L. Physical activity and the prevention of weight gain in adults: A systematic review. Med. Sci. Sports Exerc. 2019, 51, 1262–1269. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4-and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Manoogian, E.N.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef]
- Moro, T. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.J.; Langton, H.M.; Mulligan, M.; Egan, B. Effects of 8 wk of 16: 8 time-restricted eating in male middle-and long-distance runners. Med. Sci. Sports Exerc. 2021, 53, 633–642. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve months of time-restricted eating and resistance training improves inflammatory markers and cardiometabolic risk factors. Med. Sci. Sports Exerc. 2021, 53, 2577. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Using big data to emulate a target trial when a randomized trial is not available: Table 1. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef]
- Guo, F.; McGee, E.E.; Chiu, Y.-H.; Giovannucci, E.; Mucci, L.A.; Dickerman, B.A. Evaluating recommendation-based dietary and physical activity strategies for prostate cancer prevention: A target trial emulation in the health professionals follow-up study. Am. J. Epidemiol. 2024, 194, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Elahy, V.; Jiang, L.; Lee, S.; Odegaard, A.O. A hypothetical intervention of the timing of dietary intake on weight and body composition after initial weight loss. Obesity 2023, 31, 1095–1107. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chavarro, J.E.; Dickerman, B.A.; Manson, J.E.; Mukamal, K.J.; Rexrode, K.M.; Rimm, E.B.; Hernán, M.A. Estimating the effect of nutritional interventions using observational data: The american heart association’s 2020 dietary goals and mortality. Am. J. Clin. Nutr. 2021, 114, 690–703. [Google Scholar] [CrossRef]
- Westerberg, M.; Garmo, H.; Robinson, D.; Stattin, P.; Gedeborg, R. Target trial emulation using new comorbidity indices provided risk estimates comparable to a randomized trial. J. Clin. Epidemiol. 2024, 174, 111504. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.M.; Hernán, M.Á.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef]
- Greenland, S.; Robins, J.M. Identifiability, exchangeability and confounding revisited. Epidemiol. Perspect. Innov. 2009, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A. Methods of public health research—Strengthening causal inference from observational data. N. Engl. J. Med. 2021, 385, 1345–1348. [Google Scholar] [CrossRef]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef]
- Phoi, Y.Y.; Keogh, J.B. Dietary interventions for night shift workers: A literature review. Nutrients 2019, 11, 2276. [Google Scholar] [CrossRef]
- Hartman, T.J.; Masters, M.; Flanders, W.D.; Wang, Y.; Li, M.; Mitchell, D.C.; Guinter, M.; Patel, A.V.; McCullough, M.L. Self-reported eating-occasion frequency and timing are reproducible and relatively valid in the american cancer society cancer prevention study-3 diet assessment substudy. J. Nutr. 2022, 152, 2827–2836. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Mengi Çelik, Ö.; Köksal, E.; Aktürk, M. Time-restricted eating (16/8) and energy-restricted diet: Effects on diet quality, body composition and biochemical parameters in healthy overweight females. BMC Nutr. 2023, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Jacobs, E.J.; Dudas, D.M.; Briggs, P.J.; Lichtman, C.J.; Bain, E.B.; Stevens, V.L.; McCullough, M.L.; Teras, L.R.; Campbell, P.T.; et al. The american cancer society’s cancer prevention study 3 (cps-3): Recruitment, study design, and baseline characteristics. Cancer 2017, 123, 2014–2024. [Google Scholar] [CrossRef]
- Troeschel, A.N.; Hartman, T.J.; Flanders, W.D.; Wang, Y.; Hodge, R.A.; McCullough, L.E.; Mitchell, D.C.; Sampson, L.; Patel, A.V.; McCullough, M.L. The American Cancer Society Cancer Prevention Study-3 FFQ has reasonable validity and reproducibility for food groups and a diet quality score. J. Nutr. 2020, 150, 1566–1578. [Google Scholar] [CrossRef]
- McCullough, M.L.; Wang, Y.; Hartman, T.J.; Hodge, R.A.; Flanders, W.D.; Stevens, V.L.; Sampson, L.; Mitchell, D.C.; Patel, A.V. The Cancer Prevention Study-3 FFQ is a reliable and valid measure of nutrient intakes among racial/ethnic subgroups, compared with 24-hour recalls and biomarkers. J. Nutr. 2021, 151, 636–648. [Google Scholar] [CrossRef]
- Hodge, J.M.; Shah, R.; McCullough, M.L.; Gapstur, S.M.; Patel, A.V. Validation of self-reported height and weight in a large, nationwide cohort of U.S. adults. PLoS ONE 2020, 15, e0231229. [Google Scholar] [CrossRef]
- Rees-Punia, E.; Matthews, C.E.; Evans, E.M.; Keadle, S.K.; Anderson, R.L.; Gay, J.L.; Schmidt, M.D.; Gapstur, S.M.; Patel, A.V. Demographic-specific validity of the cancer prevention study-3 sedentary time survey. Med. Sci. Sports Exerc. 2019, 51, 41–48. [Google Scholar] [CrossRef]
- McCullough, M.L.; Masters, M.; Hartman, T.J.; Flanders, W.D.; Playdon, M.C.; Elahy, V.; Hodge, R.A.; Teras, L.R.; Wang, Y.; Patel, A.V. Length of overnight fasting and 6-year weight change in the Cancer Prevention Study-3. J. Nutr. 2024, 154, 2834–2842. [Google Scholar] [CrossRef]
- Hammerton, G.; Munafò, M.R. Causal inference with observational data: The need for triangulation of evidence. Psychol. Med. 2021, 51, 563–578. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Stel, V.S.; Tripepi, G.; Dekker, F.W.; Fu, E.L.; Zoccali, C.; Jager, K.J. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 2022, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A. IP weighting and marginal structural models censoring and missing data. In Causal Inference: What If; Chapman & Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Piaggi, P. Metabolic determinants of weight gain in humans. Obesity 2019, 27, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.E.; Chasens, E.R.; Bizhanova, Z.; Sereika, S.M.; Buysse, D.J.; Imes, C.C.; Kariuki, J.K.; Mendez, D.D.; Cajita, M.I.; Rathbun, S.L.; et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int. J. Obes. 2021, 45, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Comparison of four methods for handing missing data in longitudinal data analysis through a simulation study. Open J. Stat. 2014, 4, 933–944. [Google Scholar] [CrossRef]
- Sijtsma, F.P.; Meyer, K.A.; Steffen, L.M.; Shikany, J.M.; Van Horn, L.; Harnack, L.; Kromhout, D.; Jacobs, D.R. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: The coronary artery risk development in young adults study. Am. J. Clin. Nutr. 2012, 95, 580–586. [Google Scholar] [CrossRef]
- Cheng, W.; Meng, X.; Gao, J.; Jiang, W.; Sun, X.; Li, Y.; Han, T.; Zhang, D.; Wei, W. Relationship between circadian eating behavior (daily eating frequency and nighttime fasting duration) and cardiovascular mortality. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 22. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Cienfuegos, S.; Corapi, S.; Gabel, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of intermittent fasting on reproductive hormone levels in females and males: A review of human trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef]
- Ongphiphadhanakul, B.; Rajatanavin, R.; Challurkit, L.; Piaseu, N.; Teerarungsikul, K.; Sirisriro, R.; Komindr, S.; Puavilai, G. Serum testosterone and its relation to bone mineral density and body composition in normal males. Clin. Endocrinol. 1995, 43, 727–733. [Google Scholar] [CrossRef]
- Mudali, S. Effects of testosterone on body composition of the aging male. Mech. Ageing Dev. 2004, 125, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, B.; Riat, A.; Ghashang, S.K.; Eljurnazi, L.; Gutenbrunner, C. A prospective clinical trial of prolonged fasting in healthy young males and females—Effect on fatigue, sleepiness, mood and body composition. Nutrients 2020, 12, 2281. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity. JAMA Intern. Med. 2020, 180, 1491. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020, 31, 92–104.e105. [Google Scholar] [CrossRef]
- Liu, J.; Yi, P.; Liu, F. The effect of early time-restricted eating vs. later time-restricted eating on weight loss and metabolic health. J. Clin. Endocrinol. Metab. 2023, 108, 1824–1834. [Google Scholar] [CrossRef]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, controlled daytime versus delayed eating impacts weight and metabolism. Curr. Biol. 2021, 31, 650–657.e653. [Google Scholar] [CrossRef]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.-J.; Peterson, C.M. Effectiveness of early time-restricted eating for weight loss, fat loss, and cardiometabolic health in adults with obesity. JAMA Intern. Med. 2022, 182, 953. [Google Scholar] [CrossRef]
- Hernán, M.A.; Wang, W.; Leaf, D.E. Target trial emulation. JAMA 2022, 328, 2446. [Google Scholar] [CrossRef]
- Danaei, G.; Tavakkoli, M.; Hernán, M.A. Bias in observational studies of prevalent users: Lessons for comparative effectiveness research from a meta-analysis of statins. Am. J. Epidemiol. 2012, 175, 250–262. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
| Component | Target Trial Specifications | Target Trial Emulation |
|---|---|---|
| Aim | Estimate the effect of overnight fasting, before-sleep fasting, and after-sleep fasting strategies on body weight in U.S. adults across three independent interventions. | Same as target trial. |
| Eligibility criteria | Exclude:
| Same as target trial. Additional exclusion criteria: Missing data on fasting duration or other covariates at pre-baseline or baseline. |
| Treatment strategies | Individuals would be assigned to one of the following strategies:
| Same as target trial. |
| Treatment assignment | Random assignment at baseline (2016), with strategy awareness. Treatment starts at assignment and lasts 1 year with monthly adherence checks. | Emulate randomization by adjusting for pre-baseline confounders (race, sex, education, age, work status, income BMI, diet quality, alcohol intake, and respective pre-baseline fasting durations). No adherence assessment during the follow-up. |
| Follow-up | Follow-up begins from baseline (2016) when an individual is assigned to treatment. Follow-up ends at the earliest of death, loss to follow-up, or administrative end of follow-up 2 years since baseline. Participants are censored if they did not participate in the follow-up, reported pregnancy, or shift work. | Follow-up begins from baseline when an individual returns the CPS-3 DAS 2016 survey. Follow-up ends at the earliest of death, loss to follow-up, or the administrative end of follow-up (the return date of the self-administered CPS-3 2018 survey). Participants are censored if they did not participate in the CPS-3 outcome assessment survey, reported pregnancy, or shift work in 2018. |
| Outcome | Body weight (kg) assessed by self-administered questionnaire in 2018. | Same as target trial. |
| Causal contrast of interest | Intention-to-treat effect. Per-protocol effect. | Cannot distinguish between observational analog of intention-to-treat effect and observational analog of per-protocol effect. |
| Statistical analyses | Intention-to-treat analysis. Per-protocol analysis: Marginal structural models with stabilized inverse probability weight to compare body weight between treatment groups in each intervention, adjusting for baseline and pre-baseline factors related to adherence and censoring. Non-parametric bootstrap for 95% CIs. | Same as the per-protocol analysis in the target trial. |
| Total | Overnight Fasting | Before-Sleep Fasting | After-Sleep Fasting | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | <12 h | ≥12 h | <4 h | ≥4 h | <1 h | ≥1 h | ||
| n = 457 | n = 263 | n = 194 | n = 266 | n = 191 | n = 251 | n = 206 | ||
| Fasting duration range | 8.1–11.9 h | 12.1–17.6 h | 1.1–3.9 h | 4.1–9.5 h | 0–0.9 h | 1.0–8.1 h | ||
| Age, y | 51.2 (9.63) | 51.4 (9.68) | 51.1 (9.59) | 51.2 (9.45) | 51.4 (9.9) | 51.7 (9.93) | 50.7 (9.24) | |
| Male, n (%) | 162 (35.4) | 103 (39.2) | 59 (30.4) | 87 (32.7) | 75 (39.3) | 94 (37.5) | 68 (33) | |
| Race/ethnicity, n (%) | Black | 103 (22.5) | 57 (21.7) | 46 (23.7) | 57 (21.4) | 46 (24.1) | 38 (15.1) | 65 (31.6) |
| Hispanic | 63 (13.8) | 38 (14.4) | 25 (12.9) | 35 (13.2) | 28 (14.7) | 37 (14.7) | 26 (12.6) | |
| White | 291 (63.7) | 168 (63.9) | 123 (63.4) | 174 (65.4) | 117 (61.3) | 176 (70.1) | 115 (55.8) | |
| Education, n (%) | High school/some college | 95 (20.8) | 47 (17.9) | 48 (24.7) | 56 (21.1) | 39 (20.4) | 46 (18.3) | 49 (23.8) |
| College degree | 166 (36.3) | 95 (36.1) | 71 (36.6) | 103 (38.7) | 63 (33) | 92 (36.7) | 74 (35.9) | |
| Graduate degree | 196 (42.9) | 121 (46) | 75 (38.7) | 107 (40.2) | 89 (46.6) | 113 (45) | 83 (40.3) | |
| Income, n (%) | <25 K | 16 (3.5) | 8 (3) | 8 (4.1) | 6 (2.3) | 10 (5.2) | 8 (3.2) | 8 (3.9) |
| 25–<50 K | 55 (12) | 24 (9.1) | 31 (16) | 29 (10.9) | 26 (13.6) | 20 (8) | 35 (17) | |
| 50–<75 K | 67 (14.7) | 44 (16.7) | 23 (11.9) | 43 (16.2) | 24 (12.6) | 38 (15.1) | 29 (14.1) | |
| 75–<100 K | 62 (13.6) | 31 (11.8) | 31 (16) | 33 (12.4) | 29 (15.2) | 33 (13.1) | 29 (14.1) | |
| ≥100 K | 257 (56.2) | 156 (59.3) | 101 (52.1) | 155 (58.3) | 102 (53.4) | 152 (60.6) | 105 (51) | |
| Married or living with partner, n (%) | 335 (73.3) | 199 (75.7) | 136 (70.1) | 201 (75.6) | 134 (70.2) | 193 (76.9) | 142 (68.9) | |
| Current smoker, n (%) | 14 (3.1) | 7 (2.7) | 7 (3.6) | 5 (1.9) | 9 (4.7) | 8 (3.2) | 6 (2.9) | |
| Employed, n (%) | 375 (82.1) | 227 (86.3) | 148 (76.3) | 226 (85) | 149 (78) | 210 (83.7) | 165 (80.1) | |
| Post-menopausal females 2, n (%) | 116 (39.3) | 64 (40.0) | 52 (38.5) | 73 (40.8) | 43 (37.1) | 60 (38.2) | 56 (40.6) | |
| BMI, kg/m2 | 27.1 (5.55) | 26.7 (5.5) | 27.6 (5.57) | 26.9 (5.38) | 27.4 (5.78) | 26.3 (5.11) | 28.1 (5.9) | |
| HEI-2015 score 1 | 71.5 (7.84) | 71.9 (7.96) | 71 (7.67) | 71.2 (7.52) | 71.8 (8.28) | 72.8 (7.36) | 70 (8.14) | |
| Alcohol intake, mL/wk | 9.2 (13.71) | 8.8 (12.73) | 9.8 (14.94) | 8.7 (12.66) | 10 (15.03) | 8.6 (12.53) | 10 (15.01) | |
| Energy intake, kcal/d | 2023.9 (638.1) | 2065.2 (663.9) | 1967.9 (598.5) | 2022.6 (611.0) | 2025.7 (675.6) | 2049.6 (665.5) | 1992.5 (603.2) | |
| Physical activity, MET-h/wk | 6.6 (5.88) | 6.7 (5.93) | 6.4 (5.81) | 6.4 (5.68) | 6.9 (6.14) | 7.2 (5.78) | 5.8 (5.91) | |
| Sleep duration, h | 7.9 (1.02) | 7.7 (0.98) | 8.1 (1.01) | 8 (0.96) | 7.7 (1.07) | 8.1 (0.97) | 7.7 (1.04) | |
| Sleep midpoint 3, HH:MM | 03:18 | 03:18 | 03:24 | 03:12 | 03:36 | 03:24 | 03:18 | |
| Snacks, snacks/day | 1.73 (0.89) | 1.94 (0.90) | 1.45 (0.81) | 1.99 (0.85) | 1.37 (0.83) | 1.73 (0.92) | 1.74 (0.86) | |
| Meals, meals/day | 2.80 (0.42) | 2.88 (0.38) | 2.70 (0.45) | 2.84 (0.42) | 2.74 (0.42) | 2.86 (0.37) | 2.73 (0.46) | |
| Overnight fasting, n (%) | <12 h | 263 (57.5) | 200 (75.2) | 63 (33) | 181 (72.1) | 82 (39.8) | ||
| ≥12 h | 194 (42.5) | 66 (24.8) | 128 (67) | 70 (27.9) | 124 (60.2) | |||
| Before-sleep fasting, n (%) | <4 h | 266 (58.2) | 200 (76) | 66 (34) | 139 (55.4) | 127 (61.7) | ||
| ≥4 h | 191 (41.8) | 63 (24) | 128 (66) | 112 (44.6) | 79 (38.3) | |||
| After-sleep fasting, n (%) | <1 h | 251 (54.9) | 181 (68.8) | 70 (36.1) | 139 (52.3) | 112 (58.6) | ||
| ≥1 h | 206 (45.1) | 82 (31.2) | 124 (63.9) | 127 (47.7) | 79 (41.4) | |||
| Body weight, kg | 78.3 (17.9) | 77.8 (17.7) | 79.1 (18.2) | 77.5 (17.3) | 79.6 (18.7) | 76.4 (17.3) | 80.7 (18.4) | |
| Intervention Strategy | Mean Body Weight 1,2 (kg) (95% CI) | Mean Difference (kg) (95% CI) |
|---|---|---|
| Overnight fasting | ||
| <12 h | 78.9 (75.5, 82.9) | 0 (Referent) |
| ≥12 h | 79.4 (76.2, 82.2) | 0.4 (−4.1, 4.7) |
| Before-sleep fasting | ||
| <4 h | 77.5 (75.5, 79.3) | 0 (Referent) |
| ≥4 h | 79.4 (76.9, 81.5) | 1.9 (−0.4, 4.1) |
| After-sleep fasting | ||
| <1 h | 78.9 (75.6, 84.1) | 0 (Referent) |
| ≥1 h | 79.8 (77.7, 81.9) | 0.9 (−4.3, 4.4) |
| Intervention Strategy | Estimated Mean 1 and Mean Difference (95% CI) (kg), Stratified by Baseline Covariates | |||
|---|---|---|---|---|
| Stratified by Sex 3 | Male (n = 162) | Female (n = 295) | ||
| <12 h overnight fasting | 83.9 (80.4, 87.5) | 0 (Referent) | 77.1 (74.4, 79.7) | 0 (Referent) |
| ≥12 h overnight fasting | 100.9 (95.9, 105.9) | 17.0 (10.8, 23.1) | 74.3 (71.3, 77.3) | −2.8 (−6.8, 1.2) |
| <4 h before-sleep fasting | 84.6 (81.1, 88.1) | 0 (Referent) | 73.6 (71.1, 76.1) | 0 (Referent) |
| ≥4 h before-sleep fasting | 87.1 (83.3, 90.9) | 2.5 (−2.6, 7.6) | 75.4 (72.3, 78.5) | 1.9 (−2.1, 5.8) |
| <1 h after-sleep fasting | 83.4 (80.4, 86.5) | 0 (Referent) | 77.6 (74.6, 80.6) | 0 (Referent) |
| ≥1 h after-sleep fasting | 87.8 (84.1, 91.5) | 4.4 (−0.4, 9.2) | 75.2 (71.7, 78.8) | −2.4 (−7.0, 2.3) |
| Stratified by sleep midpoint 2,4 | Before 3:17 a.m. (n = 235) | 3:17 a.m. or later (n = 222) | ||
| <12 h overnight fasting | 79.0 (76.1, 81.9) | 0 (Referent) | 80.6 (77.1, 84.1) | 0 (Referent) |
| ≥12 h overnight fasting | 78.5 (74.7, 82.2) | −0.5 (−5.2, 4.2) | 80.8 (76.9, 84.7) | 0.2 (−5.0, 5.5) |
| <4 h before-sleep fasting | 75.5 (72.7, 78.3) | 0 (Referent) | 80.1 (76.6, 83.5) | 0 (Referent) |
| ≥4 h before-sleep fasting | 79.7 (75.8, 83.7) | 4.2 (−0.6, 9.1) | 78.8 (75.5, 82.2) | −1.2 (−6.0, 3.5) |
| <1 h after-sleep fasting | 79.5 (76.1, 82.8) | 0 (Referent) | 80.3 (77.0, 83.6) | 0 (Referent) |
| ≥1 h after-sleep fasting | 78.2 (74.6, 81.9) | −1.2 (−6.2, 3.7) | 82.5 (78.4, 86.6) | 2.2 (−3.1, 7.5) |
| Stratified by BMI 5 | <25 kg/m2 (n = 204) | ≥25 kg/m2 (n = 253) | ||
| <12 h overnight fasting | 65.7 (64.0, 67.5) | 0 (Referent) | 89.8 (86.9, 92.7) | 0 (Referent) |
| ≥12 h overnight fasting | 66.5 (64.0, 68.9) | 0.8 (−2.3, 3.8) | 88.2 (84.9, 91.5) | −1.6 (−6.0, 2.8) |
| <4 h before-sleep fasting | 66.1 (64.4, 67.8) | 0 (Referent) | 87.4 (84.6, 90.2) | 0 (Referent) |
| ≥4 h before-sleep fasting | 67.2 (65.1, 69.3) | 1.1 (−1.6, 3.8) | 89.3 (86.3, 92.4) | 1.9 (−2.3, 6.0) |
| <1 h after-sleep fasting | 66.5 (64.8, 68.2) | 0 (Referent) | 89.1 (85.9, 92.3) | 0 (Referent) |
| ≥1 h after-sleep fasting | 68.5 (66.3, 70.8) | 2.1 (−0.8, 4.9) | 88.2 (85.1, 91.4) | −0.8 (−5.3, 3.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elahy, V.; Wang, Y.; Flanders, W.D.; Zhong, C.; McCullough, M.L. Overnight Fasting and Body Weight: Emulated Target Trial Using Cancer Prevention Study-3 Data. Nutrients 2025, 17, 1559. https://doi.org/10.3390/nu17091559
Elahy V, Wang Y, Flanders WD, Zhong C, McCullough ML. Overnight Fasting and Body Weight: Emulated Target Trial Using Cancer Prevention Study-3 Data. Nutrients. 2025; 17(9):1559. https://doi.org/10.3390/nu17091559
Chicago/Turabian StyleElahy, Valeria, Ying Wang, W. Dana Flanders, Charlie Zhong, and Marjorie L. McCullough. 2025. "Overnight Fasting and Body Weight: Emulated Target Trial Using Cancer Prevention Study-3 Data" Nutrients 17, no. 9: 1559. https://doi.org/10.3390/nu17091559
APA StyleElahy, V., Wang, Y., Flanders, W. D., Zhong, C., & McCullough, M. L. (2025). Overnight Fasting and Body Weight: Emulated Target Trial Using Cancer Prevention Study-3 Data. Nutrients, 17(9), 1559. https://doi.org/10.3390/nu17091559





